ABSTRACT
Chitosan and chitin sources have emerged as promising groups of biological substances that can induce plant growth and resistance to diseases. This study is aimed at investigating the potential effect of chitosan and snail shell to promote cocoa growth and induce resistance against Phytophthora megakarya. The results showed that treatment of cocoa seeds with chitosan at 1.5 to 2.0% and snail shell at 2.0% increases the percentage of germination and also reduces the time of germination. 12 weeks after planting, a series of morphological changes was observed on the plants treated with chitosan and snail shell. Plant height (34 to 39.5 cm), leaf number (11 to 13 leaves/plant), leaf area (88 to 130 cm2) and fresh weight of roots and shoots (25 to 44 g/plant) increased significantly in the chitosan and snail shell treated soils. Pods inoculated with chitosan and/or snail shell treated soil suspensions presented very slight necrotic lesions. This could be linked to a decrease in the P. megakarya load of the soil suspension. Leaf inoculation showed variation among the treatments with the lowest index (highest level of resistance) recorded in plants treated either with chitosan or snail shell. The treatment of plants with chitosan and snail shell powder before and after inoculation showed higher level of phenolic compounds than in the control plants. Furthermore, the inoculation induced a significant accumulation of proteins in the cocoa plants treated with chitosan and snail shell. The level of proteins accumulation after inoculation was higher in plants treated with snail shell than those treated with chitosan. In conclusion, the effect of snail shell on cocoa growth and resistance showed that it is possible for snail shell powder to be a biofungicide and biofertilizer used in the control of cacao Black Pod Disease in nurseries.
Key words: Theobroma cacao, Phytophthora megakarya, black pod disease, chitosan, snail shell, biocontrol agents.
In Cameroon and other African countries, cocoa (Theobroma cacao) is one of the most important economical cash crops. However, its cultivation is faced with numerous problems such as parasitic attack and the insufficiency of selected genotypes. Among the parasitic constraints, Black Pod Disease (BPD) caused by several Phytophthora species has been the worst threat of cacao in West and Central Africa (Opoku et al., 2000). Reports have shown that in Cameroon, the BPD caused by Phytophthora megakarya is the highest threat; and losses of up to 50 - 80% of cocoa beans have been reported (Ndoumbe-Nkeng et al., 2004). This disease reduces the yield and quality of the product, and also increases the cost of production. The cocoa fruits, stems or roots can be infected by chlamydospores or sporangia, which may germinate to produce swimming zoospores that can be spread by drops of rain, wind-blown rain, soil and soil water. In order to prevent these losses due to P. megakarya infection, different strategies have been developed. Chemical control using metalaxyl and copper-based fungicides has been reported to be the most effective strategy to reduce the impact of BPD in nurseries and in farms (Ndoumbe-Nkeng et al., 2004; Sonwa et al., 2008). However, application of fungicides can have drastic effects on the consumer and the environment (Naseby et al., 2000). A promising and safer method for controlling cocoa BPD has been the development of resistant cultivars and the use of appropriate cultural practices (Nyasse et al., 2007; Tchameni et al., 2011). However, disease resistant cultivars are not yet available.
A biocontrol approach using chitosan or chitin sources (snail shell, crab skeleton, shrimp skeleton) is an eco-friendly alternative. Chitosan is a carbohydrate biopolymer derived from deacetylation of chitin, which is found in the shells of Crustaceans, cuticules of insects and cell walls of fungi. The positive charge of chitosan confers to it numerous and unique physiological and biological properties with great potential in a wide range of agricultural practices (Bautista-Banos et al., 2004; Tang et al., 2010). Many studies have reported the capacity of chitosan to stimulate the immune system for plant resistance to pathogen infection, to induce the accumulation of phytoalexins resulting in antifungal responses in order to enhance protection against further infection (Coqueiro et al., 2011) and also to change the soil microorganisms content (Roy et al., 2010). Moreover, chitosan has been widely used as a growth stimulator, germination accelerator and yield enhancer in many crop species such as in orchid (Uthairatanakij et al., 2007), faba bean (El-Sawy et al., 2010), cucumber (Shehata et al., 2012) and corn (Boonlertnirun et al., 2011; Lizárraga-Paulín et al., 2011).
This study is therefore aimed at investigating the ability of chitosan and snail shell powder to promote cocoa growth and induce resistance against P. megakarya in nurseries by evaluation of the rate of germination of seeds, plant agro-morphological characteristics, suppressive potential, total phenolic compounds and peroxidase activities. Our findings will contribute to the evaluation of the role of chitosan and snail shell powder as a biofungicide alternative in the control of cocoa BPD.
Soil
The soil used in this experiment was collected from Yaoundé (Centre region, Cameroon) and are often used by farmers to prepare young cocoa seedlings. The soil was air-dried and passed through a 4 mm sieve before mixing (3:1; v/v) with river sand. Chemical analysis (organic matter, nitrogen, calcium, magnesium, phosphorus contents and pH) of dry samples was carried out before the cultivation period. The contents of available nutrients in the soil were: organic matter, 3.40%; nitrogen, 1.23%; calcium, 6.48×10-3 meq.g-1 of soil; magnesium, 23.20×10-3 meq.g-1 of soil; phosphorus, 3.54 mg.g-1 of soil; and pH was 6.1. The soil-sand mix was then autoclaved three times at 121°C for 30 min before being transferred into pots.
