Full Length Research Paper
ABSTRACT
Limited access to oyster mushroom (Pleurotus Ostreatus) substrates and the high cost of production are among the constraints affecting mushroom farming in Kenya. In an effort to solve the above problem, a study was carried out in Egerton University to determine the effect of grain spawn and substrates on growth and yield of oyster mushroom grown under different cropping shelters. The study was laid out as 8×2×3 factorial experiment in a completely randomized design (CRD), where 8 levels of substrates (wheat straw, kikuyu grass, uncomposted grevillea sawdust, corn cobs, and their combinations), 2 levels of grain spawn (popcorn, rice), and 3 levels of cropping shelters (mikeka, shade net and dark house) were evaluated on their effect on growth and yield of oyster mushroom. The results showed that the substrates and cereal grain spawn significantly affected the growth and yield of Pleurotus ostreatus grown under mikeka, shade net, and dark cropping shelters at P≤0.005. The total biological efficiency showed the highest yields in interaction of mikeka shelter × corn cobs × rice spawn with 109.1 g, respectively. The study recommends corn cobs with rice spawn grown under mikeka cropping shelter to be used for the production of oyster mushroom in Kenya.
Key words: Mushroom seeds, agriculture residuals, production structure, harvest.
INTRODUCTION
Commercial mushroom farming and enterprises in Kenya are relatively few compared to other places. However, the mushroom industry in the country is rapidly growing, and production cannot currently meet increasing local demand. The total production of mushrooms is 500 tons per year against the demand of 1200 tons annually (NAFIS, 2014). In Kenya, unlike previously when consumption was confined to rural communities, urban dwellers are increasingly consuming mushrooms (Ojwang, 2014). The increase in demand for edible mushrooms has resulted in the setting up of several mushroom units in different parts of the country. Mushroom cultivation has not been given a lot of importance and the sector is underdeveloped with only two exotic species (Pleurotus ostreatus and Agaracus bisporus grown for the hotel industry) (Waiganjo et al., 2008; Odendo et al., 2009; Onyango et al., 2011). Oyster mushroom (P. ostreatus) is the second largest commercially produced mushroom in the world, second to A. bisporus (Sánchez, 2010; Pardo-Giménez et al., 2010). Among the white-rot fungi, oyster mushrooms of genus Pleurotus are well-known for the conversion of lignocellulosic materials into fruiting bodies (Porselvi and Vijayakumar, 2019).
P. ostreatus are grown on organic substances termed as substrates, which are lignocellulosic material that supports the development, growth and fruiting of mushrooms (Anike et al., 2016). Mushroom spawn is the planting material or mycelium that serves as seed of a given substrate in mushroom cultivation (Stanley and Awi-Waadu, 2010). P. ostreatus is the best mushroom crop to cultivate in developing countries for many reasons. One of the reasons is that they are grown on agricultural residuals (Kumla et al., 2020). By-product residuals that are generated at harvesting time are normally disposed by burning and the smoke produced is usually an environmental nuisance. Therefore, by using the waste to cultivate mushrooms, the environment is conserved (Singh et al., 2020). Many agriculture wastes are lignocellulosic materials, so they could be a suitable substrate for solid-state fermentation processes required by oyster mushrooms to grow and produce edible fruiting bodies (Ritota and Manzi, 2019). Compared to other types of mushrooms, oyster mushroom (Pleurotus spp.) utilizes more varied kinds of substrate materials (Yang et al., 2016) to produce biomass of high market value. Transformation of those unused agricultural residuals into useful materials in mushroom farming is the one of the solutions to reduce the threat to the environment and public health, that are increasingly associated with alternative waste disposal methods, such as burning and other forms of environmentally destructive disposal of agricultural wastes. Lignocellulosic materials such as wood materials, sawdust, cereals straws, bagasse, papers, grasses and cotton seed hull, and uncomposted grevillea sawdust are used for mushroom cultivation (Tekeste et al., 2020; Tesfay et al., 2020; Baysal et al., 2007; Nongthombam et al., 2021l).
