ABSTRACT
The antimicrobial activities of Xylopia aethiopica and Syzygium aromaticum extracts on fungi associated with rotting white and water yam was investigated. Diseased and healthy yam species of Dioscorea spp. were obtained from some markets. Fungal isolation was done from the samples using standard procedures. Leaves and fruits of X. aethiopica and S. aromaticum were obtained from the botanical garden, University of Ibadan, Ibadan. Crude aqueous and ethanol extracts of the plants were obtained using standard procedures. After pathogenicity tests, the isolated fungi were cultured on acidified potato dextrose agar (APDA) that were impregnated separately with the leaves and fruits of X. aethiopica and fruits of S. aromaticum extracts at specific concentrations for 10 days. Experimental design was completely randomized design (CRD) with three replicates. Mycelial extension of the fungi was measured daily using a meter rule. Data were subjected to statistical analysis using SAS software. Means separation was done using LSD (DMRT) at P≤0.05. The isolated fungi were identified as Aspergillus niger, Aspergillus fumigatus, and Penicillium chrysogenum. Pathogenicity test showed that the three fungi caused rotting in the yams. Growth inhibition of the fungi was significantly (P≤0.05) higher with ethanol extracts than aqueous extract. Highest mycelial growth inhibitory effect was recorded in the S. aromaticum fruit ethanol extracts on all the organisms. Likewise, X. aethiopica leaf aqueous extract showed high mycelial growth inhibition on A. fumigatus at 50 and 75% concentrations while X. aethiopica fruit ethanol and aqueous extracts was noted to have inhibitory effects on the growth of A. niger and P. chrysogenum at 50 and 75% concentrations respectively. The in vitro result underscores the antifungal abilities of these plant extracts and is also suggestive of their promising potential in vivo. Further works are underway to examine their antimicrobial potentials in the field.
Key words: Dioscorea alata, Dioscorea rotundata, postharvest rot, Syzygium aromaticum, Xylopia Aethiopica.
Yam belongs to Dioscorea family and is rated as one of the most important staple food crops in most parts of West Africa especially Nigeria (Olayemi and Ajaiyeoba, 2007). Yams are root tuber bearing plants grown and harvested annually with over 600 species out of which, six are economically and socially important as regards export purposes, medicine and food (IITA, 2009). The six edible species of yam are; white yam (Dioscorea rotundata), water yam (Dioscorea alata), bitter yam (Dioscorea dumetorum), aerial yam (Dioscorea bulbifera), Chinese yam, (Dioscorea esculenta), yellow yam (Dioscorea cayenensis) (Zaknayiba and Tanko, 2013; Lawal et al., 2014; Princewill-Ogbonna and Ibeji, 2015). The variation in taste of yam inspires it’s processing in different forms. Some are eaten as cooked starchy vegetables, some are boiled and mashed, and some are baked, roasted, fried, or pounded into thick paste after boiling and eaten with soup (Frank and Kingsley, 2014). Also, some yam tubers can be sliced and used as herbal medicine in China (Lee et al., 2003).
The crop plays an encouraging role as a guarantee for household food security. Nigeria is the largest producer of yam in the world followed by Ghana, Cote d’ Ivoire, Benin and Togo with a total global output of 67% and an annual yam production estimated at 44.11 metric tonnes out of 65.94 metric tonnes total global production in 2016 (FAO, 2013). Farmers engage in yam production for household production, production of planting materials for private uses, income from sale of yams and surplus seed yams. The superstition and ritual often associated with yam in West Africa is an indication of the antiquity of this crop (Frank and Kingsley, 2014).
The steady rise in demand and supply of yam over the years has not been zealously met as farmers encounter various major constraints in the production, harvesting and marketing of yam. Studies by Zaknayiba and Tanko (2013) revealed inadequate storage facilities, poor producers, prices, incidences of pests and diseases, lack of access to farm inputs and finances are the negative constraints faced by farmers in yam production. Many tuber crops especially yams in Nigeria are labor intensive as the high cost of labour constrains small farm holders from enhancing productivity (Ayanwuyi et al., 2011).
