ABSTRACT
To investigate the effects of quarries emissions on the leaf morpho-anatomy of Romi, Nabali and K18 olive cultivars in Taffouh village (Palestine), one set of olive cultivars of two-years old was cultivated close to quarries while the control set was cultivated almost 4 km from quarries. The morphology of six month-old leaves was examined. Leaf segments were fixed in compound fixative of formaldehyde, acetic acid and 70% ethanol (FAA). The segments were then embedded in Paraplast plus and 5  m thick sections were stained with safranin and fast green. A reduction in trichome density was evident for the three cultivars in response to exposure to quarries emission, with high density in the abaxial epidermis. Moreover, the abaxial epidermis of the three cultivars possessed elaborated and well-developed trichomes. The leaves of Romi cultivar exhibit increase in all leaf components except the adaxial epidermis while Nabali cultivar exhibited a reduction in all leaf components. Nevertheless, K18 cultivar exhibited a reduction only in palisade and spongy layers due to exposure to quarries pollutions. In conclusion, quarries emissions led to condensed palisade and spongy cells in all cultivars. In addition, Romi cultivar showed a variegated increase in all morpho-anatomical parameters concomitant with increased sclerophylly of leaves following their exposure to quarries emissions. This cultivar proved to be the most resistant to quarries stress which implies it is well suited for olive production.
m thick sections were stained with safranin and fast green. A reduction in trichome density was evident for the three cultivars in response to exposure to quarries emission, with high density in the abaxial epidermis. Moreover, the abaxial epidermis of the three cultivars possessed elaborated and well-developed trichomes. The leaves of Romi cultivar exhibit increase in all leaf components except the adaxial epidermis while Nabali cultivar exhibited a reduction in all leaf components. Nevertheless, K18 cultivar exhibited a reduction only in palisade and spongy layers due to exposure to quarries pollutions. In conclusion, quarries emissions led to condensed palisade and spongy cells in all cultivars. In addition, Romi cultivar showed a variegated increase in all morpho-anatomical parameters concomitant with increased sclerophylly of leaves following their exposure to quarries emissions. This cultivar proved to be the most resistant to quarries stress which implies it is well suited for olive production.
Key words: Adaptation, leaf anatomy, Olea europea, olive, quarries stress, trichome.
Olive (Olea europaea L), a member of Oleaceae family, is an evergreen Mediterranean tree capable of living for more than a thousand years (Bacelar et al., 2004; Tangu, 2014). The olive leaf has an average life span of about three years. Leaves of different cultivars are of different morphology. The length varied from 4 to 10 and the width from 1 to 3 cm (Ennajeh et al., 2010). The olive tree has been taken as the symbol of peace, fertility, strength, and purity and it played an enormous role in ancient civilizations (Kiritsakis, 1989, Kiritsakis and Min 1991). The most ancient evidence of edible olive cultivation has been found in Syria, Palestine and Crete, 5000-6000 years ago (Bacelar et al., 2004). Palestine, however, is one of most ancient countries of edible olive production in the world. It is about 10 million olive trees, mainly in the major rain-fed lands. Olive orchards occupy about 48% of the agricultural land in the Western Bank and Gaza strip. Olives represent 70% of fruit production in Palestine and contribute by 14% to Palestinian economy (Palestinian News and Information Agency, 2010).
Most of the harvested olives (90%) are used for oil production, and the remaining 10% is used as table olives (Mali, 2015). Olive oil has high concentration of monounsaturated fatty acids, and can promote good cholesterol (high density cholesterol: HDL) content of the human body. It can also lower the bad cholesterol (low density cholesterol: LDL) (Visioli and Galli, 1995). In addition, it protects against cardiovascular diseases, breast cancer and malaria and it can afford defending the fruit against pathogens and insects (Kubo et al., 1995; Visioli and Galli, 1995).