Fungal strains and snail shell powder production
Zoospore suspensions of P. megakarya isolate EL from the core collection of IRAD (Institut de Recherche Agricole pour le Développement, Yaounde, Cameroon) were obtained according to Tondje et al. (2006). Sterilized and unsterilized soils were then inoculated with 106 zoospore.kg-1 of soil.
Snail shell was obtained following the method of Jideowno et al. (2007). The shells (from Buea, in the South west region of Cameroon) were thoroughly washed using tap water and air-dried for two days. They were then dried in an oven and at 105°C, then pounded in a mortar using a pestle to form the snail shell powder. Sterilized and unsterilized soils were inoculated with zoospores of P. megakarya at 106 zoospore.kg-1 of soil for 48 h before, being treated with 1% chitosan and snail shell powder. Chitosan was kindly provided by Professor Carole Beaulieu, Centre SEVE University of Sherbrooke (Quebec, Canada).
Evaluation of germination rate
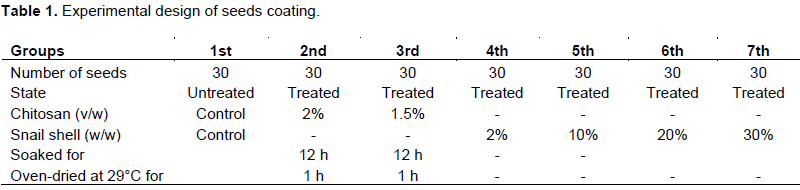
For evaluation of germination rate and plant agro-morphological characters, mature cocoa pods (♀MA12 × â™‚PA150) hybrids produced by manual pollination were collected from the SODECAO (Société de Développement du Cacao) gene banks of Mengang Station (South Region, Cameroon; Latitude 2°90’N, Longitude 11°20’E). Cocoa seeds were extracted from the pods, washed with distilled water and coated as shown in Table 1. For the germination test, 8 groups of 30 seeds each were sown per germination tray. A 2% chitosan stock solution was prepared as described by Lizárraga-Paulín et al. (2011), and the 1.5% solution was obtained by appropriate dilution of the stock solution. Experimental seeds were sown at a depth of 5 cm in sterilized soils and the trays incubated at 25 ± 1°C. Distilled water was sprinkled on the trays every two days to avoid dryness. Germination was confirmed by a shooting of the radicule and the germination rate evaluated as in Zeng et al. (2012).
Evaluation of agro-morphological characters
The agro-morphological characters that were assessed include the weight of the plant root and shoot weight, height, leaf number, length, width and area of the leaf. These parameters were assessed every 4 weeks for a period of 12 weeks. To produce the plants, a single pregerminated cocoa seedling was transplanted into each plastic pot, which contained treated and untreated soil. Each treatment was in duplicates of ten pots. All the pots were kept in the greenhouse and watered with distilled water every two days for a period of 12 weeks. During assessment at the 4 weeks interval, roots of harvested plants were washed to remove soil particles and plant height measured with a caliper tip. Length and width of leaves measured with a graduated ruler and the weight of shoots and roots of freshly harvested plants then measured separately.
The experiment was a completely randomized design with two treatments chitosan and/or snails shells at 1% w/w, and the control (treatments without chitosan and/or snail shells). The treatments without chitosan and/or snail shells are also designed: sterilize Soil (sS), sterilize Soil + P. megakarya (sS+Pm), and none sterilize Soil (nsS). The treatments with chitosan and/or snails shells are designed as: sterilize Soil (sS) + Chitosan (sS+Ch), sterilize Soil + P. megakarya + Chitosan (sS+Pm+Ch), none sterilize Soil (nsS) + Chitosan (nsS+Ch), sterilize Soil + Snail Shell (sS+SS), sterilize Soil + P. megakarya + Snail Shell (sS+Pm+SS), and none sterilize Soil + Snail Shell (nsS+SS). Each treatment consisting of three replicates were repeated twice.
Induced resistance assessment
Young cocoa leaves from two-month-old plants were collected from the nursery in the greenhouse washed thoroughly with tap water and sterilized with 70% ethanol for 30 s. Sixteen leaf discs of 1.5 cm diameter from each treatment were made with a cork borer and replicated three times. These leaf discs were placed with their abaxial surface upwards in side trays. On the other hand, six whole leaves of cocoa were set apart in a single tray and arranged according to the protocol described by Djocgoue et al. (2007). These discs and whole leaves were all simultaneously inoculated with 10 µl of 106 zoospore ml-1 suspension of P. megakarya. In each treatment, one control made up of six whole leaves and leaf discs inoculated with 10 µl of sterilized distilled water in a separate tray was included. Inoculation was performed on the underside of each leaf, and the trays were incubated in a dark room at 25 ±1°C. Disease expression was rated six days after, using the rating scale developed by Nyasse et al. (1995). This experiment was repeated twice, and the severity of disease was determined for each treatment by calculating the ratio of the sum of individual scores to the total number of discs leaves used. The disease severity index used to express the resistance level (Paulin et al., 2008) was as follows: Highly Resistant (HR: 0 < index ≤ 1); Resistant (R: 1 < index ≤ 2); Moderately Resistant (MR: 2 < index ≤ 2.5); Susceptible (S: 2.5 < index ≤ 3.5); and Highly Susceptible (HS: 3.5 < index ≤ 5).
