ABSTRACT
The variation of morphological and physiological traits of blackberry (Rubus subgenus Rubus Watson) is vital for successful breeding of the fruit crop. The objective of this study was to characterize blackberry accessions in-situ using morphological descriptors in Kenya. Each blackberry accession was nested within its county of collection. A phylogenetic tree was then constructed using the Gower’s coefficient which clustered the accessions into two classes; I and II consisting of 1 and 89 accessions, respectively. The clustering of accessions did not show an association between the origin of collection and the accessions. Principal Component Analysis (PCA) revealed ten axes of which seven had a cumulative variation of 96.30% with the first two axes having a discriminatory variance of 52.71%. This suggests that variables identified in this study could be used to differentiate blackberry accessions morphologically. This study demonstrated that the number of internodes per average growing shoots, thorniness of the plant and length of internode were associated with the first axis with Eigenvalue of 27.79%. Plant thorniness was also associated with the second axis with Eigenvalue of 24.92%. These results suggest that there exists qualitative and quantitative variation among blackberry accessions in Kenya that can be utilized in breeding.
Key words: Morphological diversity, Rubus subgenus Rubus Watson, accessions, cluster analysis.
The assessment and monitoring of diversity of plant genetic resources in-situ and ex- situ is essential for germplasm management and for establishing core breeding stocks (Oyoo et al., 2015; Orobiyi et al., 2017). Knowledge of morphological variability of germplasm collections improves understanding of the relationship between the structural morphology of plants and their corresponding functional botany (Lauri and Normand, 2017). Characters that show diversity within each species are commonly used in the characterization process. Attributes of the edible part of the plant such as leaf shape, length, persistence and total foliage cover are taken in by many crops (Chweya and Edmonds, 1997). For those crops whose fruit is the edible part, fruit size, texture, colour, length and weight are used. In addition to the nutritive aspects of each species, phenology and attributes related to storability of the harvested part for consumption are often considered (Human and Rheeder, 2004). Breeding in blackberry focus on specific characters which include adaptation, pest and disease resistance, plant habit, primocane fruiting, thornlessness, fruit size and shape, fruit quality and yield (Clark et al., 2007).
Blackberry (Rubus subgenus Rubus Watson) is a cross-pollinated, fruiting plant species formerly of subgenus Eubatus. The fruits aggregate around a receptacle and consist of fleshy drupelets, each with a single seed (Finn, 2008). Blackberry is a perennial plant with biennial canes and is of three types in reference to cane architecture; erect, semi-erect and trailing (Clark et al., 2007). There are 84 wild species of blackberry in Kenya (Chittaranjan, 2011) and only two plant introductions; one hybrid berry (definitive genetic origin unknown but is believed to be an interspecific cross between European Raspberry, Rubus idaeus and another European blackberry, Rubus fruticosus) (Wood et al., 1999) and the other, a European berry, Rubus fruticosus cultivated mainly for export market. Blackberries have a complex reproductive (sexual, facultatively apomictic to obligately apomictic), ploidy (autoploidy and alloploidy) and inheritance strategies (disomic and tetrasomic) (Clark et al., 2007). Thus, there is difficulty in identifying superior berry as well as designation to definite groups which are sometimes misclassified. Blackberry fruits have varied health benefits and are rich in natural phytochemicals (Rao and Snyder, 2010), vitamin C and E (Hirsch et al., 2013), and contains phenolic compounds that are secondary plant metabolites integral in human and animal diets (Siriwoharn et al., 2004; Lee et al., 2011) due to their antioxidant properties (Hirsch et al., 2013). They are also used to prevent lifestyle diseases like diabetes, cancer, cardiovascular diseases and other pathogens (Bravo, 1998; Hollman et al., 1996). Blackberry fruits are consumed fresh or processed as individually quick frozen (IQF), canned, pureed, juiced or freeze-dried (Finn, 2008). The crop is gaining prominence in Kenya and Africa at large due to its possible health benefits and the influx of a more informed, aggressive middle-class population.