Plants may encounter various kinds of stress, both biotic and abiotic. On the one hand, biotic stress occurs as a result of damage done to plants by other living organisms, such as bacteria, viruses, fungi, parasites, beneficial and harmful insects, weeds, and cultivated or native plants (Newton et al., 2011). On the other hand, abiotic stress is defined as environmental condition that reduces growth and yield below optimum levels (Cramer et al., 2011). Severe abiotic stresses, such as salinity and drought, are more harmful to crop plants than the biotic stress. While the later causes less than 10% loss of crop yields, the former can reduce yields up to 65% (Al-Tardeh and Iraki, 2014; Albino et al., 2001; Greenway and Munns, 1980). Indeed, quarries are considered one of the newly recognized abiotic stresses that negatively affect both animals and plants (Sayara et al., 2016).
Quarries negatively affect biodiversity of the terrestrial life. The biodiversity reduction and/or loss is obvious for many species of animals, that is, deer, birds, etc. In addition, the loss of plant biodiversity reveals the worst to come for domestic life. The most endangered plants are the sage (Salvia officinalis), thyme, wild flowers as well as the fruit plant, that is, almonds, apples, figs, etc. (Sayara et al., 2016). Furthermore, quarries are considered the main source of air pollution via gases released from the digging machines beside the huge clouds of dust containing heavy metals that affect the life of every living organism in the area. The incidence of quarries on agriculture in Taffouh is evident since they reduce the land area preserved for agriculture. Moreover, the effect of quarries on humans is not only the reduction of land area for construction, but also the destruction of the already existing human habitat (Tiimub et al., 2015).
Internationally, Palestine contributes by 4% to the world's production of stone and marble, being ranked the twelfth in the world. Quarries participate by 2% of the capital industrial plants in Western Bank and Gaza strip. This industry is concentrated mainly in Nablus, Jerusalem and Hebron (Palestinian Ministry of Economy, 2014(. In this regard, quarries negatively affect both environment and agriculture. In this work, we will investigate quarries stress on leaf morphology and anatomy of three local olive cultivars to identify the most pollution-resistant cultivar, which would be the best for olive production.
Two year-old seedlings of three olive cultivars, that is, Romi, K18 and Nabali, were collected from a local nursery at Hebron city. Sets of seedlings were planted close to quarries (almost 5 m away) and sets were planted 4 km far away (control) under identical environmental conditions.
Trichome density
Six month-old leaves were collected and examined for trichome density. The leaves were painted with a transparent nail polish and left to dry out. Then, a transparent adhesive tape was added on the painted leaf surface. After few minutes, the tape was peeled, mounted on a glass slide containing copper grid of 1799.2 µm2 surface area and the number of hairs was counted under light microscope. The differences between trichome density of control sets and those exposed to quarries were calculated. The change (reduction) percentage of trichome density was calculated as a difference between the control and those exposed to the quarries effect divided by the control value and multiply 100%.
Light microscopy
Segments from the leaf midrib of the three olive cultivars were fixed in a compound fixative overnight at 4°C. The fixative is composed from 5ml formaldehyde, 5ml acetic acid and 90ml 70% ethanol (5% FAA). The segments were double dehydrated in 50% ethanol for 45 minutes and dehydrated in solutions of graded proportions of tertiary butanol, ethanol and distilled water till 100% tertiary butanol. Then the segments were left overnight in tertiary butanol and Histoclear (1:1). Then, segments were infiltrated in 1/3, 2/3 and 100% Paraplast Plus in Histoclear, as well as 2 to 3 changes with clean Paraplast Plus for 3 to 4 h in the oven at 60°C. the segments were embedded in freshly prepared “Paraplast Plus”. Semi-thin sections (5 μm thick) were cut using a SLEE microtome (CUT 6062, Germany) and mounted on glass slides at 37°C (Al-Tardeh and Iraki, 2013).
The sections were dewaxed in double bath of histoclear and rehydrated in decreased graded ethanol series followed by staining with 1% safranin O. The sections were dehydrated in graded ethanol series and stained with 0.5% fast green (Johansen, 1940). Micrographs of the sections were taken using an Olympus CK40 microscope (Artisan Technology Group ®, USA) supplemented by Moticam Camera 3MP, 2015 (Motic ®, Chaina).