Most of the labour costs in yam production are mostly felt during the planting process and to cut costs, family members are duly engaged from the production to the marketing of the yam produce (Zaknayiba and Tanko, 2013). In 2015, Nigeria had a total decline in yam production of about 3.4% (IITA, 2009; Ike and Noni, 2006). The reason was attributed to the various constraints like pests and diseases, inadequate storage and processing facilities, inadequate preservations, marketing and access to markets. Diseases and pests related issues have been identified as a major menace in yam production. These include; fungi such as Aspergillus niger, Penicillium chrysogenum, Botryodiplodia theobromae, Fusarium oxysporum, Aspergillus fumigatus etc. and symptoms which includes leaf spot, tuber rots; insects such as tuber and leaf beetles and parasitic nematodes (Asante et al., 2007; IITA, 2009; Zaknayiba and Tanko, 2013; Bongiorno et al., 2016).
Several methods have been adopted for controlling losses due to post harvest disease of yam. These include the use of chemicals, biological method of control, and the use of natural plant extracts, as reported by Amusa et al. (2003). Because of the low capital income of farmers in Nigeria and lack of expertise in the safe handling of chemicals, farmers resorted to the method of crop rotation, fallowing, planting of healthy material and destruction of infected crop cultivars in controlling the diseases of yam tubers, and most times, these are done poorly (Nwakiti, 1982). Chemical method of control has helped to reduce the rate of storage losses and also increases yield obtained. But the problem arising with the use of chemicals is that it is expensive, can cause environmental pollution and may also induce pathogen resistance. Biological control method has been preferred in some cases because it is selective with no side effect and cheap. Resistance to biological control is rare and biological control agents are self-propagative and self-perpetuating (Okigbo and Ikediugwu, 2000). Some plants are known to synthesize phytochemicals with antimicrobial activities and are used successfully in the control of diseases in humans and crops like yam, cowpea, rice, etc. (Bediako et al., 2007).
There had been increased attention on management of plant diseases using biological control measures (Okigbo and Nmeka, 2005). The extracts of Xylopia aethiopica and Syzygium aromaticum have been reported to have high antimicrobial activity against several plant pathogens. Therefore the objectives of this work were to: Isolate and identify fungi associated with post-harvest rot of D. rotundata (white yam) and D. alata (water yam), to evaluate the effectiveness of extracts of X. aethiopica and S. aromaticum.
On growth of the isolated rot pathogens in-vitro, to examine impact of concentration on the effectiveness of the extracts, to evaluate the effectiveness of X. aethiopica and S. aromaticum extracts (in vitro) on the mycelia growth of the rot pathogens and to compare the effectiveness of X. aethiopica (Linn) and S. aromaticum plant parts extracts on the isolated fungi.
Diseased yam tubers (D. alata and D. rotundata) were obtained from Bodija market in Ibadan, Oyo state, Nigeria. Leaf and fruits of X. aethiopica and S. aromaticum were collected from the Botanical garden, University of Ibadan, Oyo state. Pieces of diseased white and water yam obtained from different markets in Ibadan were surfaced sterilized and cultured on acidified petri plates of potato dextrose agar (APDA) following standard procedures. Incubation at room temperature was done for 7 days. After pathogenicity tests and preparation of the plant extracts (leaf and fruits of X. aethiopica and S. aromaticum), their antifungal assay was examined at three different concentrations viz; 35, 50 and 75% following standard procedures (Sobowale et al., 2015). There were two controls; 0% with agar and 0% with ethanol. The experiment was conducted in a completely randomized design (CRD). All experiments were done in triplicates. Incubation was done at 28°C and diametric growths of the fungi were measured at 24 h interval using meter rule and recorded (Sobowale et al., 2015). The data collected were subjected to analysis of variance (ANOVA) using generalized linear model (GLM) procedure of SAS (version 9.2). Means were separated using Duncan's multiple range test (DMRT) at P≤0.05.