Biochemical analyses
Biochemical analyses were carried out following the assessment of infection on the whole leaves. The samples involved were cut at 1 cm beyond the necrosis point or beyond the marked scar (sections with no symptoms). Samples from the same treatments were combined. The parts of the leaves from sterilized Soil (sS) treatment were combined. For biochemical analyses, each treatment was repeated twice.
Determination of the content of total phenolic compounds
The extraction and quantitative measurement of the content of total phenolic compounds were performed as described by Djocgoue et al. (2007) with modification. Total phenolic compounds were extracted twice using 80% methanol. 1 g of fresh tissue of inoculated and healthy leaves plant was ground separately in 10 ml of 80% methanol at 4°C. After 5 min of agitation, the ground material was centrifuged at 10000 g for 5 min at 4°C. The supernatant was collected and the pellet was re-suspended in 5 ml of 80% methanol followed by agitation for 5 min. After the second centrifugation at 4°C, the supernatant was collected and mixed with the previously collected supernatant to constitute the phenolic extract. The concentration of phenolic compounds was determined spectrophotometrically at 725 nm according to the method of Marigo (1973), using the Folin-Ciocalteu reagent. Total phenolic compound contents were expressed in mg equivalent of catechin per g of fresh weight.
Determination of the content of total native protein
For the determination of total native protein content, extraction was performed as described by Priminho et al. (2008) with modification. 1 g of fresh tissue of inoculated and healthy leaves plant was ground separately in 10 ml of extraction buffer (Tris-HCl 10 mM pH 7.5, Triton X-100 1%) at 4°C, stirred for 10 min and kept on ice. The samples were sonicated (8 pulses of 3 s each with 10 s intervals) with the setting at 70% output on an Ultrasonic processor (Gex 130, 130 W), and then centrifuged at 10000 g for 25 min at 4°C. The pellet was submitted to a second extraction. Both supernatants were mixed with 0.4 volume of n-butanol and 1/10 of 3 M NaAc pH 4.5. The samples were kept on ice for 30 min with agitation every 10 min, and then centrifuged at 10000 g for 15 min at 4°C. The supernatant containing total proteins was stored at 4°C. The proteins were quantified using the Bradford (1976) method. 1 ml of Bradford reagent was added to each ml of extract. The absorbance was measured at 595 nm using a UV-VIS 1605 Shimadzu
spectrophotometer. BSA was used as the standard.
Peroxidases activity
Peroxidase activity was determined by spectrophotometry at 470 nm in the total native protein extracts according to the method of Boudjeko et al. (2005) using Tris-maleate buffer and oxygenated water (H2O2). The enzyme activity was expressed in enzyme unit per g of fresh weight.
Evaluation of the suppressive effect of snail shell
For evaluation of the suppressive effect of snail shell and chitosan in the soil, namely, P. megakarya, 3 month-old healthy pods (SNK10, susceptible clone) were harvested, washed with tap water, sterilized with 70% ethanol (for 1 min), 10% (v/v) commercial sodium hypochlorite (for 5 min) and rinsed 3 times with sterilized distilled water. The inoculation was carried out by the deposition of 500 µl suspensions of untreated and treated soils collected after 12 weeks of experiment on the scar obtained with hand utensils. The scars are then closed with cotton that has been immersed in sterilized water. The soil suspension was obtained by mixing soil with sterilized distilled water. That is, 2 g of soil sample was mixed with 10 ml of sterilized distilled water, shaken and allowed to stand for 10 min. A control constituted of pods inoculated with only sterilized distilled water was realized. The entire inoculated pods were incubated in a dark room at 25 ± 1°C in a humid chamber. The rate of necrosis was qualitatively evaluated on daily basis for 6 days using the sign + or -.
Statistical analyses
Data analysis was performed using the SPSS software version 17.0. All the results were expressed as means ± standard deviation and subjected to Analysis of Variance (ANOVA). Where significant differences were found, pairs of samples were compared by Tukey’s test at p ≤ 0.05.