Modern breeding objectives emphasize on the evaluation of the characteristics of importance to production and productivity within genetic resources and concentration of the same in one cultivar (Bozovic et al., 2016). Analysis of genetic diversity can be achieved through molecular and morphological markers. Some of the molecular markers that have been used in the assessment of genetic diversity of blackberry are random amplified polymorphic DNA (RAPD), amplified fragment length polymorphisms (AFLP), restriction fragment length polymorphisms (RFLP), ISSR-EST, and simple sequence repeats (SSRs) (Clark et al., 2007). In-situ hybridization techniques (ISH) such as genomic in-situ hybridization (GISH) and fluorescence in-situ hybridization (FISH) have also been used to infer blackberry phylogeny genus (Yan et al., 2015). Morphological markers are still useful in phenotypic descriptions of plant populations. Some morphological traits have been associated with influencing some other trait that has great economic importance but difficult to measure such as disease susceptibility (Karimi et al., 2009). Phenotypic descriptors are widely used to classify cultivars, genotypes and landraces based on discriminant variables for the plant genetic resources (PGR) studied (Orobiyi et al., 2017). Consequently, a comparative analysis is done of the composition of PGR with those of the classes obtained from principal component analysis and correlation analysis. This can better reveal the constitution of each group with respect to the landraces, cultivars or genotypes studied.
In Kenya, blackberry is still a minority crop and grown mainly for the export market or the suburban population. Smallholder farmers are still few as the crop gains prominence in the region. Statistics for yield in Africa are only available for South Africa (220 tonnes) and still, this is very low in comparison to other regions of the world (Strik et al., 2007; Finn, 2008). Being a minority crop, challenges abound; inadequate breeding programs and funding targeting blackberry in Kenya, little understanding of population structures within repositories and the available breeding program, inaccurate identification of species and misclassification in gene banks, difficulty in identification of duplicate accessions in germplasm repositories as well as unavailability of improved local cultivars. In addition, the available varieties experience pest and disease problems coupled with abiotic stresses that are not well documented. Phenotypic expression such as objective descriptions of tree and fruit characteristics discriminating against undesirable traits in the process is unreliable and may not provide an accurate indication of genetic diversity (Menkir et al., 1997). This preference for specific traits based on phenotypic descriptions has also previously led to the discarding of potentially important and advantageous germplasm. In addition, expression of morphological data are greatly influenced by environment, phenological stages of development and can be subjective in nature resulting in errors (Marinoni et al., 2003). This assessment of the morphometric diversity of the fruit tree species in core germplasm collections, wild and introduced, whether in-situ or ex-situ is however, necessary. It offers prerequisite remedies to the challenges mentioned above and is vital for the thorough understanding of these genetic resources, breeding options and subsequent conservation efforts. The objective of this study was to characterize wild blackberry types in selected counties in Kenya and Plant Introductions (PIs) using morphological markers.
Experimental site
This study was conducted in-situ in selected counties in Kenya. These counties included Kericho (0.3689° S, 35.2863° E), Nakuru (0.3031° S, 36.0800° E), Uasin Gishu (0.5143° N, 35.2697° E), Nandi (0.1036° N, 35.1777° E), Laikipia (0.3970° N, 37.1588° E), and Baringo (0.4897° N, 35.7412° E). In each county, five random districts or sub-counties were selected out of which five random locations and villages were chosen for germplasm sampling.