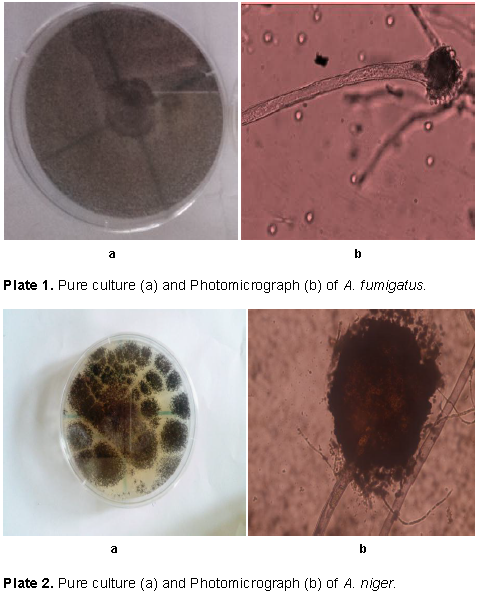
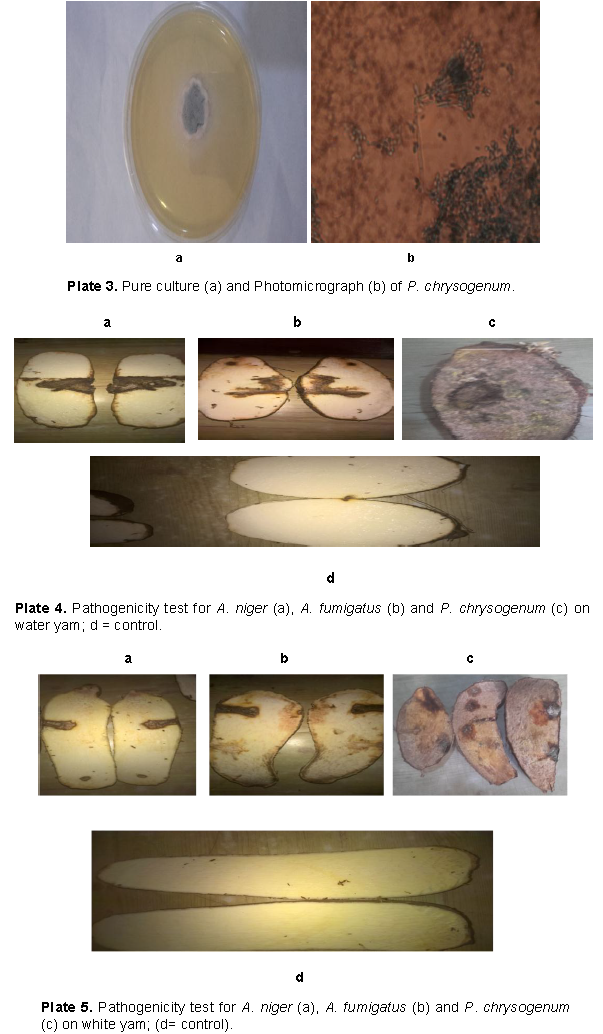
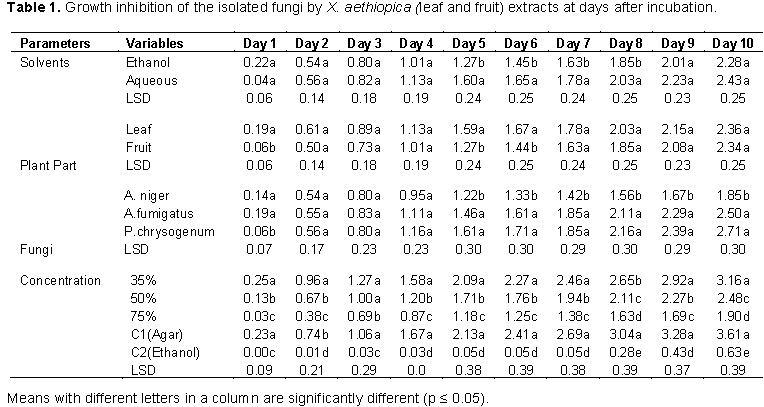
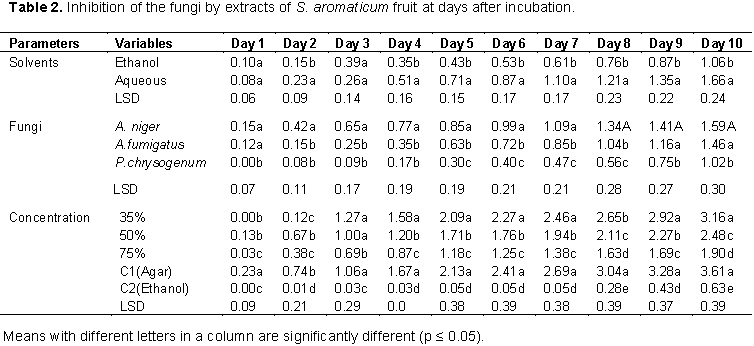
The fungi isolated from the rotting white and water yam tubers include; A. fumigatus (Plate 1), A. niger (Plate 2), and P. chrysogenum (Plate 3). The pathogenicity test conducted showed that A. niger, A. fumigatus and P. chrysogenum caused rotting on the water yam (Plate 4) and white yam (Plate 5) tubers in storage. The result showed that P. chrysogenum was more virulent on both yam tubers while the other fungi strains were not as virulent. Growth inhibition of the fungi by leaf and fruit extracts of X. aethiopica was significantly higher with ethanol extracts than aqueous extract as shown in Table 1. Growth reduction by fruit extract was better than that of leaf with significant differences on certain days after inoculation. Growth inhibition of A. niger was generally more than that of other two fungi with significant differences on days 5 to 10. Inhibition at all concentrations was significantly better than that in aqueous control. Inhibition at 75% concentration was significantly better than those at other concentrations as seen in Table 1.