Effect of chitosan and snail shell on seed germination
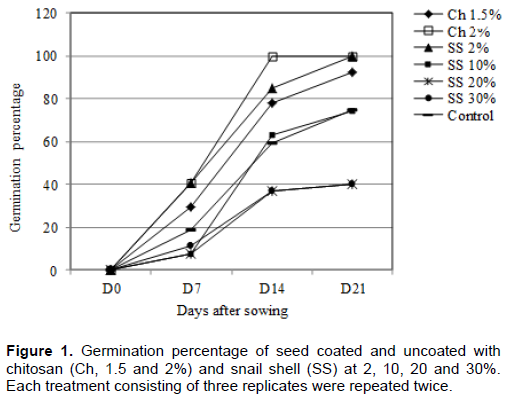
The treatment of cocoa seeds with chitosan at 1.5 to 20% and snail shell at 2% increases the percentage of germination and also reduces the time of germination compared to the control seeds (Figure 1). Treatment of seeds with chitosan at 2% increased the germination percentage after sowing from 0 to 40% within 7 days (D7) and to 100% within 14 days (D14). Treatment of seeds with snail shell (SS) coating at 2% also increased the germination percentage from 0 to 40% within 7 days (D7) but reaches 100% germination within 21 days (D21). The treatment with snail shell at 10 and 20% did not increase the germination percentage at the same rate. Hence, with these concentrations, the germination percentage increased from 0 to 60% within 14 days (D14) and reaches 75% after 21 days (D21). The effect of treatment with snail shell at 30% concentration was at the same level with the control seeds, that is, 40% of germination within 21 days (D21).
Effect of chitosan and snail shell on agro-morphological characteristics
The agro-morpho logical characteristics of cocoa seedlings (height of the plant, number of leaves, area of leaf, weight of shoots and roots) grown in soils treated with chitosan and snail shell were variably affected in different stages after 12 weeks of growth (Table 2). The heights of cocoa seedlings grown in soil treated with chitosan and snail shell powder were both significantly different from those of the control plants. The control plants (nsS, sS and sS+Pm) belonged to the same group with an average height of 26 cm. The plants grown in sterilized soil (sS) and unsterilized soil (nsS), treated either with chitosan (sS+Ch and nsS+Ch) or with snail shell (sS+SS and nsS+SS) had an average height of 38 cm and belonged to the same group. The presence of P. megakarya in the sterilized soil (sS) slowed down this growth in height as shown on Table 2, with an average height of 34 cm for the treatments sS+Pm+Ch and sS+Pm+SS. Furthermore, the number of leaves and the area of leaves of cocoa plants grown in the soils (nsS, sS and sS+Pm) treated either with chitosan or snail shell powder varied according to the treatment after 12 weeks of growth (Table 2). The average number of leaves in the control plants (nsS, sS and sS+Pm) was 7 leaves and was significantly different from those of the treated plants which had an average of 12 leaves, with no significant difference between the treatments. The area of leaves showed 2 different groups: the control plants and treated plants. The treatments without chitosan and/or snail shells (sS, sS+Pm, nsS) showed level of leaf areas significantly lower than those the treatments with chitosan and/or snails shells (sS+Ch, sS+Pm+Ch, nsS+Ch,sS+SS, sS+Pm+SS, nsS+SS).
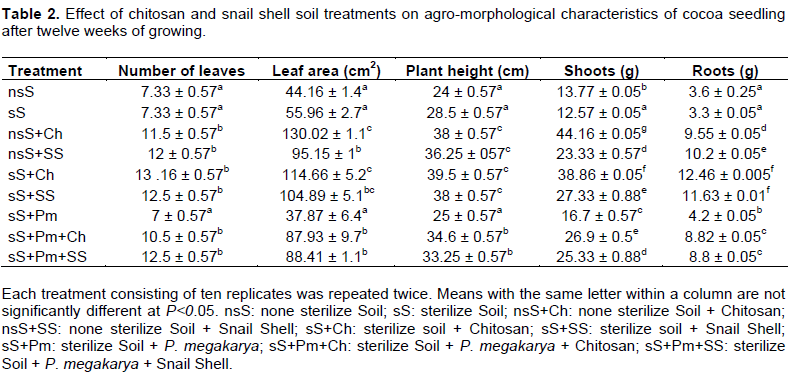
The weights of fresh shoots and roots of the cocoa plants grown in soils treated either with chitosan or snail shell powder were significantly different between the treated plants and the control (Table 2). The weight of the shoots had 3 different groups: the first group (nsS, sS and sS+Pm) had a mean of 14 g, the second group (sS+Ch, sS+Pm+Ch, nsS+Ch,sS+SS, sS+Pm+SS, nsS+SS) had a mean of 33 g and the third group (sS+Ch and nsS+Ch) had the highest. The weight of the roots had 2 different groups: the first group (nsS, sS and sS+Pm) had a mean of 4 g, the second group (sS+Ch, sS+Pm+Ch, nsS+Ch,sS+SS, sS+Pm+SS ,nsS+SS) had a mean of 10 g. Chitosan treatments consistently improved the weight of the cocoa shoots and roots more than that of snail shell treatment.
