ABSTRACT
Antioxidants are used as a persuasive remedial measure against overproduction of reactive oxygen species owing to their capacity to neutralize free radicals by oxidation. Immense interest has been observed in antioxidants of natural origin and their contribution in human health. The current investigation is undertaken to explore antioxidant capacity and reducing potential of Entoban syrup and capsules. In vitro antioxidant activity was evaluated by measuring the scavenging ability of syrup and capsules on free radical 2,2’-diphenyl-1-picryl hydrazyl (DPPH). Antiradical activity assay depends on the reduction of DPPH. The reducing ability was determined by the conversion of ferric into ferrous state by antioxidant compounds. Results showed that both formulations of syrup and capsules have excellent antioxidant potential with 8.5 and 10.3 µg/ml IC50 values respectively while butylated hydroxyanisole (BHA), the standard have 5.6 µg/ml IC50 value. When formulations of syrup and capsules were compared at various concentrations (10, 50 and 100 µg/ml), reducing ability increased in a dose dependent manner just like DPPH for both formulations just like standard BHA indicting good reducing ability. In-vitro antioxidant analysis of the polyherbal drug revealed the presence of excellent antioxidant potential and reducing capability which increases in a dose dependent manner for both formulations. It might be helpful in preventing or slowing the progress of various oxidative stress- related diseases related to gastro intestinal tract.
Key words: Antioxidant capacity, reducing potential, Entoban.
Oxidative stress is a physiological condition owing to an inequity among concentrations of reactive oxygen species (ROS) and antioxidants. ROS comprising superoxide radicals, singlet oxygen, hydroxyl radicals, and hydrogen peroxide are frequently produced as a consequence of natural reaction or due to exogenous factors (Finkel and Holbrook, 2000).
In vivo, some of these ROS participate in cell metabolism including energy production, intercellular signaling and phagocytosis. On the other hand, these ROS generated by ultraviolet light, ionizing radiation, sunlight, chemical reactions and metabolic processes have an extensive pathological effects such as DNA damage, carcinogenesis and various degenerative disorders such as cardiovascular diseases, atherosclerosis, aging and neuro-degenerative diseases (Gyamfi et al.,1999; Ganapathy et al., 2011; Gutteridge and Halliwell, 2000; Halliwell, 2001). Since ROS have potential detrimental effects, excessive ROS must be promptly eliminated from the cells (Halliwell, 2007). Though the products of ROS-induced oxidative stress are widely used to observe their biological effects, it is equally significant to assess the antioxidant capability of biological fluids, cells and extracts. Antioxidants usually counterbalance radicals via a hydrogen atom transfer (HAT) or single electron transfer (SET) mechanism. SET assays enumerate the capacity of an antioxidant to transfer one electron to reduce any compound, such as free radicals, carbonyls and metals (Shulaev and Oliver, 2006).
Naturally isolated antioxidants are adhering consideration as potential source due to their effective pharmacological activities, low toxicity and cost-effectiveness. Research has revealed that numeral plant products such as polyphenols, terpenes and different plant extracts exerted an antioxidant action (de Souza et al., 2007; Lin and Yin, 2007; Rice-Evans et al.,1996; Kaur and Kapoor, 2002). Extensive data has been generated on antioxidant properties of food plants around the globe (Djeridane et al., 2006). The investigation for natural compounds affluent in antioxidant properties is escalating because of their magnitude in controlling various chronic disorders (Auddy et al., 2003). The present study was directed to polyherbal formulation Entoban syrup and capsules which integrates an outstanding blend of herbs including Holarrhena antidysenterica , Berberis aristata, Symplocos racemosa, Querecus infectoria and Helicteres isora. This study is undertaken to explore antioxidant capacity and reducing potential of Entoban syrup and capsules.
Composition of Entoban syrup
Each 10 ml contains Aegle marmelos: syrup, oral, 10 mg; Berberis aristata: extract 30 mg; Butea frondosa: dry extract 20 mg; Holarrhena antidysenterica: dry extract 50 mg; Myrtus communis: dry extract 20 mg and Quecrus infectoria: dry extract 50 mg.