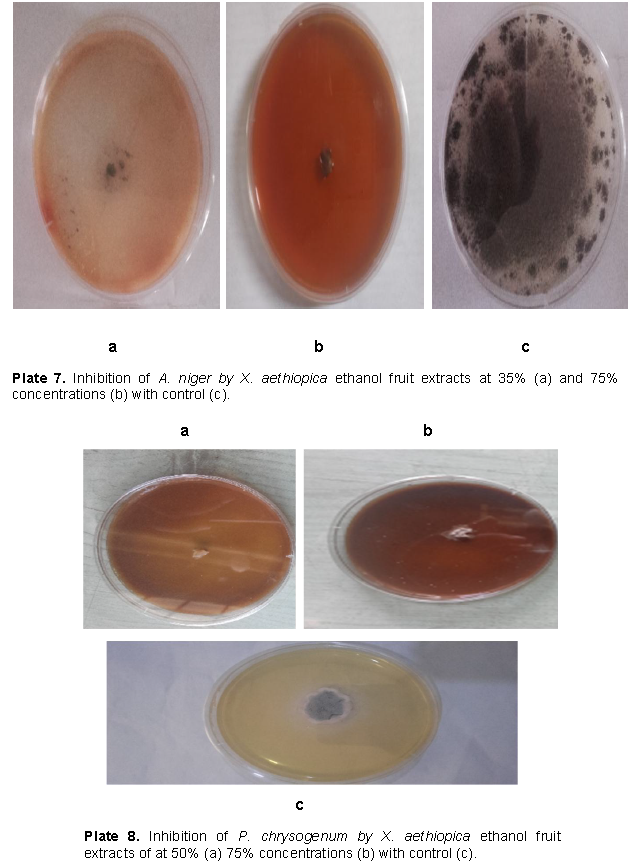
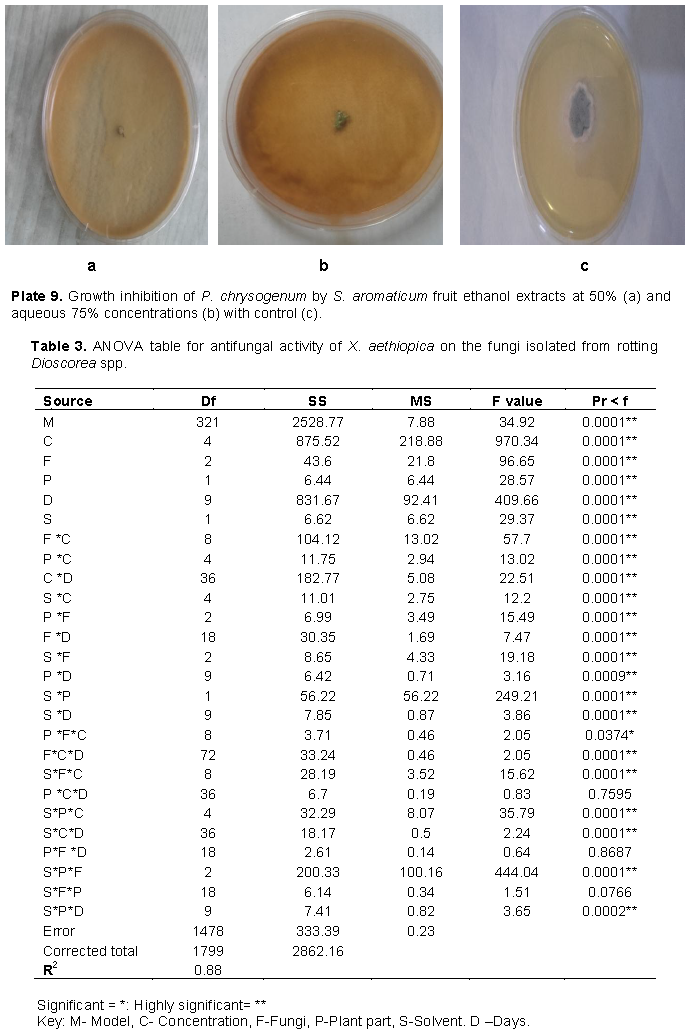
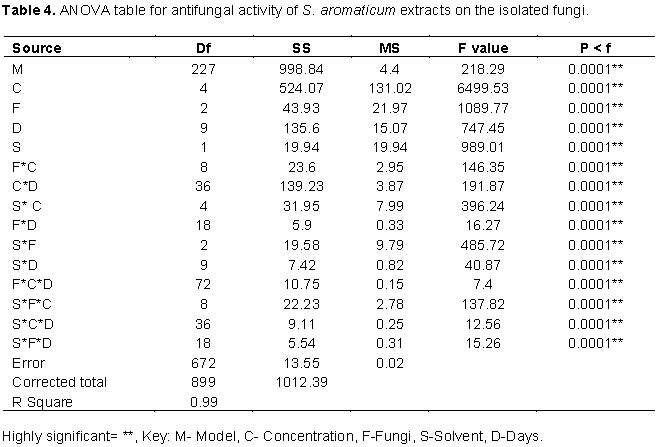
Growth inhibition of the fungi by fruit extracts of S. aromaticum was significantly higher with ethanol extracts than aqueous extract as seen in Table 2. Generally, inhibition of P. chrysogenum by the fruit extract was significantly better than that of the other two fungi. However, the impact of S. aromaticum extracts on growth of A. niger was significantly higher than that of X. aethiopica while the converse is true for P. chrysogenum as shown in Figure 1. Growth inhibitions of A. fumigatus by aqueous leaf extracts of X. aethiopica at all concentrations were significantly better than that in the controls as seen in Plate 6. Growth inhibitions of A. niger by ethanol fruit extracts of X. aethiopica at all concentrations was significantly better than that in the controls as shown in Plate 7. Growth inhibitions of P. chrysogenum by ethanol fruit extracts of X. aethiopica at all concentrations were significantly better than that in the controls as shown in Plate 8. Growth inhibitions of P. chrysogenum by ethanol fruit extracts of S. aromaticum at all