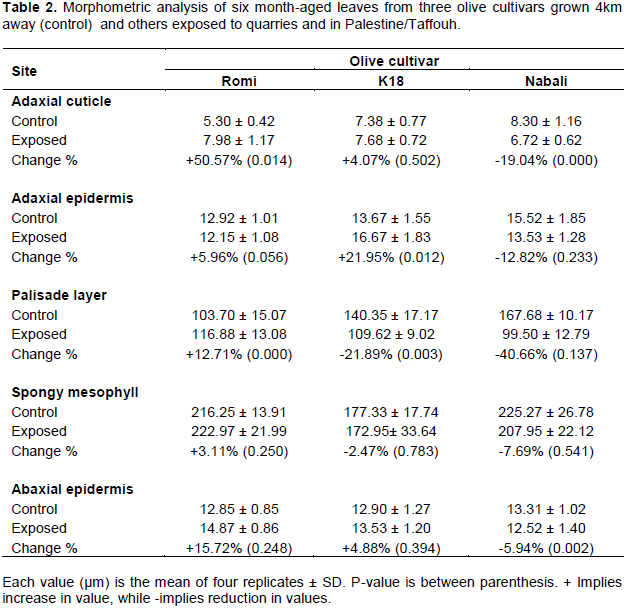
Morphometry
Micrographs of the three local olive cultivars grown 4 km far from quarries and those exposed to quarries were obtained, and measurements of all tissue layers and structure thickness were conducted. The ANOVA for the different variables was performed. Statistical analysis was performed using “R Software” (R Development Core Team, 2018). Correlation analysis was used to examine relationships among plant tissue variables. The change percentage of all olive leaf components was calculated as a difference between the control leaves components and those exposed to the quarries effect was divided by the control value and multiply 100%.
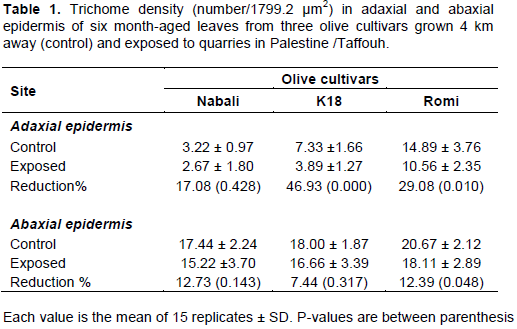
Trichome density
The three cultivars have possessed higher trichome density in the abaxial epidermis than the adaxial ones (Table 1). However, Romi cultivar reveals the highest trichome density in both adaxial and abaxial epidermis (14.89±3.76 and 20.67±2.12/1799.2 µm2, respectively) in comparison to those of K18 and Nabali. Meanwhile, it shows minimal difference between its adaxial and abaxial trichome density (Table 1).
Under quarries stress, on contrast, there was a variegated reduction in trichome density for all cultivars grown under the impact of quarries emissions, mainly for adaxial epidermis. Romi shows significant reduction (p < 0.05) in adaxial trichome and abaxial trichome densities. Meanwhile, k18 shows a high fluctuation in trichome density reduction with significant reduction (p < 0.05) in adaxial epidermis trichomes (46.93%) and the lowest reduction percentage (7.44%) in abaxial epidermis. The moderate resistant was recorded for the Nabali cultivar since the reduction in trichome density is not significant (Table 1).