Composition of capsule
Each 500 mg capsule contains H. antidysenterica: 40 mg; M. Communis: 40 mg; Symplocos racemosa: 20 mg; Aluminum silicate: 20 mg; Quercus infectoria: 10 mg; Zingiber officinalis: 10 mg; Helicteres isora: 10 mg; Berberis aristata: 10 mg; Butea frondosa: 10 mg; Aegle marmelos: 10 mg and Acacia Arabica: 10 mg.
Plant Material
Herbs used in Entoban syrup and capsules were stored in dark at 23°C. All herbs were tested for their prescribed part, macro and microscopic descriptions.
Preparation of plant extract
Individual herbs were taken into the grinder and were sieved through 60 # mesh to get the desired particle size. Individual grinded herbs were taken into extractor and water as solvent was added to the grinded herbs in the ratio of 1:10 with herb: solvent. The extractors were heated with steam for 2 to 3 h to get the desired extract in the form of decoction (individual liquid extract). The decoctions were than filtered and transferred to evaporators to remove the extra solvent, and to get the desired moisture content that is, not more than 25%. The individual extracts were stored in the form of thick extracts.
Chemicals and reagents
The solvents used were of high-performance liquid chromatography (HPLC) grade. The standard (Butylated hydroxyanisole) and chemicals used were obtained from Merck, Pakistan. 1,1-diphenyl-2-picryl hydrazyl (DPPH) radicals was purchased from Sigma-Aldrich Chemie (Buchs, Switzerland).
DPPH radical scavenging activity
The antioxidant activity was assessed by measurement of scavenging ability of the syrup and capsules on free radical 2,2’-diphenyl-1-picryl hydrazyl (DPPH; C18H12N5O6). Antiradical activity assay depends on the reduction of 1, 1-diphenyl-2-picrylhydrazyl. DPPH free radicals showed strong absorption maximum at 517 nm due to odd electrons. When this electron becomes paired off in the presence of a hydrogen donor for example any antioxidant, the absorption strength is decreased, and colour changed from purple to yellow, with respect to the number of electrons captured (Gülçin et al., 2005). 2, 2-Diphenyl-1-(2, 4, 6-trinitrophenyl) hydrazyl (M.W= 394.24) (Sigma) was prepared in ethanol in the concentration of 3 mM. Each well in 96 well plate was labelled as control, blank and test compound of various concentrations. DPPH solution (95 µl) was added in the labelled wells. The test compound (5 µl) of concentration 10- 1000 mM in DMSO) was then added in DPPH solution and reaction mixture was mixed for few seconds. The reaction was taken place in wells when 96 well plate was incubated at 37°C for 30 min. The micro titre plate was read at the absor-bance of 515 nm (Spectramax plus 384 Molecular Device, USA) after 30 min. Percentage of radical scavenging activity was calculated with respect to DMSO treated control. Butylated hydroxyanisole (BHA) was taken as standard. The DPPH radical scavenging activities were determined by means of the following equation:

Determination of the reducing power
The reducing ability was determined by the conversion of ferric into ferrous state by antioxidant compounds using the method of Oyaizu (1986). Each test compound (100 ml: 10-1000 mM) prepared in DMSO/ was mixed with phosphate buffer (250 ml: pH 6.6: 0.2 M). Potassium ferricyanide (250 ml: 1%) was then added to the contents in the test tube. This mixture was then incubated at 50°C for 20 min in water bath, and was centrifuged for 10 min at 3000 rpm. After centrifugation, upper layer of solution (250 ml) was separated in another set of test tubes and mixed with equal volume of Dimethyl sulfoxide (DMSO) (250 ml). Ferric chloride (0.1 %: 50 ml) was added to the mixture and the absorbance was determined at 700 nm on spectrophotometer (Specord 2000, Germany). Percent reduction ability was determined in terms of percentage with respect to BHA used as standard.