Germplasm sampling
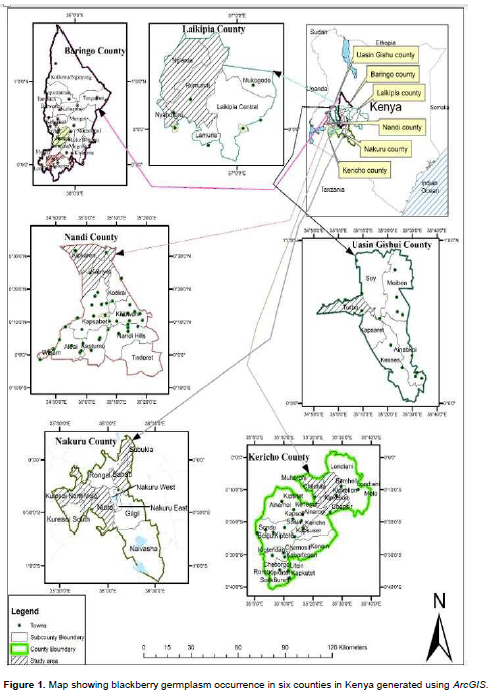
Sampling of blackberry for morphological trait analysis was carried out in the areas mentioned above. Fruit trees were coded to reflect the county, district, division, subdivision, village and the collection number (Oyoo et al., 2015). If the collection was from Baringo county, Tinet district, Torongo division, Lembus Mosop location, Makutano village and it was the first blackberry sampled, the code given was; BRG/TIN/TOR/LM/MAK/01 (Supplementary material 1). Sampling was done to reflect different agro-ecological zones in the counties where blackberry is reportedly growing. The selected agro-ecological zones were different and are designated pyrethrum (Chrysanthemum cinerelifolium)-wheat (Triticum aesitivum) zone (UH2), tea-dairy zone (LH1), wheat-maize-pyrethrum zone (LH2) wheat-barley zone (LH3), cattle-sheep-barley (Hordeum vulgare) zone (LH4), coffee (Coffea arabica) zone (UM2) and sunflower (Helianthus annus)-maize zone (UM4). The altitude varied from 1650 m to 2743 m above sea level. Samples were taken in areas where the fruit trees are morphologically different and there are marked changes in altitude, cropping systems, a formidable barrier such as a mountain, river, valley or local people are ethnically different (dialect) from previous collection sites. Here, quantitative and qualitative attributes of the plants were taken along edaphic, topographic and climatic gradients. Data stations in a location were within 200 m intervals. This was done to minimize redundancies. For each fruit tree sampled, Global Positioning System (GPS) data were taken and the plant photographed. This was vital for mapping of these areas using ArcGIS software (Figure 1). Plants with similar features growing in ecologically distinct locations were assumed to be of different eco-strain and hence, were sampled and characterized. Blackberry accessions were evaluated for population structure, architecture and fruit tree characteristics.
Evaluation of traits
Seven qualitative and three quantitative important traits to blackberry breeding were characterized (Table 1) according to Yin, (2017). These included tree, stem, leaf, reproductive characteristics and stress severity assessment. The descriptors of blackberry were as further discussed.
Pre-harvest
Vegetative observations
The cane architecture, showing the degree of creeping for each plant (denoted for each individual as erect “E,” semi-erect “S,” or trailing “T”); stem type, whether malformed, symmetrical or asymmetrical; thorniness, indicating whether the plant is thorny or not (denoted for each individual as thorny “T” or thornless “N”); overall plant health, showing the degree of infestation, apparent nutrient deficiencies and general abiotic stress susceptibility (subjectively assessed from 1 to 10, where 10 = excellent health); overall plant vigor, examining leafiness, length of current season’s growth, and relative number of actively growing shoots (subjectively assessed from 1 to 10, where 10 = extremely vigorous).
Reproductive observations
Number of internodes/actively growing shoot; internode length (mm), the average length of the fourth internode of four plants; pubescent colour (white, varied or purple); flower colour (white, varied or purple).
Data analyses
Multivariate analysis
Multivariate analysis was carried out using GENSTAT 15th Edition programme on morphological data to identify discriminant variables amongst the 90 accessions. Means of quantitative traits were first obtained using PROC GLM in Statistical Analysis System (SAS) version 9.1 (SAS Institute Inc., Cary, 2001) to determine the significant differences among the accessions. The following statistical model was used
Qualitative phenotypic variability
The accessions exhibited a wide range of differences in qualitative morphological features in plant architecture which refers to the growth habit of canes. The semi-erect type was the most dominant with 97% of the fruit trees sampled, with trailing and erect types being 1 and 2%, respectively (Figure 2). For the fruit trees sampled, 36% had small to medium thorns, sporadically spaced; 22% had medium to long thorns, evenly spaced; 13% had small, sparse visible thorns; 12% had medium to long thorns, sporadically spaced; 9% had medium thorns, evenly spaced; 6% had medium thorns, closely spaced while only 2% had long thorns that are closely spaced. White was the most frequent flower colour representing 62% of all sampled fruit trees, followed by purple (33%). Pink flower colour accounted only for 4%. The plant (leaf) pubescence colours were categorized into 3; white was the most dominant (54%), with brown and green types being 37 and 9%, respectively.