Mushroom farming can be a good source of employment as an agro-industrial activity (Thakur, 2020); and thus it can help as a source of income, employment. It also presents a good opportunity for small to middle-scale farmers, such as women and youth, in developing countries where the standard of living is very low (Amuneke et al., 2011). Mushroom farming for small farmers requires relatively little space; they can be stacked using shelf-like culture systems, other materials that can create moisture and low temperature like, mud, mikeka (a traditional mat made out of sisal fibres ), and shade nets (black shade of 60% density). Therefore, this will lead to an increase in the economy of not only small-scale farmers but other weak sections of communities as well (Shah et al., 2004). Generally, Pleurotus spp. cultivation technology is very crucial in solving the problems of pollution of the environment, shortage of food and malnutrition, which are the challenges that human beings are still facing, due to the continued increase of climate change, and natural resource degradation and pollution all over the world (Oseni et al., 2012). This study aimed at investigating the effect of different sources of mushroom substrate on the growth and yield of oyster mushroom, including: cereal grain spawn (popcorn, rice); local substrates (popcorn cobs, uncomposted grevillea sawdust, kikuyu grass, wheat straw); and cropping shelters (mikeka, shade net and dark house).
MATERIALS AND METHODS
Experimental sites
This field-based research was conducted under three shelter structures between January 2021 and July 2021 at Egerton University in three experimental fields. The site lies between longitude 35° 35 ? E and latitude 0° 23 ? S, and at an altitude of 2238 m asl. The annual mean precipitation is 1000 mm and the annual mean temperature is 15.9°C. The site is situated in the agro-ecological zone III and has thick humic topsoil (mollic andosols) (Jaetzold et al., 2007). The site has high relative humidity and low temperature, which are suitable for oyster mushroom production.
Variety description
Oyster mushroom is scientifically classified in the Kingdom – Fungi, Phylum – Basidiomycota, Class – Agaricomycetes, Order - Agaricales, Family - Pleurotaceae, and Genus – Pleurotus, Species - Pleurotus ostreatus (Randive, 2012). The pearl oyster is a common mushroom prized for its edibility. Most edible mushrooms do well within a pH range of 3 to 7 at a temperature ranging from 20°C to 25°C (Randive, 2012). The ecological requirements of P. ostreatus vary at the various stages of the growing period. The optimal temperatures for growing mycelia and pin forming are between 20 to 30°C and 10 to 20°C, respectively. Substrate moisture should be from 60 to 75%, but it should be 80 to 95% during the fruiting because 80%, or substantially more, of the fruit body is water (Nadir et al., 2016).
Substrates preparation
The dry wheat straw, uncomposted grevillea sawdust, popcorn cobs, and lawn grass were cleaned and air-dried. The straws were chopped into pieces of 2 cm width and 4 cm length, according to the suggestions made by Kimenju et al. (2009). The organic residuals were then soaked in water for 3 days. The wastes were dried to minimize the moisture content to 75%. All substrates were emended with dry wheat bran (5% by weight) to increase the amount of nitrogen and some minerals, and 1.5% by weight dry calcium carbonate to adjust the pH of organic wastes (Zakil et al., 2022; Carrasco et al., 2018). The dried mixture of organic wastes was packed in polypropylene bags (12 × 22 cm), then they were tied with a rubber band, and each bag contained 1200 g of cultivation substrate. Pasteurization of substrates was carried out using hot steam at 80°C for 6 h within a metal barrel.
Spawning
Spawning was done under aseptic conditions (Laminar airflow hood). The grain spawns (popcorn and rice) obtained for the first experiment (that is, tissue culture) were mixed in all substrates using 45 g of the total weight of the packet. After spawning, bags were kept in total darkness, and 9 small holes were pierced in the walls of the bags for aeration.
Spawn running
Room temperature, varying from 22 to 26°C, and relative humidity of 80 to 90% were maintained during the spawn run. Humidity was maintained by water spraying three times a day. After the completion of spawn run in the straw, the substrate became a compacted mass, which also stuck to the polypropylene bags. And after the complete spawn run in the bags, some of the bags were moved to an outdoor location under a semi-tunnel with soil in a shade net and mikeka shelters. Others were maintained in a dark mushroom house.