Superoxide scavenging activity by alkaline DMSO method
For this method to be performed (Sanja et al., 2009) nitro-blue tetrazolium (NBT) was prepared in the concentration of 1 mg/ml pure DMSO (AVONCHEM). Alkaline DMSO was prepared by adding 0.1 ml 5 mM NaOH solution in water and 0.9 ml DMSO. Stock solution of 1 mM of test compounds was prepared and serial dilutions were made at the time of assay in the range of 7 to 1000 µM in DMSO or methanol depending on the best solubility. 100 ml NBT (1 mg/ml) was added in each of the tubes. In tube marked as test, compound was added of different known concentrations in the volume of 300 µl. Finally, 1 ml alkaline DMSO was added to give total volume 1.4 ml. After 5 min incubation time, test compound may inhibit the purple coloured formazan formation which was generated by reaction of superoxide radicals liberated by alkaline DMSO with NBT. In the tube of control, 0.1 mM of NBT in 100 µl solvent (DMSO or methanol) compounds were made and 1 ml pure DMSO (not alkaline) was taken. Absorbance was measured at 560 nm against control and the percentage of super oxide radical scavenging by the test compounds were calculated by
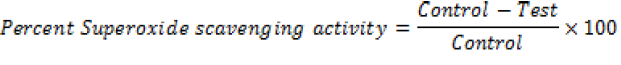
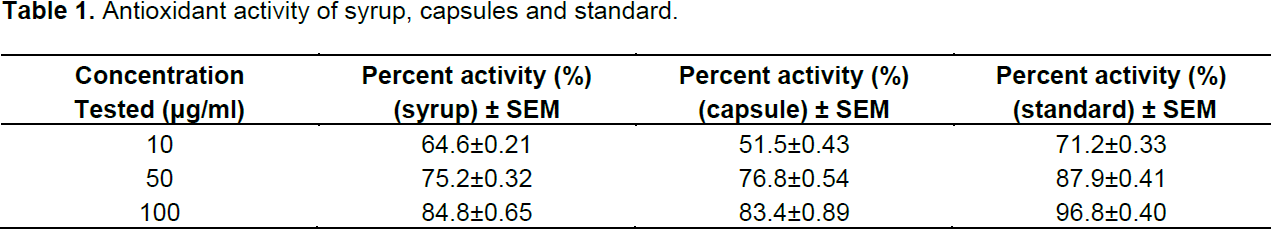
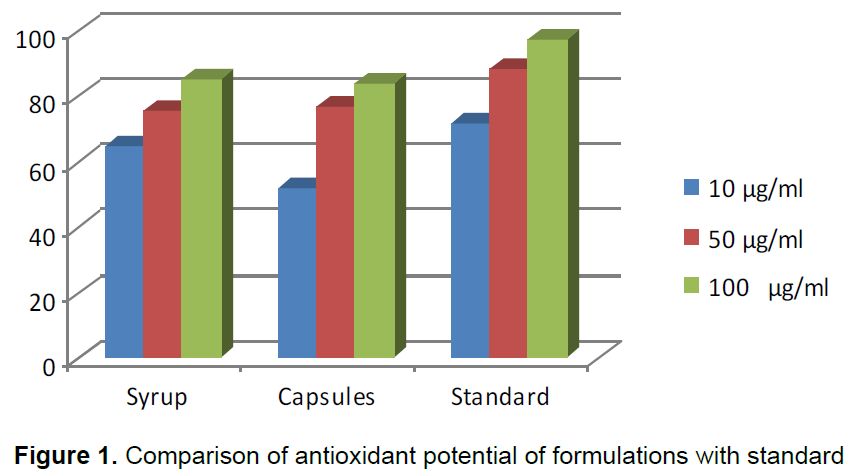
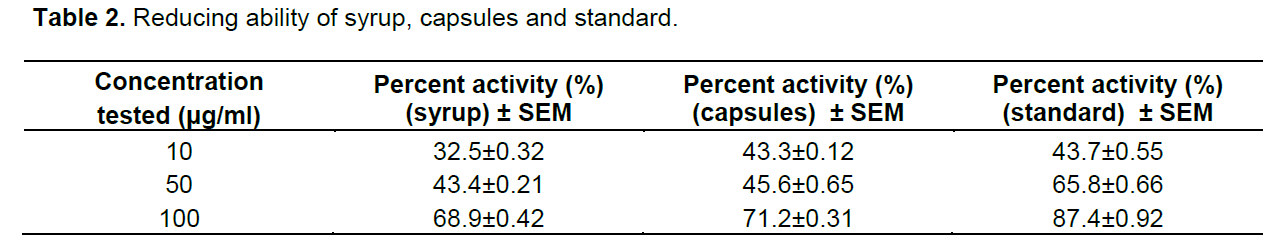
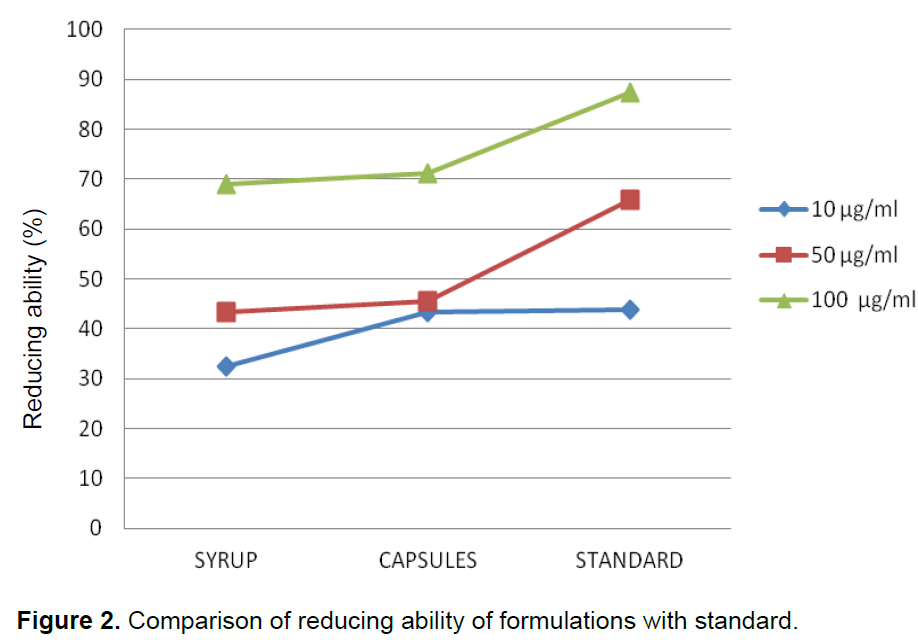
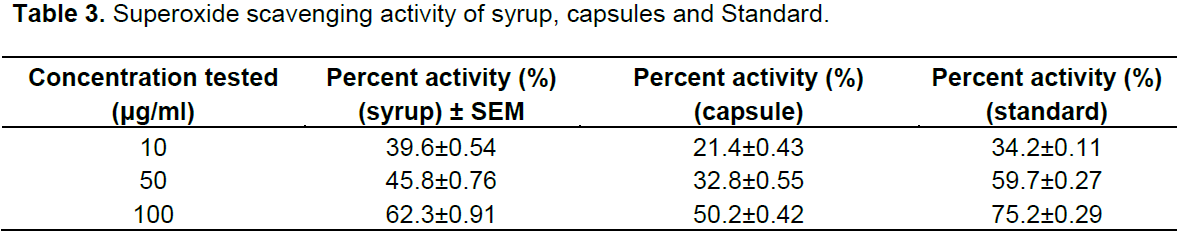
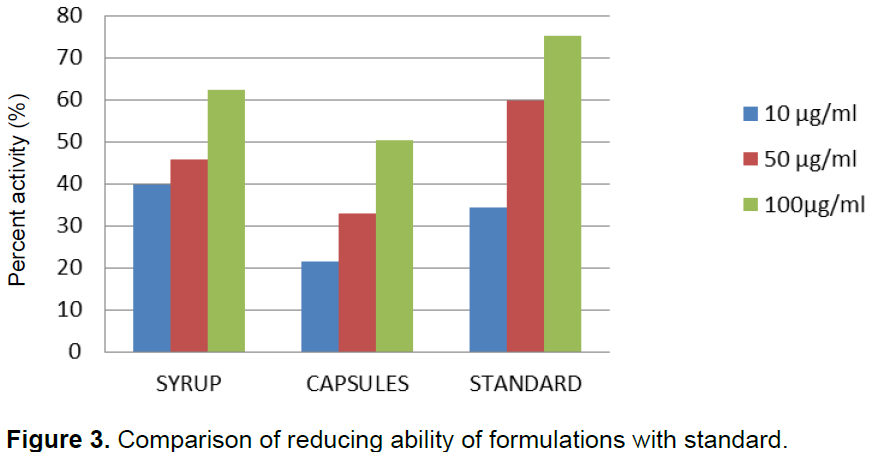
When formulations of syrup and capsules were compared at various concentrations (10, 50 and 100 µg/ml), DPPH radical scavenging activity increased in a dose dependent manner for both formulations just like standard BHA (Table 1). Results showed that both formulations of syrup and capsules have