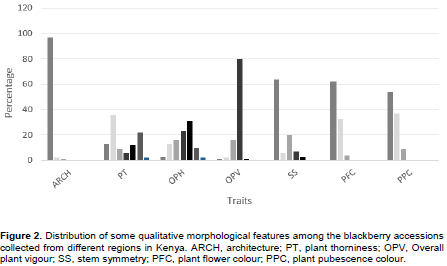
For all the germplasm sampled, 31% had their leaves mostly green with minor symptoms of biotic and abiotic stress (B/AS), 23% had apparent majority of leaves green but with obvious symptoms of B/AS, 16% had obvious symptoms of B/AS with more than 50% of leaves green, 13% had obvious symptoms of B/AS with 50% of leaves green, 10% had B/AS only observable upon close inspection; 3% had extreme B/AS with less than 50% leaves green and only 2% had no symptoms of B/AS (Figure 2). In addition, 80% of the plant genetic resources studied had vigorous growth with long primocanes. In terms of stem type, 64% were asymmetrical; 20% slightly asymmetrical; 7% very slightly asymmetrical; 6% somewhat asymmetrical while 3% were symmetrical. No malformed stems were observed.
Principal component analysis (PCA)
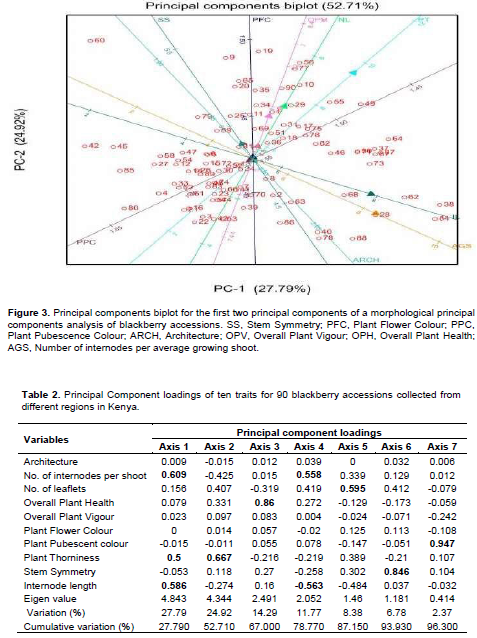
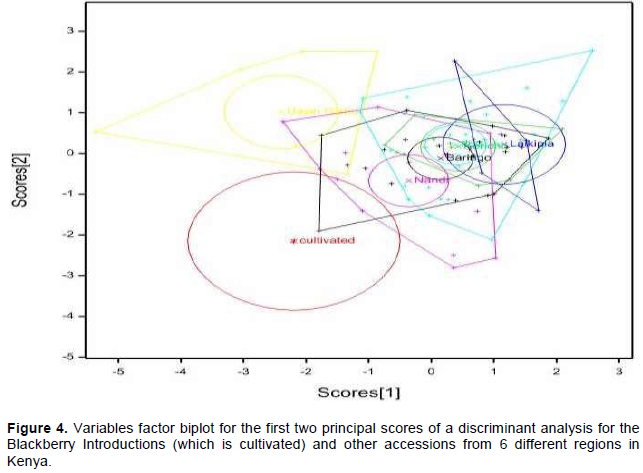
Principal component analysis allowed the association of axes to the variables and out of the ten axes generated, seven had a cumulative variation of 96.30% with the first two axes having a discriminatory variance of 52.71% (Figure 3). Three variables were associated with the first axis with Eigenvalue of 27.79% (Table 2). These were number of internodes per average growing shoots, plant thorniness and internode length. Plant thorniness was also associated with the second axis with Eigenvalue of 24.92%). One variable was associated with the third (Eigenvalue = 14.29%), fifth (Eigenvalue = 8.38%), sixth (Eigenvalue = 6.78%) and seventh (Eigenvalue = 2.37%) axes. These were overall plant health, number of leaflets, symmetry of stem and the colour of plant during pubescence (Table 2). The number of internodes per average growing shoots and length of internode were associated with the fourth axis with Eigenvalue of 11.77%. Discriminant analysis of the variables taken for all the accessions for the first two principal scores clustered the wild blackberry types together in comparison to the cultivated types (Figure 4).
