Sorghum is the third cereal after rice and wheat in India and the second major crops after Maize in Africa (Melese, 2016). The largest grain sorghum producers are the United States, India, Nigeria, Mexico, Sudan and China (Agrama and Tuinstra, 2003). It is used as a major food grain in semi-arid tropical Africa (Tadesse et al., 2008). In Ethiopia, sorghum is one of the major crops produced and the third important crop in terms of area coverage and volume of production (CSA, 2015). Generally, cereals contributed 87.48% (about 26,778, 976.40 tons) of the grain crops production of which 16.89% (5, 169, 252, 54 tons) was from sorghum (CSA, 2018). It grows in a wide range of agro-ecologies most importantly in the moisture stressed parts where other crops can least survive and food insecurity is rampant (Asfaw, 2007). It is an indigenous crop second to tef used as injera (leavened local flat bread) making (Geremew et al., 2004). As the prices of tef, a staple cereal food crop of Ethiopia, have been ascended in recent years, sorghum has become the most affordable substitute for low-income society in urban areas. It is also a preferred substitute crop for countryside communities who produce tef as a cash crop (Demeke and Di Marcantonio, 2013).
The amount of genetic variability existing in sorghum is immense (Warkad et al., 2008). Ethiopia and Niger have abundant sorghum genetic diversity and are the largest sorghum producing countries in eastern and western Africa, respectively (Tesfaye et al., 2008). Genetic enhancement in sorghum yield depends on the degree of genetic variability, heritability and genetic advance in the population as well as the nature of the correlation between yield and its components (Jimmy et al., 2017). Correlation coefficients support in deciding the way of selection and number of characters to be looked at in improving grain yield (Jimmy et al., 2017). In drought-stricken lowland sorghum growing parts of Ethiopia, sorghum landraces showed a wide range of genotypic variations in terms of plant traits, which are useful in sorghum breeding program (Beyene et al., 2016). For real use of the genetic stock in crop enhancement, information of mutual correlations among yield, malting quality and yield components are needed (Gobezayohu et al., 2019b). Sorghum landraces are farmer’s varieties cultivated for grain production over many years and they are also the basic breeding resources for developing varieties or hybrids (Shiferaw and Yoseph, 2014). The earliness and grain characteristics were the most required characters of the improved varieties sought by farmers (Hailegebrial and Adane, 2018). Demand for malt sorghum will be increased because sorghum is a climate-resilient crop and the extent of barley production is not increasing with malt demand. However, using sorghum for brewing is low due to limited availability of improved sorghum varieties and commercial production of malt sorghum in Ethiopia (Tamirat et al., 2020). Hence, it is high time to explore the genetic variability to address the increasing malting sorghum request with advanced malting quality and yield (Gobezayohu et al., 2019a).
Therefore, in germplasm collection and selection, the creation of new genetically dissimilar genotypes from the novel population is possible (Addissu, 2011). Focus on identifying varieties that meet important traits in agricultural and nutrition necessities from the great diversity of sorghums is crucial to insure food security (Dicko et al., 2006). The objectives of this study were to identify sorghum landraces with high grain yield, study phenotypic and genotypic variability and analyze the correlation of yield and malting quality traits.
Study Site
The experiment was conducted at Sheraro in the Northern Tigray regional state of Ethiopia in 2017. Sheraro is a substation of Mytseberi Agricultural Research Center, located at 14º24’N and 37º45’E, with an altitude of 1028 m above sea level. The location is categorized by hot to warm semi-arid low-land plains, with a mono-modal rain pattern between May and September (Hailegebrial et al., 2019). The minimum and maximum temperatures of the site are 18.8 and 32.9ºC, respectively and the average rainfall received annually is 677mm. The monthly minimum value of the daily maximum temperature at Sheraro during July, August and September varied from 21 to 26ºC, while the minimum value of the daily maximum temperature during March, April, May and June ranged between 26 and 36ºC from 1983 to 2016. Increasing day and night temperature, and decreasing total annual rainfall, might affect crop development and yield, requiring heat resilient and drought-tolerant crop genotypes as adaptation strategies (Abadi et al., 2020).