Fruiting management
Egerton university farm (named field three) was selected as a site where the ambient temperature can be manipulated to vary between 15 and 25°C, and the clay content of the soil at 40-cm depth is about 20%. Besides production houses for mushroom cultivation, shades were built using a net of 60% and others using mikeka, semi-tunnels were built inside the shade net. For Shade net, a trench of 2.5 m wide, 6.3 m long, and 0.4 m deep was dug and the trench was divided into 48 experimental units; whereas, for mikeka shade, a circular trench of 9-m circumference, 2.8 m in diameter and 0.4 m deep were built. Then, the floor was disinfected by treating with 2.0 kg of hydrated lime for preventing pests such as termites and moles. A portion of the top-soil (20 cm) was set aside for later use as a ‘cover’ over the mushroom substrate after digging the trench. The soil was also sterilized with hydrated lime (1 full wheelbarrow with 0.5 kg of lime). The plastic bags were removed from the substrate packs that were neatly placed vertically into the trench (3 per experimental unit) and the substrates were covered with disinfected soil to a depth of 15 mm. The plastic semi-tunnels were constructed over the filled trench and Bamboo was used to construct the semi-tunnel under the shade net. The trench containing the mushroom substrate was watered with 10 l of clean water per day and per square meter of trench for maintaining the moisture content of substrates. Then I closed the semi- tunnels using the clear tunnel and waited until the formation of the primordia (3-7 days); water was reduced to 10 l clean water within the entire trench. Once the fruit bodies (mushrooms) appeared, the amount of water applied was reduced to 5 l per day and per square meter within the entire trench. The semi-tunnels were opened between 10:30 and 11:00 am for 30 min every day for aeration; however, during one rain they were left closed for that day irrespective of the season. For the dark mushroom production house, 144 bags were used in that environment according to the 18 treatments with 3 replications, where each experimental unit had 3 bags hanging under shelves. For the initiation of pinheads and fruiting bodies, a temperature of 18-21°C and a relative humidity of 75-90% were maintained. Figure 1 shows the process of production under different structures.

Data collection
Data were collected on the following parameters:
Full colonization to pinhead formation [TFCP] (days)
After full colonization of bags, the formation of primordia were observed during every two days-intervals; and the number of days the bag took for the first primordia formation was observed and recorded.
Length of Stalks [LS] (cm)
Length was measured using a ruler (units for ruler is in cm ruler). Five fruiting bodies were randomly selected using a simple random technique. The lengths of the stalks were measured from the tip of the stalk to the base of the caps. This was done for each harvest.
Diameter of the cap [DC] (cm)
Diameter was measured using a thread; and its length was determined using a measuring rule. The thread was used to trace the diameter of the caps of the five randomly selected fruiting bodies, and the length of the thread that stretched across the diameter of the caps was measured on the tape ruler, and the value recorded. This was done for each harvest.
Average number of primordia per packet [ANP]
Number of primordia was measured by accounting for the total number of primordia and dividing by the number of packets.
Number of fruiting bodies per packet [NFB]
Only well-developed fruiting bodies per each packet were counted, while dry and pinheaded fruiting bodies were discarded. However, tiny fruiting bodies were included in the counting.
The average weight of the individual fruiting body [AWIF]
The weight of each fruiting body was calculated by dividing the total weight of the fruiting bodies per packet by the total number of the fruiting bodies per packet.
Biological yield [BY]
Biological yield per each harvest was measured by weighing the whole cluster of the fruiting body per each treatment and per each harvest, without removing the lower hard and dirty portion.
Biological efficiency [BE]
Yield of mushroom per weight of substrate (on a dry weight basis) was calculated by the formula proposed by Chang et al. (1981) as follows:
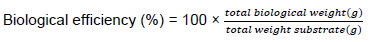
Economic yield [EY]
Economic yield per treatment was recorded by weighing all the fruiting bodies in a packet after removing the lower hard and dirty portion.