Factorial component analysis carried out using dissimilarity coefficients obtained from the usual Euclidean distance was conservative and splits the accessions into the 4 planes (Figures 5 and 6). Most of the accessions overlapped, demonstrating redundancies in the morphology of the characterized germplasm. From the PCoA plot generated (Figures 5 and 6), principal axes 1 and 2 showed that NKR/NJR/EGER/EGER/F7/01 (90), NKR/NJR/NES/NES/KIM/01 (9) from Nakuru county; BRG/TIN/TOR/LM/MAK/06 (42), BRG/TIN/TOR/LM/MAK/02 (39), BRG/ERN/IGE/MM/KIN/04 (53), BRG/ERN/TIM/MBE/KMA/O2 (56) from Baringo county; NDI/NN/KUR/CKO/SUR/03 (60) from Nandi County, LC/LKN/GMA/GMA/KBI/RK/01 (77) from Laikipia county, UG/KKB/ABK/KPG/CHES/03 (80) and UG/KKB/ABK/KBG/CHES/01 (78) from Uasin Gishu and an introduced germplasm CV/RBN/01 (85) (from South Africa) were the most distinct from the other accessions.
Cluster analysis
Cluster analysis split the accessions into two clusters, I and II (Figure 7). The Plant Introductions (CV/RBN/01) had its own cluster, I, while the other (CV/BYN/01) clustered with the rest of the wild accessions collected from different regions of Kenya. The accessions were clustered together according to the traits (quantitative and qualitative) measured. The accessions did not cluster according to counties of origin. However, most of the accessions from all the six geographical regions clustered in Group II. Cluster II had the highest number of genotypes of 89 based on the morphological descriptors used. Cluster II also had sub-clusters. Grouping of these accessions into these sub-clusters indicated a substantial level of intra-polymorphism within the wild blackberry population in the country. Cluster I only had one genotype indicating inter-polymorphism with the rest of the accessions collected.
Structure analysis was illustrated using a silhouette plot and was used to compare the minimum average dissimilarity of each accession to other clusters with the average dissimilarity to accessions in its own cluster. There were two main groups within this set of germplasm, hence objective determination in the number of stable clusters. Observations close to 1 (large si) indicate that the individual (s) is very well clustered. Clusters with observations close to 0 (small si) indicate that the germplasm lie between two clusters. Based on the silhouette plot generated, the natural number of clusters in this particular germplasm, given by the traits analysed, is k = 2. The average silhouette width (ASW) from this structure analysis is 0.32 (Figure 8). This shows that the structure of the population of the accessions under study was weak and could therefore be artificial.
The assessment of variation in morphological germplasm is the first step in the determination of genetic diversity. It is a prerequisite for conservation and utilization of plant genetic resources (Mason et al., 2017). Morphological characterization can also be useful in selection of parents for breeding (Orobiyi et al., 2017; Kagimbo et al., 2017). Therefore, there is a need to assess the diversity of any crop prior to selection and crossing to better utilize the resources in any breeding program. In this work, blackberries were studied at different agro-ecological areas and therefore, had differences in morphological expressions (Figure 2). According to tree characteristics, most of the wild blackberry accessions were semi-erect (87%) and 80% of genotypes studied had vigorous growth. This study anticipated higher morphological diversity due to the inclusion of introduced germplasm from South Africa. However, a narrow diversity was observed (ASW=0.32) as per the silhouette plot (Figure 8). This shows that the structure of the population of the accessions under study was weak and could be artificial. The introduced germplasm from South Africa had their origins from Europe and North America. Neither Kenya nor South Africa is a centre of origin for blackberry.