excellent antioxidant potential with 8.5 and 10.3 µg/ml IC50 values respectively while BHA, the standard have 5.6 µg/ml IC50 value (Figure 1). When formulations of syrup and capsules were compared at various concentrations (10, 50 and 100 µg/ml), reducing ability increased in a dose dependent manner just like DPPH for both formulations just like standard BHA (Table 2). Results showed that both formulations of syrup and capsules have good reducing ability (Figure 2). Superoxide radicals are known to be very harmful to the cellular component. Super oxide free radical was formed by alkaline DMSO which reacts with NBT to produce colored diformazan. Formulations of syrup and capsules were compared at various concentrations (Table 3). Both syrup and capsules have moderate activity to scavenge superoxide radicals (Figure 3).
Research has proved that the antioxidants may be imperative in the pathogenesis of certain diseases and ageing. The use of antioxidant supplementation in dropping the level of oxidative stress and in preventing the development of complications allied with diseases (Preethi et al., 2010). Many synthetic additives, like BHA and butylhydroxytoluene (BHT), have been used to preserve food from oxidation for long, with no apparent toxicological effects. However, their utilization is constrained because they are suspected to be carcinogenic (isora Linn, 2009).
Natural antioxidants safeguard the human body from free radicals, put off oxidative stress and related diseases and thereby play an extremely significant role in health care. Plants contain several compounds with antioxidant activity such as phenolic acids, flavonoids, anthocyanins, tannins and carotenoids. Flavonoids and other phenolic compounds of plant source have