Statistical analyses
Normality of the data was examined using the Shapiro Wilk test to determine if the data was sufficiently distributed customarily to meet the assumptions of the statistical tests and the Probability Plot at 95% of the confident interval were conducted in SAS Software 9.4 M6 (SAS Institute inc, Cary, NC 2017) prior to analysis. Analysis of variance (ANOVA) was also performed for determining if there were significant differences among the grain spawn, substrates and cropping shelters (P≤ 0.05). Tukey grouping for differences in means and post ANOVA was used to observe the differences between the substrates. Pearson correlations were used for testing the relationship between growth and yield of oyster mushroom.
Statistical model
The following is the linear model:
Yijkl = μ + Rk + Gi + Ej + EGij + Sk + GSik + ESjk + EGSijk + ?ijkl
where: Yijkl = Overall observations, μ= Overall mean, Ei= Effect due to ith environment level, Rk= Effect due to kith replication level, Sk= Effect due to kth substrate level, Gj= Effect due to jth grain spawn levels, EGij= Interaction effect due to environment and grain, ESik= Interaction effect due to environment and substrate, GSjk= Interaction effect due to grain and substrate, EGSijk= Interaction effect due to environment, grain, and substrate, ?ijkl= Random error.
RESULTS
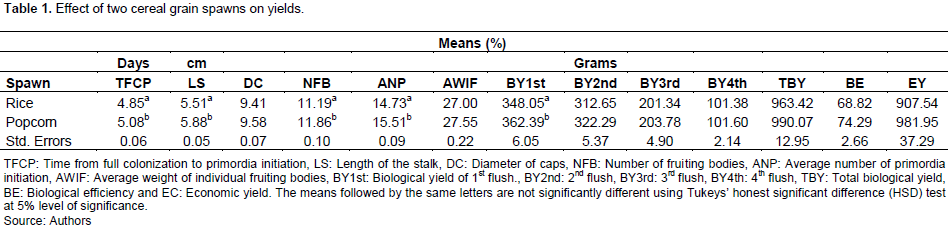
Effect of two cereal grain spawns on yield
The effect of grain spawn on growth and yield is presented in Table 1. Grain spawn significantly influenced yield (P≤ 0.05) for the variables TFCP, LS, NFB, ANP and BY4th (definition of abbreviations is seen in Table 1). Popcorn spawn (4.8 days) was better in TFCP than rice spawn (5.0 days) and were statistically significant from each other. Rice spawn (5.0cm) was better than popcorn spawn (4.8 cm) in influencing LS. Rice spawn highly influenced (11.8 cm) NFB of oyster mushroom compare to popcorn spawn (11.1 cm). The rice spawn highly affected (15.5) the ANP of mushroom while popcorn spawn affected less (14.7); whereas both cereal grain spawn were not significantly different from each other on DC, all flushes, TBY, BE and EY.