The observed low genetic diversity might be due to a number of reasons including nature of propagation of the crop, method of dispersal of the crop, effect of environment, nature of breeding and farmer to farmer exchanges of germplasm. Blackberry reproductive nature is complex. This varies from sexual to facultatively apomictic to obligately apomictic. Blackberry (Rubus subgenus Rubus Watson) are often hermaphrodites (Nybom, 1986) and outcrossing has also been observed (Antonius and Nybom, 1995). Additionally, self-fertilization is frequent (Nybom 1988). Infertility or partial fertility may also occur in some plants, and this is attributed to genetic factors such as poor pollen production, unattractive nature to pollinators, lack of pollinators and environmental effects. Open pollinators are likely to have higher diversities compared to inbreds or vegetatively propagated berries (Stafne and Clark, 2004). Most wild blackberries are clonally propagated by way of root sprouts, underground stems (rhizomes) and branches that has its root at the tips (stolons). Therefore, the number of breeding parents may be few, thus, low diversity. Low morphological diversity of blackberry could also be due to the fact that the crop is an invasive species, and propagate vegetatively very vigorously, enabling the clonal spread of single individuals in a patch of habitat. This, however, does not mean that there is a narrow genetic base or higher homozygosity of blackberries in Kenya. In retrospect, the genetic base of fruit tree crop is varied and can be attributed to the different species available.
The progress of breeding blackberry genotypes is directly affected by the plant thorniness. Thornlessness is the most bred qualitative trait in blackberry. Four genes have been detected to be responsible for thornlessness, and they can vary from recessive to dominant for the trait. Breeding progress is thus, hampered by the source of the thornless genotypes and the ploidy of the blackberry type in question.
Plant flower colour varied from white, pink to purple in which white was the most dominant color (62%). Differences in plant flower colour and plant pubescence colours are commonly noticed in natural and introduced blackberry germplasms. This was evident in the data taken and is important as it influences pollination and diversity of the accessions. Some accessions, for instance CV/RBN/01, were more divergent than others and this may be attributed to their outcrossing nature (Figures 4 and 7).
Cluster analysis on the characteristics split the accessions into two groups, I and II. These groups were in a random manner irrespective of their geographical origin. The two distinct groupings were due to the availability of introduced germplasm (CV/RBN/01) that singly constituted Group I. The rest of the landraces grouped together, albeit with subgroups. Although there was no clear association between the subgroups and counties of origin, most germplasm from Nakuru county tended to cluster together. This may be explained in terms of gene pool concept where the wild types formed the primary gene pool, which consists of the crop species itself and other species that can be easily crossed with it. The cultivated type may have grouped alone (CV/RBN/01) as variability in cultivated plant species depends on how evolutionary forces impact on natural populations. The sole grouping of CV/RBN/01 over the rest of the accessions in this study may also be due to the selective advantage it has over the rest of the accessions. This can be by way of mutation, genetic drift that is as a result of random changes in allele frequencies for generations due to the finite size of populations, gene exchanges or gene flow among populations and selections (both natural and artificial selection). The high similarity of the wild types in morphometry and agro-morphological traits across the different agro-ecological zones may be attributed to the invasive nature of the accessions characterized.
Principal Component Analysis (PCA) also did not associate the accessions with their regions of origin. Of the ten traits subjected to PCA, eight were able to differentiate the collected accessions and were considered as variables that are capable of discriminating accessions on the basis of morphology. It was evident that PCA also categorized assessed phenotypic traits in the population into several related groups (Figure 3). This can also be explained by the reproductive and often invasive nature of the fruit tree species over wide ethno-geographical regions as well as the folk nomenclature that exists in these areas. Apomixis also occurs in some blackberry species (wild and introduced). Therefore, clones dispersed by man or birds spatially across habitats can be a cause. This often results in misclassification of genotypes and existence of duplicates (Mason et al., 2015: Agre et al., 2017).
This work is important as a treatise to breeding of blackberry and can be used with DNA genotyping information to understand the morphological variations that are present in blackberries. Although the study covered only six counties in Kenya, this work is of great significance in management of these genetic resources. It highlights the contribution of key qualitative and quantitative traits to morphological differences of blackberry and their variation across environments. The agro-morphological characterization is however, ambiguous. Moreover, the phenotypic characters are influenced by the environments in which each plant was collected. A more detailed high throughput phenotyping study should be carried out including fruit characteristics such as yield, fruit quality and quantity variables to determine the health benefits of blackberry with possibility of improvement. It is also important to exploit molecular techniques to generate non-ambiguous information to know more about the genetic variation that really exists in these materials.
The authors have not declared any conflict of interests.