concentrations were significantly better than that in the controls as seen in Plate 9. Inhibitions at 75% concentration were significantly better than that at other concentrations as seen in Table 2. The F values for the model, concentration, fungi, plant part, solvent and days were all highly (P>0.0001) significant for the antifungal activities of both X. aethiopica and S. aromaticum. Different interactions among the variables were also highly significant (P>0.0001) as shown in Tables 3 and 4.
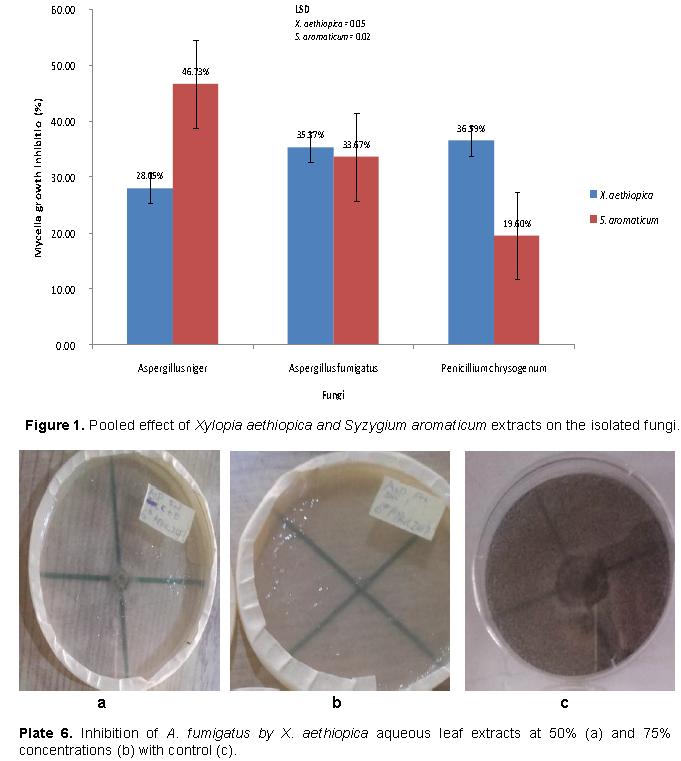
The antimicrobial potentials of X. aethiopica and S. aromaticum evaluated on A. niger, A. fumigatus and P. chrysogenum obtained from rotting yam tubers (D. rotundata and D. alata) showed inhibitory potentials on the mycelial growth of the fungi. A. niger, A. fumigatus and P. chrysogenum amongst others have been reported to be the causal agents of post-harvest rot of yam tubers in storage (Okigbo and Nmeka, 2005). The extracts of X. aethiopica and S. aromaticum have been reported to have anti-microbial and anti-fungal properties of which their derivatives are of great importance in public health, cosmetics, medicine and agriculture (Coyne et al, 2012).