revealed their activity as scavengers and inhibitors of lipid peroxidation (Kalim et al., 2010). Consequently, the researchers have focused on natural antioxidants and various crude extracts, and pure natural compounds have been reported to possess antioxidant activities.
The present study was directed to polyherbal formulation entoban syrup and capsules which integrates an outstanding blend of herbs including H. antidysenterica, B. aristata, S. racemosa, Q. infectoria and H. isora. The antioxidant activity of these herbs have already reported in the study of Kaur et al. (2008), Vijayabaskaran et al., (2010), Suthar et al. (2009). Further more, gallic acid is a common phytoconstituent present in ingredients which has been reported to possess antioxidant activity (Soong and Barlow, 2006). Therefore the present study was aimed to evaluate the antioxidant activity of the polyherbal formulation. When formulations of syrup and capsules were compared at various
concentrations (10, 50 and 100 µg/ml), DPPH radical scavenging activity increased in a dose dependent manner for both formulations just like standard BHA (Table 1). Results showed that both formulations of syrup and capsules have excellent antioxidant potential with 8.5 and 10.3 µg/ml IC50 values respectively while BHA, the standard have 5.6 µg/ml IC50 value. The scavenging activity on DPPH radicals is commonly used as screening method for assessing the antiradical activity of a range of compounds (Sharma and Bhat, 2009). DPPH is a stable free radical that possesses a distinguishing absorption maximum between 515 and 517 nm, which is diminished in the presence of a compound competent of reducing it to its hydrazine form by a hydrogen/electron transfer reaction (Huang et al., 2005).