This work was funded by RUFORUM Grant award (RUFORUM-RU/CGS/CRG/30/03/14) in collaboration with Egerton University, Kenya. The authors would like to thank Prof. John R. Clark (University of Arkansas, Fayetteville), Dr. Chad Finn (USDA-ARS) and Dr. P. Okwiri (Egerton University) for reviewing this manuscript; KALRO-Njoro (Food Crops Institute) staff for their insights and assistance during field work; along with colleagues and staff from the Department of Crops, Horticulture and Soils, Egerton University for their support during the research.
REFERENCES
|
Agre AP, Bhattacharjee R, Dansi A, Becerra Lopez-Lavalle LA., Dansi M, Sanni A (2017). Assessment of cassava (Manihot esculenta Crantz) diversity, loss of landraces and farmers preference criteria in southern Benin using farmers' participatory approach, Genetic Resources and Crop Evolution 64(2):307-320.
Crossref
|
|
|
|
Antonius K, Nybom H (1995). Discrimination between sexual recombination and apomixis/automixis in a Rubus plant breeding program. Hereditas 123(3):205-213.
Crossref
|
|
|
|
|
Bozovic D, Lazovic B, Ercisli S, Adakalic M, Jacimovic V, Sezer I (2016). Morphological characterization of autochthonous apple genetic resources in Montenegro. Erwerbs-Obstbau 58(2):93-102.
Crossref
|
|
|
|
|
Bravo L (1998). Polyphenols: Chemistry, Dietary Sources, Metabolism, and Nutritional Significance. Nutrition Reviews 56(11):317-333.
Crossref
|
|
|
|
|
Chittaranjan TW (2011). Blackberry and blueberry pests and diseases. Indian Journal of Fruit Science 25(2):54-68.
|
|
|
|
|
Chweya JA, Edmonds J (1997). Nightshades and related species.
|
|
|
|
|
Clark JR, Stafne ET, Hall HK, Finn CE (2007). Blackberry breeding and genetics. Plant Breeding Reviews 29(1):82-101.
Crossref
|
|
|
|
|
Finn CE (2008). Blackberries. In: Temperate Fruit and Crop Breeding. (J.F Hancock, ed.). Library of Congress Control Number: 2007939760.
|
|
|
|
|
Gower JC (1971). A General Coefficient of Similarity and Some of Its Properties. Biometrics 27(4):857-862.
Crossref
|
|
|
|
|
Hirsch GE, Vizzotto M, Aboy AL, Henriques T, Emanuelli T (2013). Antioxidant Activity of Blackberry (Rubus sp.) Genotypes from the Southern Region of Brazil. B. CEPPA Curitiba 31(1):83-98.
Crossref
|
|
|
|
|
Hollman PCH, Hertog MGL, Katan MB (1996). Analysis and health effects of flavonoids. Food Chemicals 57(1):43-46.
Crossref
|
|
|
|
|
Human CF, Rheeder S (2004). Mango breeding: Results and Successes. Acta Horticulturae 645(9):331-335.
Crossref
|
|
|
|
|
Kagimbo FM, Shimelis H, Sibiya J (2017). Diversity assessment of sweet potato germplasm collections for yield and yield-related traits in western Tanzania. Acta Agriculturae Scandinavica, Section B - Soil and Plant Science 68(2):121-129.
Crossref
|
|
|
|
|
Karimi HR, Zamani Z, Ebadi A, Fatahi MR (2009). Morphological diversity of Pistacia species in Iran. Genetic Resources and Crop Evolution 56(4):561-571.
Crossref
|
|
|
|
|
Lauri P, Normand F (2017). Are leaves only involved in flowering? Bridging the gap between structural botany and functional morphology. Tree Physiology 37(9):1137-1139.
Crossref
|
|
|
|
|
Lee J, Dossett M, Finn CE (2011). Rubus fruit phenolic research: The good, the bad, and the confusing. Food Chemistry 130(4):785-796.
Crossref
|
|
|
|
|
Marinoni D, Akkak A, Bounous G, Edwards KJ, Botta R (2003). Development and characterization of microsatellite markers in Castanea sativa (Mill.). Molecular Breeding 11(12):127-136.
Crossref
|
|
|
|
|
Mason AS, Zhang J, Tollenaere R, Teuber PV, Dalton-Morgan J, Hu L, Yan G, Edwards D, Redden R, Batley J (2015). High-throughput genotyping for species identification and diversity assessment in germplasm collections. Molecular Ecology Resources 15(5):1091-1101.