The results obtained with fruit and leaf extracts of X. aethiopica are suggestive of higher antifungal potency of the former than the latter. It may thus be advisable to pay more attention on the fruit extract when field experiment is to be done. Extract concentration is also a key consideration for such a field experiment. The highly significant F values (P>0.0001) for the models in all the experiments show their appropriateness or ‘goodness of fit’. This means effective growth inhibitions of the three fungi depend to a large extent on the fungi, plant part, concentration and interactions amongst them.
The highly significant F value for concentration, fungi, plant part, solvent, days as well as the various interactions among them in the case of both X. aethiopica and S. aromaticum are suggestive of the significant impacts played by these factors on the antifungal activities of the plant parts. It means the same plant part will most likely exert different antifungal effect on different fungi. This is also corroborated by the results obtained in the pooled effect of X. aethiopica and S. aromaticum extracts on the isolated fungi. This agrees with the works of Suleiman and Falaiye (2013) who reported that extracts from different plant parts are used in controlling different fungi. The highly significant F values (P>0.0001) for plant parts may also be suggesting that the different plant parts employed might contain certain phytochemicals that are capable of inhibiting the growth of several fungal pathogens. The highly significant F values (P>0.0001) for fungi shows that the different fungi had significantly different growth responses in the presence of extracts of S. aromaticum.
The significant F values (P>0.0001) for days means that the growth inhibitory effects of the S. aromaticum on A. niger, A. fumigatus and P. chrysogenum among incubation days differed significantly. This is thus suggesting that contact period between plant extracts and the fungi is also critical for effective inhibition. The highly significant F value (P>0.0001) for solvent, indicates that method of extraction can also impact on the effectiveness of extracts against the fungal growth. This agrees with the work of Azwanida (2015) who reported that different plant parts require certain extraction methods in order that their antifungal potentials could be obtained. The results obtained with the aqueous and ethanol extracts showed that both solvents are good for extraction of extracts from X. aethiopica and S. aromaticum.
The highly significant F value (P>0.0001) for interaction between fungi and concentration means that any particular concentration of extract did not impact similar antifungal effect on any two fungi. This means the antifungal effect of the extracts at any particular concentration differed significantly from one fungus to the other. The highly significant F value (P>0.0001) for plant part and concentration (P>0.0001) means that any particular extract concentration of any particular plant part exerted significantly different antifungal effect on two different fungi. In other words the antifungal effect of extract of any particular concentration differed significantly from one fungus to the other. It can thus be said that appreciable growth reduction of the isolated fungi is dependent amongst other factors on the type of extract engaged as well as the concentration of the extracts. Onuh et al., (2015) reported that the higher the concentration, the more effective the plant extract on mycelial growth inhibition.