Experimental materials and design
The study was conducted using 34 landraces and two malt sorghum varieties. The landraces were collected by Melkassa Agricultural Research Center from different sorghum growing regional states of Ethiopia: namely, Tigray, Afar, Amhara, Benshangul-Gumuz, Oromia, Southern Nation Nationalities People Regional State (SNNPRS), Dire Dawa city administration and from Ethiopian Institute of Biodiversity (Table 1). The trial was planted in a 6x6 alpha-lattice design with three replications. Each replication consisted of 6 blocks spaced 1 m apart and the distance between replications was 1.5 m. The spacing between rows and between plants was 75 and 15 cm, respectively. The experimental plot area was 7.5 m2 having two rows of 5 m length.
Data collection
The data were recorded on seven agronomic traits according to the sorghum descriptors (IBPGR/ICRISAT, 1993) while malting quality parameters were recorded at the Assela Malt Factory.
Days to flowering (DF)
DF was recorded as the number of days from the date of emergence to the date when 50% of the plants in a plot produce flowers. Plant height (PH): PH in centimeter was measured as an average height from ground level to the tip of five randomly taken plants at maturity per plot.
Days to maturity (DM)
DM was recorded as the number of days from emergence to the stage when grains developed a black layer at the base.
Head Length (HL)
HL was measured in centimeter from the base to the tip of the panicles of five randomly selected plants at the middle of the rows and averaged.
Head Width (HW)
HW was measured in centimeter at the widest part of the panicles of five randomly selected plants and averaged.
Thousand Kernel Weight (TKW)
TKW was measured as the weight of a sample of thousand kernels and the obtained weight was adjusted to 12.5% moisture content.
Grain yield per plot (GY)
GY was expressed in kilogram weight units and adjusted to 12.5% standard moisture content of each accession grain after harvested from each plot. The adjusted yield was converted to ton ha-1. Plot yield adjustment was done based on stand count taken just before harvest and the required plant population per plot using the following equation.
Germination energy (GE)
The germination energy was estimated by putting a hundred grains on moist double filter paper with 4 ml water in a Petri dish and germinated seeds were counted at 72 h.
Hectoliter weight (HLW)
Hectoliter weight was determined on dockage free samples using a standard laboratory hectoliter weight apparatus according to method no 55-10 (American Association of Cereal Chemists, 2000).
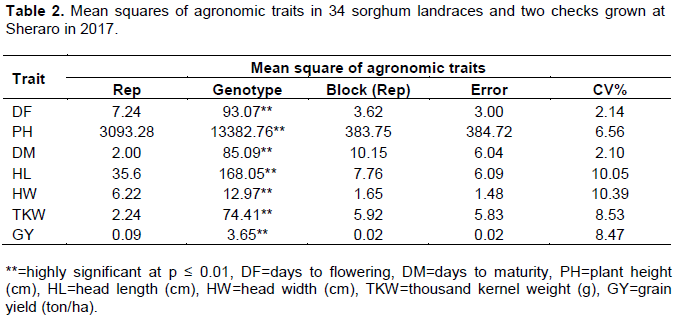
Extract fine and extract coarse grind
These were investigated at Assella Malt Factory by using the method of the American Society of Brewing Chemists (ASBC, 2008). The extract was measured based on the following procedure a 50.0 gram of milled malt placed in a mash beaker and 200 ml of distilled water was added at 45ºC for 30 min. Then, after 30 min, the temperature of the mash was raised to 70ºC over a 25 min period. 100 ml of distilled water was added to each mash beaker after a temperature of 70ºC was achieved or at 55 min. The scarification rate was started to measure after 10 min of water spray at 65 min. Subsequently, after 65 min, the temperature of the mash was kept at 70ºC for 1 h. During the next 115 min of mashing, the temperature of mash was cooled to room temperature within 10-15 min. At 125 min of mashing the mash, the beaker was picked out of the mash bath and dried the exterior well and distilled water was added until the content of the beaker weighed 450.0 gram. The contents of the mash beaker were stirred with a glass rod and immediately poured into the fluted filter paper. The obtained extract was measured for its specific gravity using a DMA density meter and reported in degrees Plato.

Where, GA=genetic advance and ?? = the grand mean for the trait considered.