A direct association between antioxidant capability and reducing potential of certain plant extracts has been reported. The reducing powers are usually correlated with the presence of reductones, which exert antioxidant action by breaking the free radical chain by donating a hydrogen atom (ElmastaÅŸ et al., 2006). When formu-lations of syrup and capsules were compared at various concentrations (10, 50 and 100 µg/ml), reducing ability increased in a dose dependent manner just like DPPH for both formulations just like standard BHA. It has been reported that the phenol and polyphenolic compound (flavonoids) constituents of the plant-possess antioxidant properties primarily due to their redox properties, which permit them to act as reducing agents, hydrogen donors and singlet oxygen quenchers (Hossain et al., 2011).
The relation between antioxidant and antidiarrheal activities has already been explored. Research has shown that children with crohn disease have alterations in circulating antioxidant defenses, possibly related to an ongoing oxidant stress (Hoffenberg et al., 1997). Rahman and Wilcock reported that medicinal plants were having antidiarrheal properties due to their reducing potential containing polyphenolic compounds (Rahman and Wilcock, 1991).
In this study, plant extract showed conspicuous antioxidant capacity owing to the polyphenolic compound constituents of the plants used in the preparation. This capability may partly contribute the antidiarrheal activity. It was revealed that the extract did show the proton donating ability and could serve up as free radical inhibitor or scavenger. Indeed, the radical scavenging capability of phenolic compounds in the prepared extract is due to their hydrogen donating ability/number of hydroxyl groups present.
In-vitro antioxidant analysis of the polyherbal drug revealed the presence of excellent antioxidant potential, and reducing capability which increases in a dose dependent manner for both formulations. It might be helpful in preventing or slowing the progress of various oxidative stress- related diseases related to gastro intestinal tract.
The authors have none to declare.
REFERENCES
|
Auddy B, Ferreira M, Blasina F, Lafon L, Arredondo F, Dajas F, Tripathi PC, Seal T, Mukherjee B (2003). Screening of antioxidant activity of three Indian medicinal plants, traditionally used for the management of neurodegenerative diseases. J. Ethnopharmacol. 84(2):131-138.
Crossref
|
|
|
|
de Souza MCR, Marques CT, Dore CMG, da Silva FRF, Rocha HAO, Leite EL (2007). Antioxidant activities of sulfated polysaccharides from brown and red seaweeds. J. Appl. Psychol. 19(2):153-160.
Crossref
|
|
|
|
|
Djeridane A, Yousfi M, Nadjemi B, Boutassouna D, Stocker P, Vidal N (2006). Antioxidant activity of some Algerian medicinal plants extracts containing phenolic compounds. Food Chem. 97(4):654-660.
Crossref
|
|
|
|
|
ElmastaÅŸ M, Gülçin I, Beydemir Åž, Ä°rfan KüfrevioÄŸlu Ö, Aboulâ€Enein HY (2006). A study on the in vitro antioxidant activity of juniper (Juniperus communis L.) fruit extracts. Anal letter 39(1):47-65.
Crossref
|
|
|
|
|
Finkel T, Holbrook NJ (2000). Oxidants, oxidative stress and the biology of ageing. Nature 408(6809):239-247.
Crossref
|
|
|
|
|
Ganapathy PS, Ramachandra Y, Rai SP (2011). In vitro antioxidant activity of Holarrhena antidysenterica Wall. methanolic leaf extract. J. Basic Clin. Pharm. 2(4):175-178.
|
|
|
|
|
Gülçin I, Berashvili D, Gepdiremen A (2005). Antiradical and antioxidant activity of total anthocyanins from Perilla pankinensis Decne. J. Ethnopharmacol. 101(1):287-293.
Crossref
|
|
|
|
|
Gutteridge J, Halliwell B (2000). Free radicals and antioxidants in the year 2000: a historical look to the future. Ann. NY Acad. Sci. 899(1):136-147.
Crossref
|
|
|
|
|
Gyamfi MA, Yonamine M, Aniya Y (1999). Free-radical scavenging action of medicinal herbs from Ghana: Thonningia sanguinea on experimentally-induced liver injuries. Gen. Pharmacol. Vasc. System 32(6):661-667.
Crossref
|
|
|
|
|
Halliwell B (2001). Role of free radicals in the neurodegenerative diseases. Drugs aging 18(9):685-716.
Crossref
|
|
|
|
|
Halliwell B (2007). Biochemistry of oxidative stress. Biochem. Soc. Transactions 35(5):1147-1150.