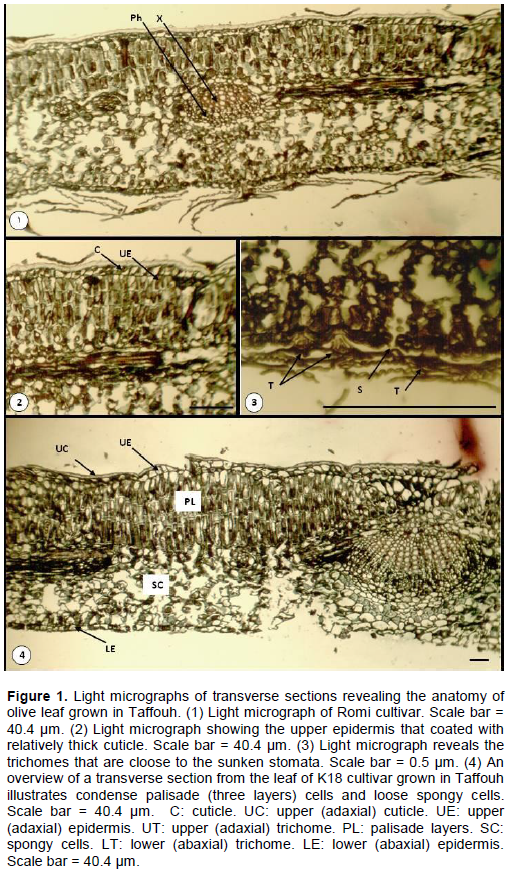
Anatomical features of the olive leaves
The anatomy of the leaf of three olive cultivars grown in Taffouh is illustrated in Figure 1. The leaf is covered by a relatively thick cuticle in adaxial epidermis (Figure 1 (1)). The epidermal cells are isodiametric to elongated in shape with thicker external cell wall than the internal ones (Figure 1 (2)). The stomata are sunken and protected by trichomes on both adaxial and abaxial epidermis Figure 1 (13). In addition, the olive leaf mesophyll is characterized by three distinct palisade layers which are tightly compacted together and loose spongy cells of undefined shape (Figure 1 (4)). The intercellular space is large between spongy cells in order to save space for gas exchange for better photosynthesis. The vascular system in the leaf is a typical one of the dicot plant with xylem tissue towards the adaxial epidermis (Figure 1 (1-4)).
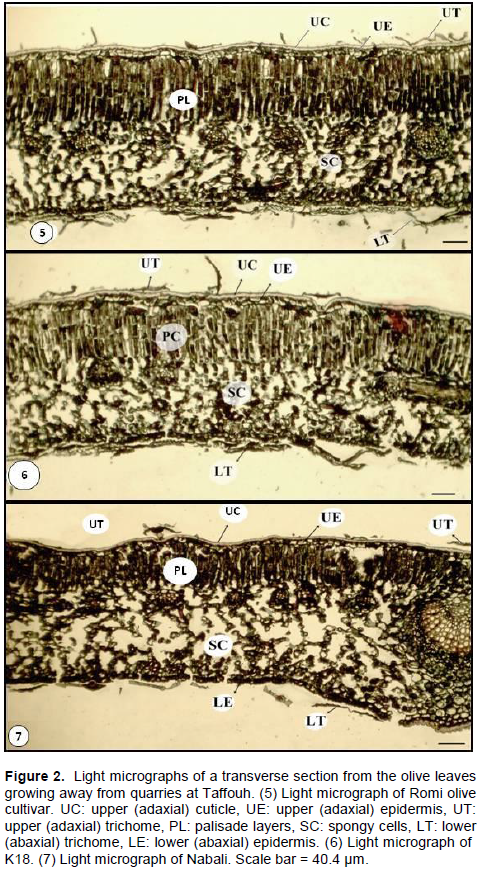
Comparative effect of quarries on morpho-anatomy of olive leaves
The leaf morpho-anatomy of the three olive cultivars grown in Taffouh is comparatively obvious in Figure 2 and Table 2. Leaf of Romi cultivar possesses the lowest values for all leaf components except for spongy cells (Table 1 and Figure 2 (5)). However, the leaf of K18 possesses moderate values of its components (Figure 2 (6)). On contrast, leaf of Nabali cultivar (Figure 2 (7)), possesses the thickest adaxial cuticle layer (8.30 ± 1.16 µm), adaxial epidermal cells (15.52± 1.85 µm), three condense layers of palisade cells (167.68 ± 10.17 µm), loose spongy layer (225.27 ± 26.78 µm), and abaxial epidermis (13.31 ± 1.02 µm).