Crossref
|
|
|
|
|
Mason AS, Chauhan P, Banga S, Banga SS, Salisbury P, Barbetti MJ Batley J (2017). Agricultural selection and presence-absence variation in spring-type canola germplasm. Crop and Pasture Science 69(1):55-64.
Crossref
|
|
|
|
|
Menkir A, Goldsbrough P, Ejeta G (1997). RAPD based assessment of genetic diversity in cultivated races of sorghum. Crop Science 37(2):564-569.
Crossref
|
|
|
|
|
Nybom H (1986). Chromosome Numbers and Reproduction in Rubus subgen. Malachobatus. Plant Systematics and Evolution 152(3-4):211-218.
Crossref
|
|
|
|
|
Nybom H (1988). Apomixis versus sexuality in blackberries." Plant Systematics and Evolution 160(3-4):207-218.
Crossref
|
|
|
|
|
Orobiyi A, Loko LY, Sanoussi F, Agré AP, Korie N, Gbaguidi A, Adjatin A, Agbangla C, Dansi A (2017). Agro-morphological characterization of chilli pepper landraces (Capsicum annuum L.) cultivated in Northern Benin. Genetic Resources and Crop Evolution 65(2):555-569.
Crossref
|
|
|
|
|
Oyoo ME, Muhammed N, Githiri SM, Ojwang PO, Muniu FK, Masha E, Owuoche J (2015). In-situ morphological characterization of coconut in the coastal lowlands of Kenya. African Journal of Crop Science 9(2):65-74.
Crossref
|
|
|
|
|
Perrier X, Flori A, Bonnot F (2003). Data analysis methods. In: Hamon P, Seguin M, Perrier X, Glaszmann JC, editors. Genetic Diversity of Cultivated Tropical Plants. Montpellier, France: Science Publishers pp. 43-76.
|
|
|
|
|
Perrier X, Jacquemoud-Collet JP (2006). DARwin software.
View
|
|
|
|
|
R Development Core Team (2015). R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing Vienna Austria. Available at: Austria.
View
|
|
|
|
|
Rao AV, Snyder DM (2010). Raspberries and human health: A review. Journal of Agricultural and Food Chemistry 58(7):3871-3883.
Crossref
|
|
|
|
|
SAS Institute (2001). SAS procedure for personal computers. Version 8 SAS Institute, Cary, NC, USA.
|
|
|
|
|
Siriwoharn T, Wrolstad RE, Finn CE, Pereira CB (2004). Influence of cultivar, maturity and sampling on blackberry (Rubus L. hybrids) anthocyanins, polyphenolics, and antioxidant properties. Journal of Agricultural and Food Chemistry 52(26):8021-8030.
Crossref
|
|
|
|
|
Stafne ET, Clark JR (2007). Genetic relatedness among eastern North American blackberry cultivars based on pedigree analysis. Euphytica 139(2):95-104.
Crossref
|
|
|
|
|
Strik BC, Clark JR, Finn CE, Banados MP (2007). Worldwide blackberry production." Horticulture Technology 17(2):205-213.
Crossref
|
|
|
|
|
Wood GA, Andersen MT, Forster RL, Braithwaite M, Hall HK (1999). History of Boysenberry and Youngberry in New Zealand in relation to their problems with Boysenberry decline, the association of a fungal pathogen, and possibly a phytoplasma, with this disease. New Zealand Journal of Crop and Horticultural Science 27(4):281-295.
Crossref
|
|
|
|
|
Yan W, Wang X, Chen Q, Zhang L, Tang H, Luo Y, Liu Z (2015). Phylogenetic insight into subgenera Idaeobatus and Malachobatus (Rubus, Rosaceae) inferring from ISH analysis." Molecular Cytogenetics 8(1):11-24.
Crossref
|
|
|
|
|
Yin M (2017). Studies in Blackberry : Development and Implementation of a Phenotyping Protocol for Blackberry Seedling Populations and Impact of Time of Day of Harvest on Red Drupelet Reversion for University of Arkansas Blackberry Genotypes. Theses and Dissertations. 2417, 2017. Available at:
View
|
|