Principal component and clustering analysis
Principal components (PCs) with an Eigen value greater than one were used as criteria to determine the number of PCs. Clustering was performed by average linkage method and using the SAS computer software facilities.
The analysis of variance for agronomic traits revealed highly significant variation (P ≤ 0.01) among 34 landraces and two checks for all studied traits (Table 2). This indicated that the landraces were genetically diverse and provides opportunities for improvement through breeding. Sorghum landraces are used as a genetic resource for breeding programs. Girma et al. (2019) noted that Ethiopia and its surroundings countries are considered as the center of origin and diversity for sorghum, and has contributed to global sorghum genetic improvement. The germplasm from this region harbors enormous genetic variation for various traits.
Crop phenology and plant growth
Days to flowering ranged from 67.33 for variety Macia to 102 days for landrace ETSL 100575 with a mean of 80.94 days. Fuad et al. (2018) reported that the earliest days to flowering was Meko variety on two locations with 68.67 and 66.67 days at Fedis and Erer respectively, which was almost similar with Macia variety in the days to flowering. Early maturity is the most required characters of a crop grown by farmers in areas with low rainfall Hailegebrial et al. (2019). The days to maturity ranged from 103.67 for variety Macia to 126 for landrace ETSL 101622 with a mean of 117.29 days.
The high yielding Gambella 1107, Debar and Macia genotypes flowered and matured early while landraces 87BK4250, ETSL 101650, ETSL 100141, ETSL 100954 and ETSL 100568 flowered and matured early but gave lower grain yield (Table 3). Days to flowering and maturity determine adaptability and yield potential of sorghum genotypes in areas where the growing period is limited by the availability of adequate rainfall. In this study, early flowering and maturing landraces gave a higher yield than late flowering and maturing landraces. This indicates that Sheraro is not suitable for the production of late-maturing sorghum landraces. Geremew et al. (2004) reported that in dry lowland areas, the growing period is short, and highly erratic dry spells may occur at vegetative and grain formation stages of crop growth; therefore, the genotype cultured in these areas should be early maturing.
Plant height ranged from 149 cm for genotype Macia to 386.73 cm for genotype IS 38279 with a mean of 299.174 cm. The highest plant height was recorded from landraces IS 38279, ETSL 100582 and ETSL 100735 while the lowest plant height was measured from Macia, 87BK4250 and Debar (Table 3). Farmers prefer taller landraces to harvest high above-ground biomass for animal feed. However, landraces with tall plant height, flowering and maturing late gave lower grain yield than landraces that had medium plant height. Therefore, landraces with reasonable plant height and early maturity were better than tall landraces in dry lowland areas. As Amare et al. (2019) mentioned plant biomass is a vital trait to sorghum growing farmers and the variety 2005MI5064 is preferred primarily for its tall plant height (190.79 cm). It also has a favorable grain yield (3810.96 kg/ha), with a benefit of 7% over the standard check Melkam.
Yield and yield components
The landraces ETSL 100141, ETSL 101466 and ETSL 101006 had the longest head lengths of 43.80, 36.60 and 36.20 cm respectively, whereas the shortest head lengths were recorded on landraces ETSL 101622 (11.73 cm), ETSL 100582 (13.00 cm) and ETSL 101061 (13.87 cm) (Table 3). Moreover, the largest panicle width was recorded on 01MS7013, 05MI5069 and ETSL 100974 with values of 16.10, 15.47 and 14.80 cm respectively, while the smallest panicle width was recorded on the landraces ETSL 100575 (6.67 cm), ETSL 100738 (8.53 cm) and ETSL 100735 (8.67 cm). Bhagasara et al. (2017) reported the range of panicle length from 6 to 45 cm and panicle width from 3 to 19 cm of sorghum crop. In this study, landraces with larger width and compacted panicle had high grain yield. Among the tested landraces, ETSL 101650 had the highest thousand kernel weight (TKW) of 38.22 g followed by ETSL 100582 (35.94 g) and ETSL 100974 (33.72 g).