In an effort to explore the changes in the morpho-anatomy of the leaves under quarries stress, micrographs were analyzed for thicknesses of each leaf component (Figure 3). The parameters for olive seedling 4 km away from quarries and those under quarries stress are listed in Table 2. The leaf morpho-anatomy of the three cultivars reveals variegated responses and behavior under their exposure to the quarries stress. Romi cultivar shows an increase in all leaf components, that is, adaxial cuticle, adxial epidermis, palisade layers, spongy cells, abaxial epidermis (Table 2, Figure. 3 (8). The significant increases in percentage (p < 0.05) were recorded for adaxial cuticle (+50.57%) and palisade layers (+12.71%). The lowest increase percentage was recorded for spongy cells (+ 3.11%) while fair increase in palisade layers (+12.71%). On contrast, Nabali cultivar shows reduction in all the aforementioned components (Table 2 and Figure 3 (10)). The highest reduction percentage was recorded for palisade layer (-40.66%) and little reduction percentage in spongy cells (-7.69%). However, the significant reduction percentage (p< 0.05) was recorded for both the adxial and abaxial cuticle. K18 reveals increases in both adaxial (significant increase, p <0.05) and abaxial epidermis and reduction in palisade (significant, p <0.05) and spongy layers (Table 2 and Figure 3 (9). In addition, the abaxial epidermis is interrupted by repeated occurrence of stomata and trichomes. These phenomena explain absence of continuous layer of cuticle on the abaxial epidermis (Figure 3). Based on the aforementioned findings, Romi cultivar is supposed to be the best olive cultivar suited for resistant against quarries stress in Palestine/Taffouh village.
In general, trees act as a sink for air pollutants and thereby can reduce the concentration of pollutants in the environment especially in urban regions (Woo and Je, 2006; Tiwari et al., 2006; Rawat and Banerjee, 1996). The deposition of quarries dust on plant surface depends on several factors including tree canopy, phyllotaxy, and leaf external characteristics such as hair density, leaf shape and length of petioles. Moreover, speed and direction of wind, weather conditions and anthropogenic activities are also affecting the dust accumulation (El-Khatib et al., 2011, 2012). In the present study, the accumulation of dust on olive leaves is evident.
Quarries exert effects and consequences on agriculture due to the accumulation of dust on plant leaves. Therefore, quarries are responsible for ecosystem distortion, loss of natural habitat, plant canopy destruction, reduction of agricultural and rural areas, restriction of the biodiversity in the area of quarries and the nearby areas. Such effects were recorded in a village known as Se’er in Hebron governorate, where almost 30 species were endangered (Sayara et al., 2016; Halaka, 2010). On one hand, olive can withstand drought and heat stress as well as the mechanical effect of violent winds (Loussert and Brousse, 1978), on the other hand, its withstand the queries stress according to the findings of the present study.
It is well-known that trichomes are located around the stomata in order to create a microclimate with low water vapor pressure gradient between the leaf interior and its surrounding, thus reducing transpiration rate in the arid and semi arid regions. Nabali cultivar shows minimal differences and lowest reduction percentages between adaxial and abaxial trichome density in comparison with the other olive cultivars (Table 2). In this context, Nabali cultivar seems to be the least affected by quarries stress. On the other hand, the high fluctuation in trichome density for K18, is interpreted as an effort for the plant to withstand the dust accumulation and water deficiency. However, Romi cultivar perceived moderate affects by the quarries stress. The reduction in trichome density is a good sign coping with dust accumulation and water deficiency, but this interferes with gas exchange which affects negatively on plant capacity for photosynthesis. Therefore, Romi cultivar possesses the highest number of trichome density which implies the highest stomata density. Therefore, when it reduces the trichome density, it means that it still has large number of trichome and stomata for better gas exchange and better photosynthesis. Such situation indicates that Romi cultivar possesses an inherent mechanism to adapt to such stresses and this inherent mechanism is working and investigated for drought stress (Tangu, 2014).