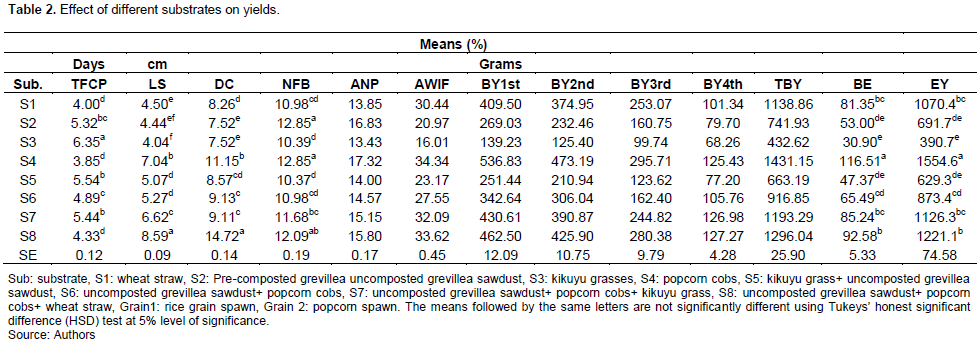
Effect of different substrates on yields
The effects of different substrates on oyster mushrooms are presented in Table 2. The results indicated that the substrates significantly influenced (P≤ 0.05) the TFCP, LS, DC, NFB, ANP, AWIF, BY1st, BY2nd, BY3rd, BY4th, TBY, BE and EC. Growth and yield for oyster mushrooms widely varied under different substrate levels. The highest TFCP (6.35 days) was obtained with S3; whereas the lowest (3.85 days) was observed under S3. Therefore, the TFCP Tukey grouping for means of substrates (P≤0.05) showed that S1, S2, S5 and S8; S3, S7, S1, and S6 were significantly different from each other; whereas S5, S7 and S2; S2, S6 and S8, S1, S4 were not significantly different from each other. The LS of oyster mushroom was highly influenced by S8 (8.5cm) followed by S4 (7.0 cm). And the least was S3 with 4.0 cm of LS; and substrates S8, S4, S7, S6, S1, and S3 showed significant differences from each other, while S5, S6 and S2, S3 did not show any significant difference from each other. The highest DC values of oyster mushroom caps were found in S8 substrate (14.7 cm), and the smallest was S3 with 7.5 cm of oyster DC. Moreover, S8, S4, S6, S1, and S2 were significantly different, while S6, S7; S5, S1 and S2, S3 were not significantly different from each other. The substrate S4 highly affected the NFB (12.8) while the less substrate was found with S5 of 10.3 fruiting bodies. Moreover, the results showed that S4, S7; S8, S6 and S8, S1 were statistically different from each other, while S4, S2; S8, S7; S6, S1 and S5, S3 were not significant. S4, like NFB, had the greatest influence on the average number of primordia with 17.3, followed by uncomposted grevillea sawdust with 16.8 fruiting bodies, while S3 had the least influence on the average number of primordia per cluster, with 13.4 primordia. Therefore, S4, S7, S5; S2, S8, S1 and S3, S7 were significantly different; whereas S4, S2; S8, S7; S7, S6 and S5, S3, S1 were not different from each other. The maximum and minimum AWIF were found in S4 (34.3 g) and S3 with 16g. Therefore, the mean separation showed that S4, S7, S6; S5, S2 and S3 were significantly different from each other; whereas S4, S8; S8, S7; S7, S1 were not significant from each other. The biological yields for all flushes showed that S4 mostly influenced the yields of oyster mushrooms unless on the 4th flush, where S8 mostly affected the yields; and S3 was the least in influencing the yields for all flushes. The biological yield of 1st flushes Tukey grouping for means of substrates showed that S4, S8, S6, S2 and S3 were significantly different; whereas S8, S7 and S1 were not. For the 2nd flush, S4, S8, S1, S2 and S3 were significantly different; whereas S2 and S3 were not. For the 3rd flush, S4, S6 and S3 were significantly different; whereas S6 and S2; S8 and S1 were not. For 4th flush, S8, S6 and S2; S7, S1and S5 were significantly different; whereas S8, S7 and S4; S6 and S1; S2, S5 and S3 were not. The TBY and BE for oyster mushroom showed the highest yields on S4 (143.1 and 116.5 g of yields, respectively); whereas S3 substrates was the least in influencing TBY and BE (432.6 and 30.9 g yields, respectively). Therefore, S4, S8, S6, S2 and S3 were significantly different; whereas S8, S7 and S1 were not for TBY, respectively. However, S4, S1 and S5 were significantly different from each other; whereas S7, S1 and S6; S2, S5 and S3 were not for BE. Substrate (S4) with 1554.6 g was the best in influencing the EC of oyster mushrooms; whereas the least was kikuyu grass with 390.7 g of yields. The mean separation indicated that S4, S8 and S3 were significantly different, while S7, S1, S6; S2, S5 and S3 were not significantly different from each other.