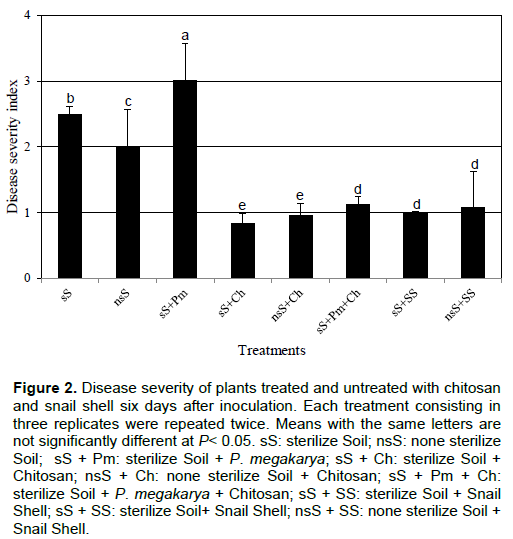
Evaluation of disease severity
The discs of cocoa leaves inoculated with 10 µl of 106 zoospore.ml-1 suspension of P. megakarya developed a clear lesion six days after, while no symptom was seen on discs of leaves inoculated with sterilized distilled water. The disease severity was significantly (p < 0.05) different among the treatments. The highest level of disease severity was observed with the control treatments (sS, nsS and sS+Pm). While, the lowest level of disease severity was recorded in plants treated with either chitosan or snail shell, showing a disease severity index of 0.83 to 1.15 for chitosan treatments and 1 to 1.4 for snail shell treatments (Figure 2).
Biochemical analyses
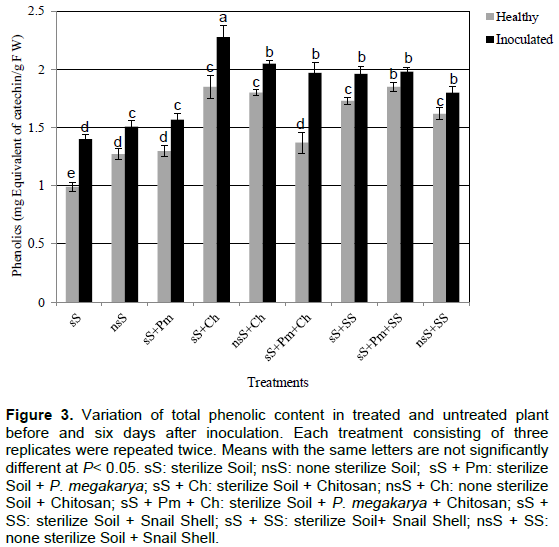
The amount of total phenolic compounds in non-inoculated plants was lower than the inoculated ones. The inoculations of leaves had a significant effect on total phenolic contents in all the treatments. The treatment of plants with chitosan and snail shell powder before and after inoculation showed higher level of phenolic compounds than in the control plants. Chitosan treatment had a more significant effect as compared to snail shell treatment (Figure 3). The presence of P. megakarya in the soil seemed to slow down the increase of the amount of phenolic compounds except for the chitosan treatment. Chitosan treatment showed an increase of the amount of phenolic compounds after inoculation, with an increase of 14, 23 and 44% for the treatments nsS+Ch, sS+Ch and sS+Pm+Ch, respectively. This was not the case with the snail shell treatment which showed an increased level of phenolic compounds after inoculation that was significantly lower, regardless of the nature of treatment.
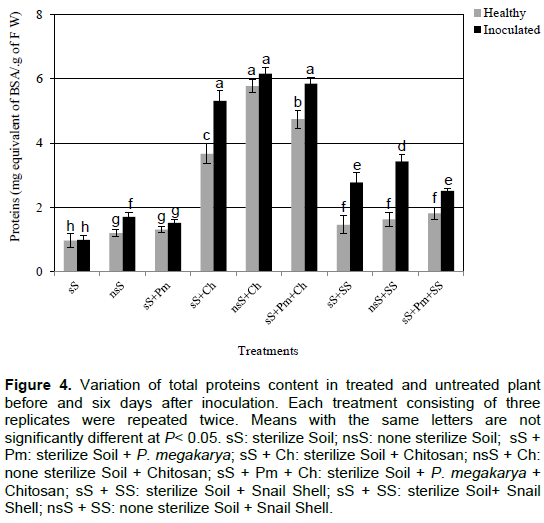
The amount of proteins was much lower in the plants grown in control soils (nsS, sS and sS+Pm) before and after inoculation. The treatment with snail shell and chitosan increased the protein level in healthy and inoculated plants (Figure 4). The presence of P. megakarya in the soil had no significant effect on the protein in the plants compared to the other treatments. The inoculation induced a significant accumulation of proteins in the cocoa plants treated with chitosan and snail shell. The proteins accumulation after inoculation was higher in plants treated with snail shell than those treated with chitosan (Figure 4).