The highly significant F value (P>0.0001) for plant part and fungi means that extract from any particular plant part will most likely exert significantly different antifungal effect on two different fungi. The highly significant F value (P>0.0001) for concentration and day means that two different concentrations of the same extract did not exert similar antifungal impact at the same incubation day. It thus means that the antifungal effects of two different extract concentrations on the same incubation day differed significantly. The highly significant F value (P>0.0001) for plant part and day means that the antifungal impact of extract from any particular plant part differed significantly from one incubation day to the other. This suggests that length of time or contact period between extract and fungus will most likely be the key to effective fungal control in field experiment. The highly significant F value (P>0.0001) for solvents and fungi means that extracts by different solvents exerted significantly different antifungal impact on the same fungus. Solvent for extraction should therefore be carefully considered for plant extract to be used for antifungal purposes. The significant F value (P>0.0374) for interactions among plant part, fungi and concentration is suggestive. This means effectiveness of any particular concentration of extract of any particular plant part on growth of any fungus does not mean effectiveness on another fungus. Thus the 75% concentration of X. aethiopica extract which was most effective against P. chrysogenum, A. niger and A. fumigatus may not necessarily be effective against other fungi
The highly significant F values (P>0.0001) for interactions among fungi, concentration and days means exposure period of any of the three fungi to any specific extract concentration played a key role in the effectiveness of such extract (of both S. aromaticum and X. aethiopica). This fact was also validated by the highly significant F value (P>0.0001) for interactions among solvent, fungi and days in the case of S. aromaticum. It means at any incubation day, a specific extract concentration (of S. aromaticum or X. aethiopica) exerted significantly different impact on the three isolated fungi.
The highly significant F value (P>0.0001) for interactions among solvent, plant part and concentration shows that the antifungal effectiveness of any particular concentration of a specific X. aethiopica plant part was not the same among extraction solvents. It means 75% aqueous and ethanol extracts, for instance, of the same plant part (either fruit or leaf) will most likely have significantly different antifungal activities. The highly significant F value (P>0.0001) for interactions among solvent, plant part and fungi shows that X. aethiopica extracts of the same part but of different extraction solvent significantly differed in effectiveness on the three fungi. The highly significant F value (P>0.0002) for interactions among solvent, plant part and days means that extract from a specific part of X. aethiopica and of a specific extraction solvent exerted different antifungal activity on different days of incubation.
The highly significant F values (P>0.0001) for interactions among solvent, fungi and concentration means that the same concentration of S. aromaticum extract of the same extraction solvent had significantly different effectiveness against the three fungi. The antifungal potentials of both S. aromaticum and X. aethiopica might not be unconnected with certain phytochemicals like tannins, alkaloids, flavonoids, phenols and glycosides contained in them. Volatile compound known as eugenol which occurred in large quantities in certain fruits has been reported to have antimicrobial activity against some pathogens (Ayoola et al., 2008; Mishra et al., 2014). Fleischer (2003) submitted that the fruit of certain plants contains higher amounts of flavonoids than the leaves and that it was responsible for the antimicrobial activity of the fruit.
CONCLUSION AND RECOMMENDATIONS
The authors have not declared any conflict of interests.
REFERENCES
|
Amusa NA, Baiyewu AR (2003). Storage and market diseases of yam tubers in South-Western Nigeria, Ogun state. Journal of Agricultural Research 11:211-255.
|
|
|
|
Asante SK, Mensah GW, Wahaga E (2007). Farmers' knowledge and perception of insect pests of yam (Dioscorea spp.) and their indigenous control practices in the Northern Ghana. Journal of
Crossref
|
|
|
|
|
Agricultural Science 40(2):185-192.
|
|
|
|
|
Ayanwuyi E, Akinboye AO, Oyetoro JO (2011). Yam production in Orire local government area of Oyo State, Nigeria: farmers perceived constraints. World Journal of Young Researchers 1(2):16-19.
|
|
|
|
|
Ayoola GA, Lawore FM, Adelowotan T, Aibinu IE, Adenipekun E, Coker HAB, Odugbemi TO (2008). Chemical analysis and antimicrobial activity of the essential oil of Syzygium aromaticum (clove) on African Journal of Microbiological Research 2:162-166.
|
|
|
|
|
Azwanida NN (2015).A review on the extraction methods use in medicinal plants, principle, strength and limitation. Medicinal and Aromatic Plants. Faculty of Agro- based industry, University of Malaysia Kelantan, Jeli Campus, 17600 Jeli, Kelantan, Malaysia 4:196.
|
|
|
|
|
Bediako FA, Showemimo YO, Asiama DH, Amevowor AK (2007). Improving Sprouting Ability of White Yam Minisetts (Dioscorea alata Poir) Var Pona and Dente Using Different Disinfectants and Protectants in Sterilized Saw Dust. Journal of Applied Sciences 7(20): 3131-3134.