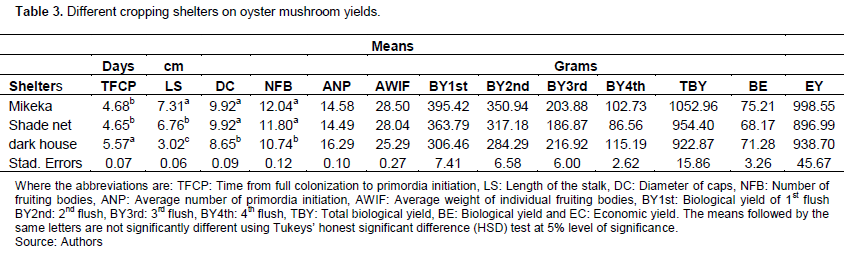
Effects of different cropping shelters on oyster mushroom yields
The effects of different cropping shelters are presented in Table 3. The findings showed that cropping shelters statistically affected (P≤ 0.005) the TFCP, LS, DC, NFB, ANP, AWIF, BY1st, BY2nd, BY3rd, BY4th, and TBY. Mikeka shelter structure took fewer days (4.6) for TFCP, followed by shade net shelter structure (4.67 days) and the least was dark house shelter (5.56 days); and both dark house shelters were significantly different from others. Mikeka shelter (7.3 cm) highly affected the LS of oyster mushroom; whereas dark house shelters (3.0 cm) affected less. Therefore, all shelters were significantly different from each other. The oyster mushroom produced under mikeka structure had a high DC (9.9 cm), while dark shelter structure produced small mushrooms (DC=8.6 cm). The dark house shelters were statistically significantly different from others. The production carried under mikeka shelter was the best in NFB (12.0); while the dark shelter (10.7) was the least and the dark house shelter was significantly different from others. Dark shelter mostly influenced the ANP (16.2) followed by mikeka structure(14.5), while the shade net structure (14.4) was less to influence the ANP and dark house shelter was significantly different from others. Mikeka shelter mostly contributed the highest yields (28.4g) of AWIF of oyster mushroom whereas dark shelter was the least with 25.2g and dark house shelter was significantly different from others. The TBY of oyster mushroom produced under mikeka shelter was the highest with 1052.9 of yields. The dark house shelter was significantly different from others, whereas none of them were different from each other for BE. All Cropping shelters (mikeka, shade net and dark house) were not significantly different from each other for EY.
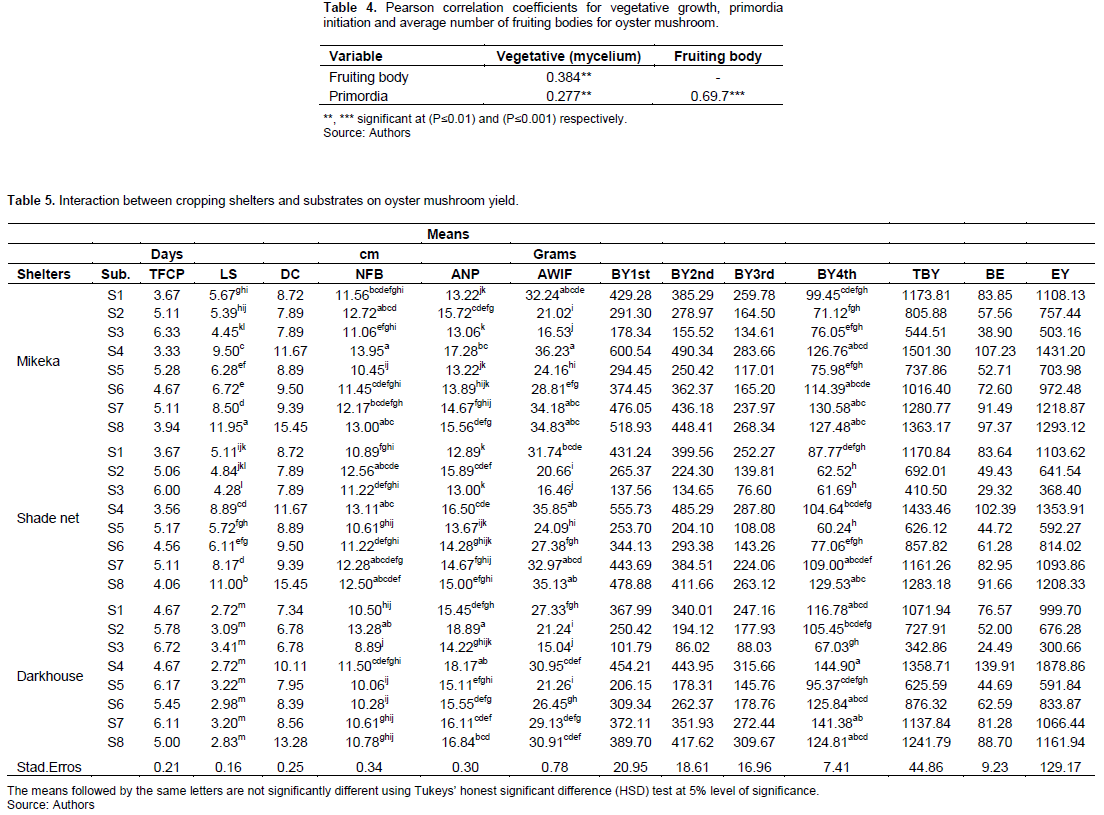
Interaction of cropping shelters× substrates on oyster mushroom yields