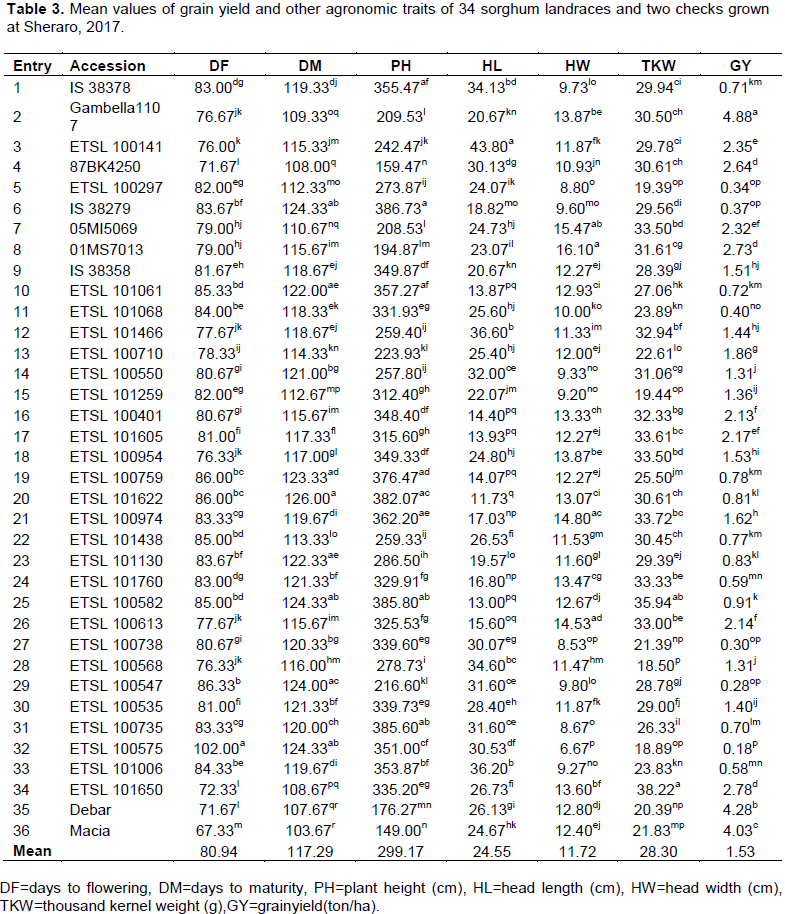
The landraces ETSL 100568 (18.50 g), ETSL 100575 (18.89 g) and ETSL 100297 (19.39 g) had the lowest TKW values (Table 3). The highest value of thousand kernel weight of sorghum in this study is similar to the maximum TKW (36.00 g) reported by Fuad et al. (2018). The average thousand kernel weight was 28.30 g and the result is comparable with 28.37 g reported by Kashiri et al. (2010). Abuajah et al. (2016) reported that genotypes with higher TKW may have more extract potential than genotypes with smaller TKW. Therefore, landraces which had high thousand kernel weight, low in grain protein content and produced high grain yield were preferable for sorghum malt.
The supply of sufficient quantity of grain with acceptable malting quality is important to satisfy the demand of malting industries. Among the tested landraces, Gambella 1107, gave the highest grain yield (4.88 ton/ha) followed by the check varieties Debar (4.28 ton/ha) and Macia (4.03 ton/ha). The lowest yielding landraces were ETSL 100575, ETSL 100547 and ETSL 100738 (Table 3). The landrace, Gambella 1107 was the top yielder followed by varieties Macia, Meko and Melkam at Fedis as reported by Fuad et al. (2018).
Phenotypic and genotypic variation for agronomic traits
The range, mean, standard error, phenotypic variance, genotypic variance, phenotypic and genotypic coefficient of variations for agronomic traits are presented in Table 4. Days to flowering and maturity, plant height, head length and thousand kernel weight exhibited high phenotypic (δ2P) and genotypic (δ2g) variances. Phenotypic variance (δ2p) and phenotypic coefficient variation (PCV) were higher than their corresponding genotypic variance (δ2g) and genotypic coefficient of variation (GCV), respectively for all the traits recorded (Table 4). This indicates that the expression of these characters was influenced by the environment. Bhagasara et al. (2017) noted the role of environment in the expression of characters that are demonstrated when phenotypic coefficient of variation (PCV) was slightly greater than corresponding genotypic coefficient of variation (GCV).