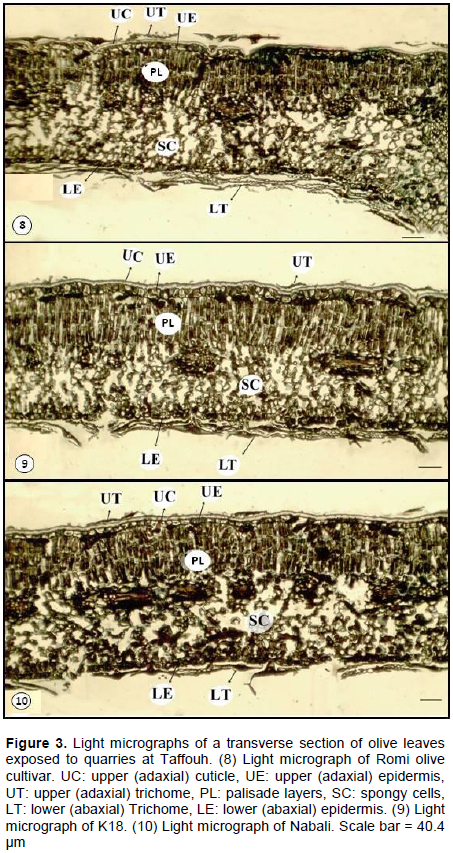
Olive leaf of Romi cultivar responds to quarries stress by increase of values for all components mainly, the spongy cells and intercellular spaces (Table 2 and Figures 2 to 3) to save space for better gas exchange and high rate of photosynthesis. These values support the idea that the Romi cultivar is the best adapted for Palestinian climate of being semi-arid environment. On one hand, such anatomical features assist the plant for compromising with shortage in water availability and precipitation and/or drought. On the other hand, Romi cultivar possesses the lowest values in comparison to K18 and Nabali for all components, except the spongy cells (control group). This is interpreted as an adaptive strategy to shorten the distance that the carbon dioxide has to travel to reach the photosynthetic cells (palisade and spongy cells). Correspondingly, it reduces the exposure time for gas exchange and it reduces the water loss through the opened stomata (Bosabalidis and Kofidis, 2002). In fact, such a reduction of the blade layers emphasizes on the total reduction in leaf area and volume (Tangu, 2014). The later, is also corresponding with an adaptation for the plant to resist drought and water shortage stress (Bacelar et al., 2004). In this regard, the quarries exert stress on olive in the same way of drought stress and this is due to the accumulation of dust over the plant leaves which disturb the gas exchange and the photosynthesis as well.
Similar findings were reported for olive response to abiotic stresses exerted by environmental conditions. In a similar study, Ennajeh et al. (2010) emphasized on olive subjected to water stress and recognized as a water stress resistant, the leaves increased the thickness of their upper palisade and spongy parenchyma by 17% and 22%, respectively. In addition, the photosynthetic capacity of olive leaves is reduced under dry-farming, that is, water stress deficiency in comparison to drip irrigation sufficient to meet the crop water requirements (DÍAZ-ESPEJO et al., 2016). Accordingly, Romi cultivar shows an increase in all leaf components including palisade and spongy parenchyma, a site for photo-synthesis activity in C3 plants. While a thicker palisade parenchyma could contain larger numbers of CO2-fixation sites, a thicker spongy parenchyma could result in easier diffusion of CO2 to these sites. Accumulatively, Romi cultivar possess a compensation mechanisms to withstand quarries stress thus the moderate reduction in trichome (hence, stomata) density is balanced by an increase in palisade and spongy layers as well as increase in the cuticle layer. Moreover, this may implies that the Romi possesses an inherent mechanism to adapt to quarries stresses in the same manner to the inherent mechanisms investigated for drought stress (Tangu, 2014).