Crossref
|
|
|
|
|
Bongiorno VA, Rhoden SA, Garcia A, Polonio JC, Azevedo JL, Pereira JO, Pamphile JA (2016). Genetic diversity of endophytic fungi from Coffea arabica cv. IAPAR-59 in organic crops. Annals of Microbiology 66(2):855-865.
Crossref
|
|
|
|
|
Coyne RS, Stover NA, Miao W (2012). Whole Genome studies of Tetrahymena 109:53-81.
Crossref
|
|
|
|
|
Food and Agriculture Organization (FAO) (2013). FAOSTAT database. [Online]. Available at: http://bit.ly/NmQzZf.
|
|
|
|
|
Fleischer T (2003). Xylopia aethiopica A Rich:A chemical and biological perspective. Journal of Universal Science Technology (23):24-31.
|
|
|
|
|
Frank CO, Kingsley CA (2014). Role of Fungal rots in post-harvest storage losses in some Nigerian varieties of Dioscorea species. British Microbiology Research Journal 4(3):343.
Crossref
|
|
|
|
|
Ike PC, Noni OE (2006).Determinants of yam production and economic efficiency among small-holder farmers in South-eastern Nigeria. Journal of Central European Agriculture 7(2):337-342.
|
|
|
|
|
International Institute for Tropical Agriculture (IITA) (2009). Yam (Dioscorea species). Available at: http://www.iita. org/yam.
|
|
|
|
|
Lawal B, Ossai P, Shittu O, Abubakar A, Ibrahim A (2014). Evaluation of phytochemicals, proximate, minerals and anti-nutritional compositions of yam peel, maize chaff and bean coat. International Journal of Applied Biological Research 6(2):21-37.
|
|
|
|
|
Lee MH, Lin YS, Lin YH, Hsu FL, Hou WC (2003). The mucilage of yam (Dioscorea batatas Decne) tuber exhibited angiotensin converting enzyme inhibitory activities. Botanical Bulletin of Academia Sinica P 44.
|
|
|
|
|
Mishra RP, Sharma K (2014). Antimicrobial activities of Syzygium aromaticum L. (Clove). International Research Journal of Biological sciences. Innovation Life sciences, NSRI Campus, Lucknow, India 3(8):22-25.
|
|
|
|
|
Nwakiti OA (1982). Studies on the Aetiology and Control of Arithrenose / Brown blotch disease Complex of D. alata in Nigeria. University of Nigeria, Nsukka. (MSc Project).
|
|
|
|
|
Okigbo RN, Nmeka IA (2005). Control of yam tuber rot of yam with leaf extracts of Xylopia aethiopica and Zingiber officinale. African Journal of Biotechnology 4(8):804-807.
|
|
|
|
|
Okigbo RN, Ikediugwu FEO (2000). Studies on Biological Control of Postharvest Rot of Yam (Diocorea spp.) with Trichoderma viride. Journal of Phytopathology 148:351-355.
Crossref
|
|
|
|
|
Olayemi J, Ajaiyeoba E (2007). Anti-inflammatory studies of yam (Dioscorea esculenta) extract on wistar rats. African Journal of Biotechnology 6(16).
Crossref
|
|
|
|
|
Onuh J, Shrikiti D, Ubwa S, Shambe T (2015). Isolation of six microorganisms from rotten Dioscorea alata (water yam), and Antimicrobial sensitivity test with nine plant extracts. Food and Nutrition Sciences 6:1381-1394.
Crossref
|
|
|
|
|
Princewill-Ogbonna I, Ibeji C (2015). Comparative study on nutritional and anti- nutritional composition of three cultivars (red, green and yellow) of aerial yam (Dioscorea bulbifera). IOSR Journal of Environmental Science and Toxicological Food Technology 9:79-86.
Crossref
|
|
|
|
|
Sobowale AA, Oludare AA, Amusa NA, Oguntade O (2015). Mycoparasitic capability of Trichoderma asperellum and its metabolite. Nigeria Journal of Mycology 7:125-140.
|
|
|
|
|
Suleiman MN, Falaiye TN (2013). In-vitro control on fungus associated with biodeterioration of sweet potato (Ipomea batatas (L) Lam) Tubers Futa. Journal of Research in Sciences 9(1):1-7.
|
|
|
|
|
Zaknayiba DB, Tanko L (2013). Costs and returns analysis of yam production among small scale farmers in Karu local government area, Nasarawa State, Nigeria 9(1):73-80.
|
|