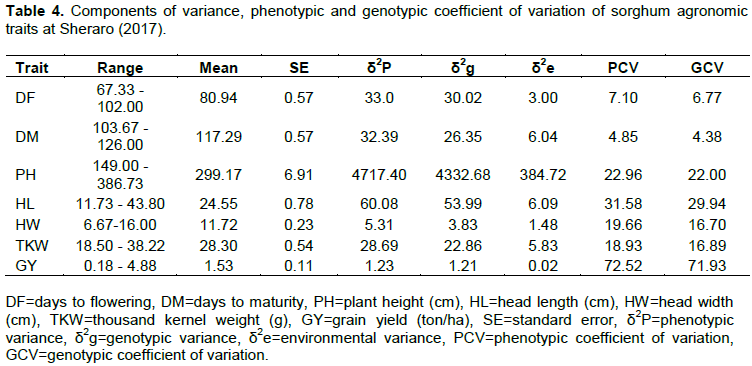
According to Deshmukh et al. (1986), phenotypic coefficient variation (PCV) and genotypic coefficient of variance (GCV) with values less than 10% are regarded as low, whereas values greater than 20% are considered as high and those between 10 – 20% are accounted medium. Based on the above explanation, high PCV values were recorded for grain yield (72.52%), head length (31.58%) and plant height (22.96%). Bhagasara et al. (2017) reported that estimates of PCV were slightly greater than the corresponding GCV which indicated that the effect of the environment was high. Days to physiological maturity and days to flowering exhibited low PCV of 4.85 and 7.10% respectively, indicating they were less affected by environmental factors (Table 3). Belay and Meresa (2017) reported a relatively high phenotypic coefficient of variation (21.73%) for the grain yield of sorghum. Jimmy et al. (2017) reported high values of PCV for plant height of sorghum.
Grain yield, head length and plant height revealed high GCV of 71.93, 29.94 and 22.00% respectively, whereas low GCV of 4.38 and 6.77% were recorded for days to physiological maturity and flowering, respectively. Belay and Meresa (2017) reported high GCV values (> 20%) for plant height and grain yield of sorghum. Jimmy et al. (2017) reported that GCV measures the variability of any trait due to genetic factors and higher GCV estimates than the PCV estimates of the genotypes showed large variation in phenotypic expression due to genetic factors.
The PCV was relatively greater than GCV for the traits days to flowering and days to maturity and the difference was low (Table 4). The results showed that the influence of environmental factors for the phenotypic expression of these traits was low. Therefore, the chance of improvement of these traits through selection based on the phenotypic performance of the landraces will be high. High GCV was estimated for characters head length and plant height, revealing that the genotypes have a broad base genetic background as well as good potential for improvement through selection. Similar results were reported by Bello et al. (2007). Belay and Meresa (2017) reported a high phenotypic and genotypic coefficient of variations for grain yield of sorghum. Low GCV and PCV value for days to flowering of sorghum was also reported by Kalpande et al. (2018).
Heritability and expected genetic advance
Estimates of heritability
According to Robinson et al. (1949), broad-sense heritability values greater than 60% are high, 31 to 60% are moderate and 0 to 30% is low. Based on the above heritability range, in this study, high broad-sense heritability was observed for days to flowering (90.92%), plant height (91.84%), and days to maturity (81.35%), head length (89.86%), head width (72.13%), thousand kernel weight (79.68%) and grain yield (98.37%). Similar estimates of heritability were reported by Bello et al. (2007) for panicle length (96%), plant length (93%) and date of flowering (95%) of sorghum crop.
Kalpande et al. (2014) reported that high heritability values were recorded for days to flowering of sorghum. Bello et al. (2007) stated that the characters with high broad-sense heritability would have a positive response to selection. A high degree of heritability estimates for most of the traits suggested that they were under genetic control and selection could be fairly easy for variety improvement. High estimates of heritability in a broad-sense were obtained for days to flowering and plant height of sorghum (Kalpande et al., 2018).
Selection may be considerably difficult for characters with low heritability. In this study high heritability was observed for days to maturity (81.35%) with low genetic advance as percent of the mean (8.13%). Kalpande et al. (2014) and Warkad et al. (2008) indicated that the high heritability estimates of days to physiological maturity of sorghum was accompanied by low genetic advance indicating the significant effect of non-additive gene action where the breeding method like heterosis breeding may be important for these traits to exploit non-additive gene action.