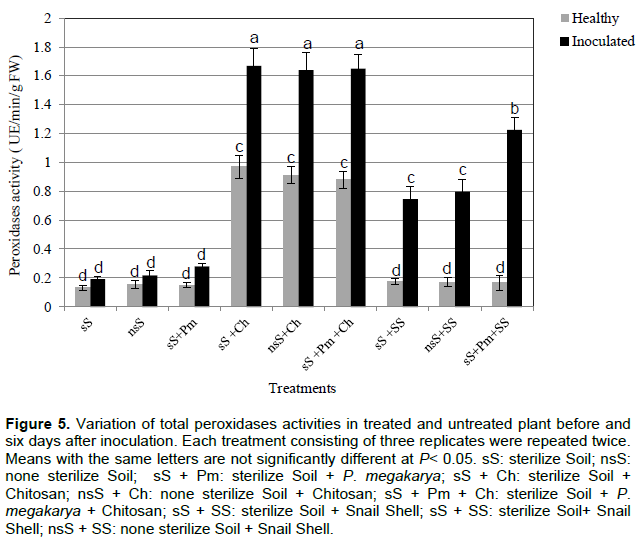
The peroxidase activity of plant leaves grown in control soils (nsS, sS and sS+Pm) before and after inoculation was not significantly different from that of the treatments nsS+SS before and after inoculation (Figure 5). In plants treated with chitosan, the peroxidase accumulation was higher in the non-inoculated plants with an average of 0.92 UE/min/g fresh weight, and this amount significantly rose to 76% in all the plant leaves after inoculation (sS+Ch, sS+Pm+Ch and nsS+Ch). Furthermore, there was a significant difference in peroxidase accumulation between the leaves of plants treated with chitosan and those treated with snail shell.
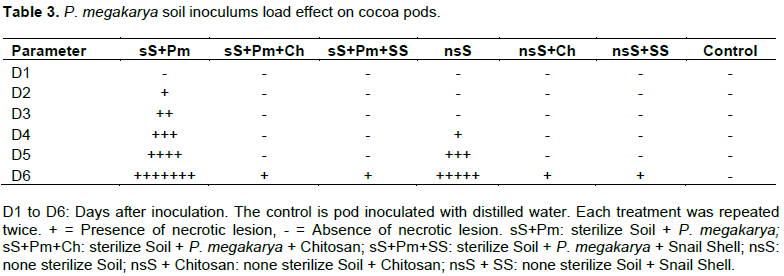
Effect of P. megakarya soil inoculum load on cocoa pods
The cocoa pods inoculated with sterile soil containing P. megakarya and non-sterile soil treated with chitosan and snail shell, respectively showed very low levels of necrotic lesions that appeared only 6 days (D6) after inoculation. For the treatments without chitosan and snail shell (nsS and sS+Pm), necrotic lesions appeared and spread on the inoculated cocoa pods from D4 and D2 after inoculation till D6. The pods inoculated with sterilized soil and with zoospores of P. megakarya (sS+Pm) got completely rotten (Table 3).
The aim of this study was to assess the ability of chitosan and snail shell powder to induce growth and resistance of cocoa against P. megakarya,the causalagent of BPD in nurseries and fields in Cameroon. Differences that emerged from seed germination, measurement of plant growth and quantitative production of selected biochemical molecules, following the infection of the plants with P. megakarya, provided a number of underlying evidence to show that soil treatment with organic matter could improve growth of cocoa seedlings and induce resistance to BPD. In our study, chitosan (up to 2%) and snail shell (up to 2%) seed coating increased the percentage of germination compared to control. These results confirm the earlier findings that seeds soaked with chitosan increased the germination percentage (Zeng et al., 2012). This could be due to the excellent film-forming capacity of chitosan, making it easy to form a semi-permeable film on the seed surface which can maintain the seed moist and absorb the moisture from the soil. Furthermore, Tahereh et al. (2012) reported that seeds coated with chitosan increased the lipase and β-1,3 glucanase enzyme activity which caused better germination. However, despite the evidence of its activity from many studies, the mechanism of chitosan effect on seed germination is still unknown. The plant growth promoting effects of chitosan observed in this study are consistent with the results of many authors who reported the positive effects of chitosan incorporated into soil on early growth stages of soybean, mini-tomato, upland rice and lettuce (Hilal et al., 2006). Like chitosan, the promoting effect of snail shell powder on the growth of the cocoa seedlings, is in agreement with the study of Beauséjour et al. (2003) who showed that soil amendment with chitinous material stimulated gram-positive bacteria which reduced common scab incidence and induced potato growth.
The absence of necrotic lesions on pods inoculated with soil treated with organic matter could be due to a decrease of P. megakarya load in the soil. This decrease could be correlated to the healthier condition observed in the treated plants compared to the control plants. Several studies have also reported that chitosan and chitinous soil amendment, contribute to plant protection by modifying the microbial community and stimulating plant defense mechanisms (Benhamou et al., 1994; Roy et al., 2010). Furthermore, Xing et al. (2013) showed that non-treated and heat-treated oyster shell powder exhibited antifungal activities.