Olive plant seems to be well adapted for environmental stresses including the quarries stress. The reason behind such adaptation is related to its genetic makeup. However, in the present study, the Romi cultivar proved to be the most resistant to quarries stress from the leaf responses point of view. This resistance is supported by (1) increase in thicknesses of all blade layers, (2) the more condensed palisade and spongy cells, (3) well-developed and elaborated trichomes, (4) thick cuticle and (5) reduction in trichome density and the presence of sunken and amphistomaty. In addition, further research is needed to determine the particulate matter of air pollution, quantitative and qualitative analysis of heavy metals in quarries dust, exploring the effect of quarries stress on olive quality and production as well as the resilience and eutrophication of quarries and their landfills.
The authors have not declared any conflict of interests.
The authors gratefully acknowledge the Deanship of Scientific Research at Palestine Polytechnic University for grant to carry out this research study. In addition, the authors also gratefully acknowledge Dr Monjed Samuh for his help in section of statistical analysis and Mrs Maysa Aljuneidi for English revision of the manuscript.
REFERENCES
|
Albino M, Hasegawa PM, Bressan RA, Consiglio MF, Joly RJ (2001). Unravelling the functional relationship between root anatomy and stress tolerance. Australian Journal of Plant Physiology 28:999-1004.
Crossref
|
|
|
|
Al-Tardeh S, Iraki N (2013). Morphological and anatomical responses of two Palestinian tomato (Solanum lycopersicon L.) cultivars to salinity during seed germination and early growth stages. African Journal of Biotechnology 12:4788-4797.
Crossref
|
|
|
|
|
Bacelar EA, Correia CM, Moutinho-Pereira JM, Gonçalves BC, Lopes JI, Torres-Pereira JM (2004). Sclerophylly and leaf anatomical traits of five field-grown olive cultivars growing under drought conditions. Tree Physiology 24:233-239.
Crossref
|
|
|
|
|
Bosabalidis AM, Kofidis G (2002). Comparative effects of drought stress on leaf anatomy of two olive cultivars. Plant Science163:375-379.
Crossref
|
|
|
|
|
Cramer GR, Urano K, Delrot S, Pezzotti M, Shinozaki K (2011). Effects of abiotic stress on plants: a systems biology perspective. BMC Plant Biology 11:163-177.
Crossref
|
|
|
|
|
Díaz-Espejo A, Walcroft AS, Fernández JE, Hafidi B, Palomo MJ, Girón IF (2016). Modeling photosynthesis in olive leaves under drought conditions. Tree Physiology 26(11):1445-1456.
Crossref
|
|
|
|
|
El-Khatib AA, Abd El-Rahman AM, Elsheikh OM (2011). Leaf geometric design of urban trees: potentiality to capture airborne particle pollutants. Journal of Environmental Studies 7:49-59.
|
|
|
|
|
El-Khatib AA, Radwan DEM, Alramah-Said AA (2012). Morpho-Anatomical Characteristics of Olive (Olea europaea L.) Trees Leaf as Bio-indicator of Cement Dust Air Pollution in Libya. Journal of Environmental Studies 9:65-72.
|
|
|
|
|
Ennajeh M, Vadel AM, Cochard H (2010). Comparative impact of water stress on leaf anatomy of a drought- resistant and a drought - sensitive olive cultivar. The Journal of Horticultural Science and Biotechnology 85:289-294.
Crossref
|
|
|
|
|
Greenway H, Munn R (1980). Mechanism of salt tolerance in nonhalophytes. Annual Review of Plant Physiology 31:149-190.
Crossref
|
|
|
|
|
Halaka H (2010). The impacts of stone quarrying and cutting industries on the economic, social and environmental conditions in Hebron District. MSc dissertation, Birzeit University, Palestine.
|
|
|
|
|
Kiritsakis A (1991). Olive oil: American oil chemist's society, Champaign.
|
|
|
|
|
Kiritsakis A, Min D (1989). Flavour chemistry of olive oil In: David BM, Thomas HS (eds.) Flavour chemistry of lipid food. American oil Chemists Society, Champaign pp. 196-221.
|
|
|
|
|
Kubo A, Lunde CS, Kubo I (1995). Antimicrobial activity of the olive oil flavor compounds. Journal of Agricultural and Food Chemistry 43:1629-1633.