Genetic advance
Genetic advance as percent of mean ranged from 8.13 for days to maturity to 146.96 for grain yield. The genetic advance days to flowering, plant height, days to maturity, head length, head width, thousand kernel weight and grain yield are presented in Table 5. Johnson et al. (1955) classified genetic advance as percent of mean (GAM) values < 10% is low, 10 to 20% is moderate and > 20% is high. Based on the above GAM classified range, days except to flowering and days to maturity of all the characters showed high genetic advance in this study (Table 4). Thus, the improvement of these traits can be made through selection. The days to flowering of sorghum had moderate genetic advance as percent of the mean (Kalpande et al. 2018). This implies improvement of this character in genotypic value for the new population compared with the base population with one cycle of selection is not rewarding.
In this study the genetic advance as percent mean for grain yield was 146.96% and heritability was 98.37%. Similarly, Kalpande et al. (2014) has reported a high estimate of heritability together with high genetic advance as percent of the mean for grain yield of sorghum, revealing the influence of additive gene action for this trait. Jimmy et al. (2017) reported that high heritability and high genetic advance as per cent of mean (GAM) due to highly additive gene effect was observed for panicle diameter and grain yield of sorghum. The result indicated high heritability accompanied with high genetic advance was observed in the case of plant height. This indicated that these traits were highly heritable and the selection of superior genotypes is possible to improve these characters (Kalpande et al., 2018; Jimmy et al. 2017). In this study high heritability and low genetic advance were estimated for head width (Table 5). This result is in agreement with the result reported by Chavan et al. (2010) who revealed high heritability combined with low genetic advance for panicle width in sorghum.
Correlations of grain yield and yield-related traits of sorghum landraces
Phenotypic correlations
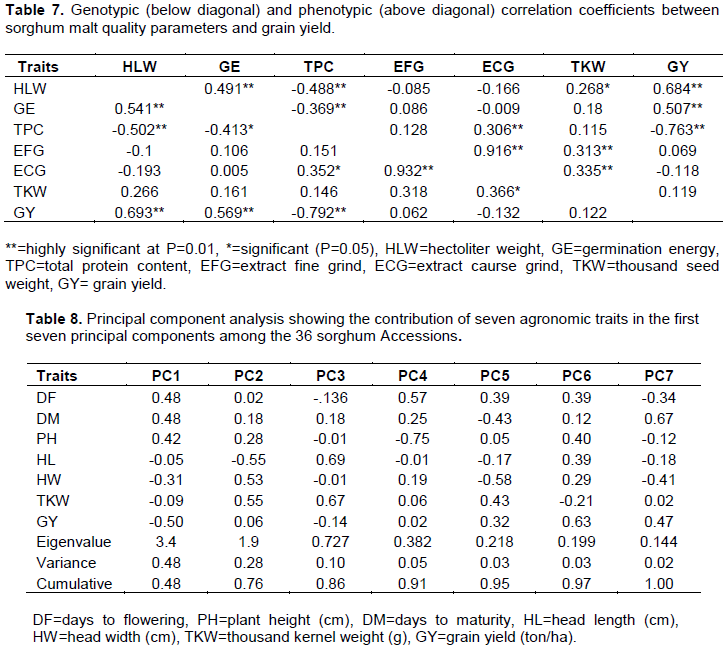
The phenotypic correlation coefficients between grain yield and other agronomic traits are presented (Table 6). Deshmukh et al. (2018) stated that correlation coefficient helps in defining the way of selection and number of traits to be considered in improving the grain yield. Grain yield had a highly significant (P=0.01) positive phenotypic correlation with head width (0.5). This shows that genotypes with larger panicle width produced high grain yield at Shiraro. Jimmy et al. (2017) reported that the positive association between grain yield and panicle width. This indicated width to use as selection criterion in sorghum to proportionally increase grain yield of sorghum.