This study shows that the incidence of disease was significantly reduced in plants obtained from soils treated with chitosan or snail shell. Under controlled conditions, chitosan and snail shell were efficient elicitors of some defense reactions in cocoa. This effect was higher in plants obtained from soil treated with chitosan. The induction of systemic resistance could be explained by the capacity of chitosan and snail shell to stimulate plant defense mechanisms and higher synthesis of plant defense metabolites like phenolic compounds and pathogen-related proteins (Benhamou and Picard, 2000; Mbouobda et al., 2010; Coqueiro et al., 2011). This induction of systemic resistance by snail shell could be due to its composition. It is well known that snail shell is mainly composed of calcium carbonate. Recently, Arfaoui et al. (2015) demonstrated that pretreatment with calcium base formulation enhanced defense-related genes’ expression in soybeans response to Slerotinia sclerotiorum. This phenomenon was confirmed in this study by higher production of phenolic compounds, total native protein contents and higher peroxidase activity in the leaves of cocoa seedlings following chitosan and snail shell soil treatment. At the concentration used, chitosan and snail shell does not affect the development of the cocoa plant. This efficacy at low concentrations suggests that this compound was recognized by plant cells and the observed protection was at least partly due to the induction of plant defense responses. In summary, this study contributes to show that chitosan and snail shell soil treatment initiated a series of morphological as well as biochemical changes in the plants which are considered to be part of the plant defense response. The effect of snail shell on cocoa growth and resistance showed that it is possible for snail shell powder to be a biofungicide and biofertilizer used in the control of cocoa Black Pod Disease in nurseries.
The authors have not declared any conflict of interests.
REFERENCES
|
Arfaoui A, El Hadrami A, Adam LR, Daayf F (2015). Pre-treatment with calcium enhanced defense-related genes' expression in the soybean's isoflavones pathway in response to Slerotinia sclerotiorum. Physiol. Mol. Plant Pathol. 93:12-21.
Crossref
|
|
|
|
Bautista-Banos S, Hernandez-Lopez M, Bosquez-Molina E (2004). Growth inhibition of select fungi by chitosan and plant extracts. Mex. J. Phytopathol. 22:178-186.
|
|
|
|
|
Beauséjour J, Clermont N, Beaulieu C (2003). Effect of Streptomyces melanosporofaciens strain EF-76 and of chitosan on common scab of potato. Plant Soil 256:463-468.
Crossref
|
|
|
|
|
Benhamou N, Lafontaine PJ, Nicole M (1994). Induction of systemic resistance to Fusarium crown and root rot in tomato plants by seed treatment with chitosan. Phytopathology 84:1432-1444.
Crossref
|
|
|
|
|
Benhamou N, Picard K (2000). La résistance induite: une nouvelle stratégie de défense des plantes contre les agents pathogènes. Phytoprotection 80:137-168.
Crossref
|
|
|
|
|
Boonlertnirun S, Suvannasara R, Promsomboon P, Boonlertnirun K (2011). Application of chitosan for reducing chemical fertilizer uses in waxy corn growing. Thai. J. Agric. Sci. 44:22-28.
|
|
|
|
|
Boudjeko T, Omokolo ND, Driouich A, Balangé AP (2005). Peroxidase and Pectin Methylesterase activities in Cocoyam (Xanthosoma sagittifolium L. Schott) Roots upon Pythium myriotylum Inoculation. J. Phytopathol. 153:409-416.
Crossref
|
|
|
|
|
Bradford M (1976). A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principles of protein dye binding. Anal. Chem. 72:248-254.
|
|
|
|
|
Coqueiro DSO, Maraschin M, Di Piero RM (2011). Chitosan reduces bacterial spot severity and acts in phenylpropanoid metabolism in tomato plants. J. Phytopathol. 159:488-494.
Crossref
|
|
|
|
|
Djocgoue PF, Boudjeko T, Mbouobda HD, Nankeu DJ, EL Hadrami I, Omokolo ND (2007). Heritability of phenols in the resistance of Theobroma cacao against Phytophthora megakarya, the causal agent of black pod disease. J. Phytopathol. 159:529-534.
Crossref
|
|
|
|
|
El-Sawy NM, El-Rehim HAA, Elbarbary AM, Hegazy EA (2010). Radiation-induced degradation of chitosan for possible use as a growth promoter in agricultural purposes. Carbohydr. Polym. 79:555-562.
Crossref
|
|
|
|
|
Hilal AA, Nada MGA, Wafaa HZ (2006). Induced resistance against Sclerotinia sclerotiorum disease in some umbelliferous medicinal plants as a possible and effective control mean. Egypt. J. Phytopathol. 34:85-101.
|
|
|
|
|
Jideowno A, Okuo JM, Okolo PO (2007). Sorption of some heavy metal ions by chitosan and chemically modified chitosan. Trends Appl. Sci. Res. 2(3):211-217.
Crossref
|
|
|
|
|
Lizárraga-Paulín E, Torres-Pacheco G, Moreno–Martinez E, Miranda–Castro SP (2011). Chitosan application in maize (Zea mays) to counteract the effects of abiotic stress at seedling level. Afr. J. Biotechnol. 10:6439-6446.
|
|
|
|
|
Marigo G (1973). Sur une méthode de fractionnement et d'estimation des composes phénoliques chez les végétaux. Analusis 12:106-110.
|
|
|
|
|
Mbouobda HD, Fotso, Djocgoue PF, Omokolo ND, El Hadrami I, Boudjeko T (2010). Benzo-(1,2,3)-thiadiazole-7-carbothioic S-methyl ester (BTH) stimulates defence reactions in Xanthosoma sagittifolium. Phytoparasitica 38:71-79.