Crossref
|
|
|
|
|
Loussert R, Brousse GL (1978). Techniques agricoles et production méditerranéenne. G. P. Maisonneuve et Larousse P 465.
|
|
|
|
|
Mali SB (2015). Olive oil and Health. Oxford Book Company pp. 1-17.
|
|
|
|
|
Palestinian Ministry of Economy (2014). Available at:
View.
|
|
|
|
|
R Development Core Team (2018). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available at:
View
|
|
|
|
|
Rawat JS, Banerjee SP (1996). Urban forestry for improvement of environment. Energy Environment Monitoring 12:109-116.
|
|
|
|
|
Sayara T, Hamdan Y, Basheer-Salimia R (2016). Impact of Air Pollution from Quarrying and Stone Cutting Industries on Agriculture and Plant Biodiversity. Annual Review of Environment and Resources 6:122-126.
|
|
|
|
|
Tangu NA (2014). Effects on Plant Morphology of drought in olive. Turkish Journal of Agricultural and Natural Sciences 6:900-904.
|
|
|
|
|
Tiimub BM, Sarkodie PA, Monney I, Maxwell O (2015). Heavy Metal Contamination of soil by Quarry Dust at Asonomaso in the Ashanti Region of Ghana. Chemistry and Materials Research 7:42-51.
|
|
|
|
|
Tiwari S, Agrawal M, Marshall FM (2006). Evaluation of ambient air pollution impact on carrot plants at a sub urban site using open top chambers. Environmental Monitoring and Assessment 119: 15-30.
Crossref
|
|
|
|
|
Visioli F, Galli C (1995). Natural antioxidants and prevention of coronary heart disease: the potential role of olive oil and its minor constituents. Nutrition, Metabolism and Cardiovascular Diseases 5:306-314.
|
|
|
|
|
WAFA Palestinian News and Information Agency (20110).
|
|
|
|
|
Woo SY, Je SM (2006). Photosynthetic rates and antioxidant enzyme activity of platanus occidental is growing under two levels of air pollution along the streets of Seoul. Journal of Plant Biology 49:315-319.
Crossref
|
|
![]() m thick sections were stained with safranin and fast green. A reduction in trichome density was evident for the three cultivars in response to exposure to quarries emission, with high density in the abaxial epidermis. Moreover, the abaxial epidermis of the three cultivars possessed elaborated and well-developed trichomes. The leaves of Romi cultivar exhibit increase in all leaf components except the adaxial epidermis while Nabali cultivar exhibited a reduction in all leaf components. Nevertheless, K18 cultivar exhibited a reduction only in palisade and spongy layers due to exposure to quarries pollutions. In conclusion, quarries emissions led to condensed palisade and spongy cells in all cultivars. In addition, Romi cultivar showed a variegated increase in all morpho-anatomical parameters concomitant with increased sclerophylly of leaves following their exposure to quarries emissions. This cultivar proved to be the most resistant to quarries stress which implies it is well suited for olive production.
m thick sections were stained with safranin and fast green. A reduction in trichome density was evident for the three cultivars in response to exposure to quarries emission, with high density in the abaxial epidermis. Moreover, the abaxial epidermis of the three cultivars possessed elaborated and well-developed trichomes. The leaves of Romi cultivar exhibit increase in all leaf components except the adaxial epidermis while Nabali cultivar exhibited a reduction in all leaf components. Nevertheless, K18 cultivar exhibited a reduction only in palisade and spongy layers due to exposure to quarries pollutions. In conclusion, quarries emissions led to condensed palisade and spongy cells in all cultivars. In addition, Romi cultivar showed a variegated increase in all morpho-anatomical parameters concomitant with increased sclerophylly of leaves following their exposure to quarries emissions. This cultivar proved to be the most resistant to quarries stress which implies it is well suited for olive production.