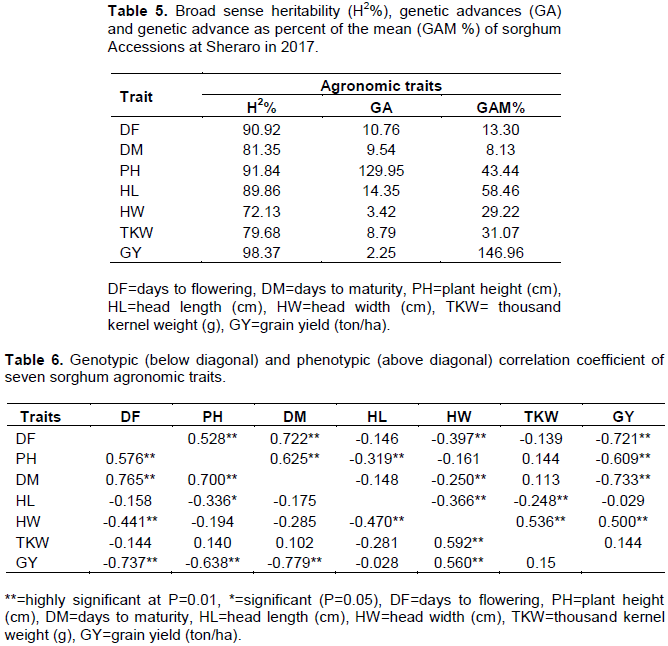
Highly significant (P=0.01) negative phenotypic correlation of grain yield was observed with plant height (r= -0.609), days to flowering (r= -0.721) and maturity (r= -0.733). Jimmy et al. (2017) reported that negative significant associations between plant height and grain yield. This shows that sorghum genotypes which are late flowering and maturity with taller plant height produced low grain yield at Sheraro which is characterized by low amount and erratic rainfall. Therefore, early maturing sorghum varieties with reasonable plant height, heavy thousand seed weight and bigger panicle width are required. Abate (2017) reported a similar results from a study conducted at moisture stressed locations of Ethiopia indicating that days to flowering, days to maturity and plant height had a negative relationship with the major yield components including grain yield of sorghum.
Genotypic correlation
Grain yield had highly significant (P=0.01) and positive genotypic correlation with head width (r=0.560), and it had highly significant (P=0.01) negative correlations with days to flowering (r= -0.737), plant height (r= -0.638) and days to maturity (r= -0.779). Thousand kernel weight had significant (P=0.01) and positive correlation with head width (r=0.592). Highly significant (P=0.01) positive correlations were observed between days to maturity and flowering (r=0.765) and between days to maturity and plant height (r=0.700). Kalpande et al. (2014) reported that days to maturity exhibited significant positive association with plant height, and days to flowering and plant height at physiological maturity; while a significant negative association was observed for grain yield. High significant (P=0.01) positive correlation was also obtained between plant height and days to flowering (r=0.576). Abate (2017) reported that days to flowering was positively and significantly correlated with plant height (r=0.27) of sorghum.
Correlations of grain yield with malt quality related traits of sorghum landraces
Phenotypic and genotypic correlations
Grain yield showed highly significant and positive phenotypic correlations with hectoliter weight (r=0.684) and germination energy (r=0.507) (Table 7). This shows that landraces with high hectoliter weight of seeds produced high grain yield. As Gobezayohu et al. (2019b) reported that grain yield exhibited positive and highly significant phenotypic correlation with hectoliter weight. Highly significant (P=0.01) and negative correlation of grain yield was exhibited with total protein content (r= -0.763). In this study, the results showed landraces with high grain yield had low protein content. Ross et al. (1981) reported similar result protein and grain yield had strong negative genetic correlations. Also, Matthieu et al. (2010) reported that a highly significant negative correlation was observed between mean grain yield and grain protein content in wheat.
Grain yield had highly significant (P=0.01) and positive genotypic correlations with hectoliter weight (r=0.693) and germination energy (r=0.569) (Table 7). These indicated accessions, with high grain yield and thousand kernel weight are better in hectoliter and germination energy. As Gobezayohu et al. (2019b) reported, grain yield showed a positive and highly significant genotypic correlation with hectoliter weight. The results obtained in the study showed high significant and negative genotypic correlations of grain yield with total protein content (r= -0.792).