Crossref
|
|
|
|
|
Naseby DC, Pascual JA, Lynch JM (2000). Effect of biocontrol strains of Trichoderma on plant growth, Pythium ultimum populations, soil microbial communities and soil enzyme activities. J. Appl. Microbiol. 8:161-169.
Crossref
|
|
|
|
|
Ndoumbe-Nkeng M, Cilas C, Nyemb E, Nyasse S, Bieysse D, Flori A, Sache I (2004). Impact of removing diseased pods on cacao black pod caused by Phytophthora megakarya and on cacao production in Cameroon. Crop Prot. 23:415-424.
Crossref
|
|
|
|
|
Nyasse S, Cilas C, Herail C, Blaha G (1995). Leaf inoculations as an early screening test for cacao (Theobroma cacao L.) resistance to Phytophthora Black Pod Disease. Crop Prot. 14:657-663.
Crossref
|
|
|
|
|
Nyasse S, Efombagn MIB, Kebé BI, Tahi M, Despréaux D, Cilas C (2007). Integrated management of Phytophthora diseases on cacao (Theobroma cacao L): impact of plant breeding on pod rot incidence. Crop Prot. 26:40-45.
Crossref
|
|
|
|
|
Opoku IY, Appiah AA, Akrofi AY, Owusu GK (2000). Phytophthora megakarya: a potential threat to the cacao industry in Ghana. J. Agric. Sci. 33:237-248.
Crossref
|
|
|
|
|
Paulin D, Ducamp M, Lachenaud P (2008). New sources of resistance to Phytophthora megakarya identified in wild cacao tree populations of French Guiana. Crop Prot. 27:1143-1147.
Crossref
|
|
|
|
|
Priminho PC, Heliana Argôlo SC, Regina Cele Reboucas M, Dayane Santos G, Fatima Cerqueira A, Fabienne M (2008). Protein extraction for proteome analysis from cacao leaves and meristems, organs infected by Moniliophthora perniciosa, the causal agent for the witches broom disease. Electrophoresis 29:2391-2401.
Crossref
|
|
|
|
|
Roy J, Lafontaine P, Chabot R, Beaulieu C (2010). Dehydrated pork manure by-product: Effects of a chitosan amendment on bacterial community and common scab incidence. Phytoprotection 90:107-115.
Crossref
|
|
|
|
|
Shehata SA, Fawzy ZF, El-Ramady HR (2012). Response of cucumber plants to foliar application of chitosan and yeast under greenhouse conditions. Aust. J. Basic Appl. Sci. 6:63-71.
|
|
|
|
|
Sonwa DJ, Coulibaly O, Weise SF, Adesina AA, Janssens MJJ (2008). Management of cacao: Constraints during acquisition and application of pesticides in the humid forest zones of southern Cameroon. Crop Prot. 27:1159-1164.
Crossref
|
|
|
|
|
Tahereh G, Bahman H, Zohreh J (2012). Improving salvia sclarea seeds germination under in vitro condition. Int. J. Agric. Res. Rev. 2:1051-1058.
|
|
|
|
|
Tang H, Zhang P, Kieft TL (2010). Antibacterial action of a novel functionalized chitosan-arginine against Gram-negative bacteria. Acta Biomater. 6:2562-2571.
Crossref
|
|
|
|
|
Tchameni SN, Ngonkeu MEL, Begoude BAD, Wakam Nana L, Fokom R, Owona AD, Mbarga JB, Tchana T, Tondje PR, Etoa FX, Kuaté J (2011). Effect of Trichoderma asperellum and arbuscular mycorrhizal fungi on cacao growth and resistance against black pod disease. Crop Prot. 30:1321-1327.
Crossref
|
|
|
|
|
Tondje PR, Hebbar KP, Samuels G, Bowers JH, Weise S, Nyemb E, Begoube D, Foko J, Fontem D (2006). Bioassay of geniculosporium species for Phytophthora megakarya biological control on cacao pod husk pieces. Afr. J. Biotechnol. 5:648-652.
|
|
|
|
|
Uthairatanakij A, Teixeira da Silva JA, Obsuwan K (2007). Chitosan for Improving orchid production and quality. Orchid Sci. Biotechnol. 1:1-5.
|
|
|
|
|
Xing R, Qin Y, Guan X, Liu S, Yu H, Li P (2013). Comparison of antifungal activities of scallop shell, oyster shell and their pyrolyzed products. Egypt. J. Aquat. Res. 39:83-90.
Crossref
|
|
|
|
|
Zeng D, Luo X, Tu R (2012). Application of bioactive coatings based on chitosan for soybean seed protection. Int. J. Carbohydr. Chem. 2:1-5.
Crossref
|
|