Crossref
|
|
|
|
|
Hoffenberg EJ, Deutsch J, Smith S, Sokol RJ (1997). Circulating antioxidant concentrations in children with inflammatory bowel disease. Am. J. Clin. Nutr. 65(5):1482-1488.
|
|
|
|
|
Hossain MS, Alam MB, Asadujjaman M, Zahan R, Islam MM, Mazumder MEH, Haque ME (2011). Antidiarrheal, Antioxidant and Antimicrobial Activities of the Musa sapientum Seed. Avicenna J. Med. Biotechnol. 3(2):95.
|
|
|
|
|
Huang D, Ou B, Prior RL (2005). The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 53(6):1841-1856.
Crossref
|
|
|
|
|
Isora Linn H (2009). In-vitro antioxidant activity of hot aqueous extract of Helicteres isora Linn. fruits. Nat. Prod. Radiance 8(5):483-487
|
|
|
|
|
Kalim MD, Bhattacharyya D, Banerjee A, Chattopadhyay S (2010). Oxidative DNA damage preventive activity and antioxidant potential of plants used in Unani system of medicine. BMC Complement Altern. Med. 10(1):77.
Crossref
|
|
|
|
|
Kaur C, Kapoor HC (2002). Antiâ€oxidant activity and total phenolic content of some Asian vegetables. Int. J. Food Sci. Technol. 37(2):153-161.
Crossref
|
|
|
|
|
Kaur G, Athar M, Alam MS (2008). Quercus infectoria galls possess antioxidant activity and abrogates oxidative stress-induced functional alterations in murine macrophages. Chem. Biol. interactions 171(3):272-282.
Crossref
|
|
|
|
|
Lin CC, Yin MC (2007). B vitamins deficiency and decreased anti-oxidative state in patients with liver cancer. Eur. J. Nutr. 46(5):293-299.
Crossref
|
|
|
|
|
Oyaizu M (1986). Studies on products of browning reaction—antioxidative activities of products of browning reaction prepared from glucosamine. Jap J. Nutr. 44(6):307-315.
Crossref
|
|
|
|
|
Preethi R, Devanathan VV, Loganathan M (2010). Antimicrobial and antioxidant efficacy of some medicinal plants against food borne pathogens. Adv. Biol. Res. 4(2):122-125.
|
|
|
|
|
Rahman M, Wilcock C (1991). A report on flavonoid investigation in some Bangladesh Asclepiads. Bangladesh J. Bot. 20(2):175-178.
|
|
|
|
|
Rice-Evans CA, Miller NJ, Paganga G (1996). Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 20(7):933-956.
Crossref
|
|
|
|
|
Sharma OP, Bhat TK (2009). DPPH antioxidant assay revisited. Food Chem. 113(4):1202-1205.
Crossref
|
|
|
|
|
Shulaev V, Oliver DJ (2006). Metabolic and proteomic markers for oxidative stress. New tools for reactive oxygen species research. Plant Physiol. 141(2):367-372.
Crossref
|
|
|
|
|
Soong YY, Barlow PJ (2006). Quantification of gallic acid and ellagic acid from longan (Dimocarpus longan Lour.) seed and mango (Mangifera indica L.) kernel and their effects on antioxidant activity. Food Chem. 97(3):524-530.
Crossref
|
|
|
|
|
Suthar M, Rathore G, Pareek A (2009). Antioxidant and antidiabetic activity of Helicteres isora (L.) fruits. Indian J. Pharm. Sci. 71(6):695.
Crossref
|
|
|
|
|
Sanja SD, Sheth NR, Patel NKD, Patel B (2009). Characterization and evaluation of antioxidant activity of Portulaca oleracea. Int. J. Pharm. Sci. 74-84.
|
|
|
|
|
Vijayabaskaran M, Yuvaraja K, Babu G, Sivakumar P, Perumal P, Jayakar B (2010). Hepatoprotective and antioxidant activity of Symplocos racemosa bark extract on DMBA induced hepatocellular carcinoma in rats. Int. J. Curr. Trends Sci. Technol. 1(3):147-158.
|
|