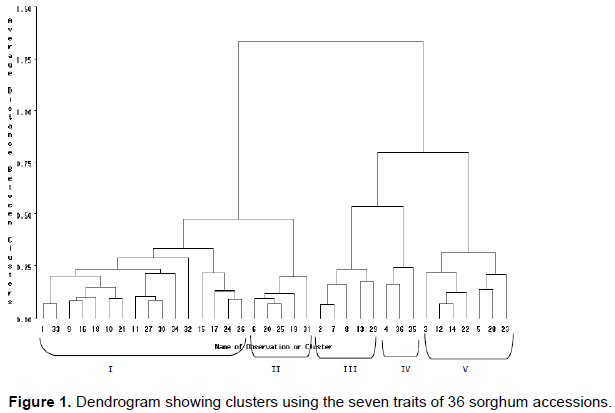
Principal component analysis
Principal component analysis was executed with the aim of decrease a large set of phenotypic traits to a more meaningful smaller set of traits and to know which trait is contributing to maximum variability (Kassahun, 2017). The principal components (PCs), with eigenvalues greater than one were considered sufficient for inclusion in the analysis. The largest and the smallest Eigen values were (3.4) and (1.9), correspondingly, and two principal components are found between these Eigen values (Chatfied and Collins, 1980). The first principal component (PC1) alone explained 48% of the total variation, mainly due to variation in days to sorghum flowering, days sorghum maturity, plant height and head width (HW). Traits which contributed more to the second principal component (PC2) accounted for 28% of the total variation and were dominated by head length (HL), head width (HW) and thousand kernel weight (TKW) (Table 8). Mesfin (2016) also reported similar results from their experiment. Generally, a maximum and minimum cumulative contribution is 76 and 48%, respectively.
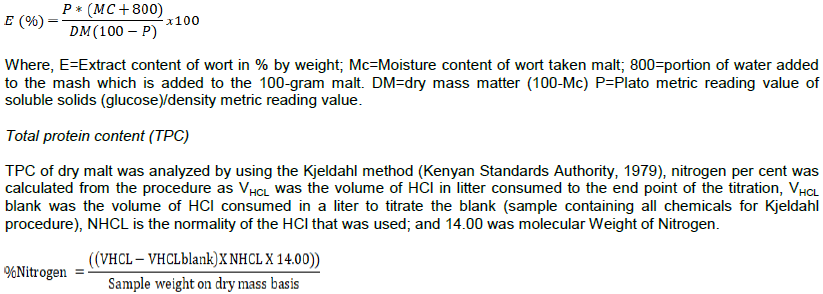
Cluster analysis
The 34 sorghum accessions along with the two standard checks formed five clusters. The result of the hierarchical cluster analysis indicated that 36 sorghum accessions were grouped into five different clusters with a range of accessions that are categorized based on their similar performance of the trait under study. The clustering pattern indicated the existence of a significant amount of variability among the sorghum landraces. Among the different clusters, the cluster size varied from 3 to 16. The maximum number of accessions was included in cluster I having 16 accessions and the minimum number in cluster IV having 3 accessions. Accession IS 38378, IS 38358, ETSL 101061, ETSL 101068, ETSL 101259, ETSL 100401, ETSL 101605, ETSL 100954, ETSL 100974, ETSL 101760, ETSL 100613, ETSL 100738, ETSL 100535, ETSL 100575, ETSL 101006 and ETSL 101650 were grouped into cluster I, accessions IS 38279, ETSL 100759, ETSL 101622, ETSL 100582 and ETSL 100735 were grouped into cluster II, accessions Gambella1107, 05MI5069, 01MS7013, ETSL 100710 and ETSL 100547 were grouped into cluster III, accessions 87BK4250 and the two checks Debar and Macia varieties were grouped into cluster IV and accessions ETSL 100141, ETSL 100297, ETSL 101466, ETSL 100550, ETSL 101130 and ETSL 100568 were grouped into cluster V. The two standard checks used in this study were grouped into cluster IV, along with one other sorghum accession that performed in a similar way for the studied quantitative characters. The grouping pattern of the accessions in the dendrogram showed similarity with the matrix plot. Figure 1 show the dendrogram clusters using the seven traits of 36 sorghum accessions.