The effect of different local cropping shelters× substrates on oyster mushroom yields are presented in Table 5. The findings showed that cropping shelters statistically affected (P≤0.005) the TFCP, LS, NFB, ANP and AWIF. The minimum and maximum TFCP were obtained in mikeka shelter × S4 (3.3 days) and dark house shelter (6.7 days). The interaction between mikeka shelter × S8 was the highest with 11.9 cm of stalk; whereas the lowest was found in the interaction of dark house× S1 with 2.7 cm of the stalk length. The maximum and minimum cap diameters were found in the interaction between mikeka and S8 with 1.9 cm and interaction between between dark shelter and S2 with 6.7 cm, respectively. The interaction of mikeka× S4 was the highest with 13.9 for fruiting bodies; whereas the lowest was observed in the interaction of dark house× S3 with 9.32 for oyster mushroom fruiting bodies. The highest and lowest ANP were obtained from the interaction of dark shelter× S2 (18.8) and interaction of shade net× S1 (12.8) for oyster mushroom primordia, respectively. The interaction of shade net × S4 substrate were the highest with 35.8g for fruiting bodies; and the lowest were found in the interaction of dark shelter× S3 with 115.4 g for oyster mushroom fruiting body. Based on the interaction, the maximum yields were observed in the interaction of dark shelter× S4 with 1501.3g for TBY and with 107.2g of BE; whereas the minimum yields were observed in the interaction of S3× dark shelter with 436.07g for TBY and 31.15g for BE. Both spawn were not significantly different from each other on TBY and BE. The highest and smallest EY of oyster mushroom were found on the interaction of mikeka shelter× S4 and mikeka shelter× S5 with 162.4 and 44.4g of yields, respectively.
Interaction of cropping shelters× grain spawn, substrates × grain spawn, substrates × shelters × grain spawn on oyster mushroom yields
The findings showed that the cropping shelters × grain
spawn, substrates × grain spawn, and substrates × shelters × grain spawn had no statistically significant influence (P ≥ 0.005) on the TFCP, LS, DC, NFB, ANP, AWIF, BY1st, BY2nd, BY3rd, BY4th, TBY, BE, and EY.
Pearson correlation for vegetative growth (mycelium growth), primordia initiation and average number of fruiting bodies
The Pearson Correlation for vegetative growth (mycelium growth), primordia initiation and average number of fruiting bodies revealed that there was no strong positive correlations between mycelium growth and number of fruiting bodies (r = 0.38.**); between primordia initiation and mycelium growth (r = 0.27**); whereas there was a slightly strong correlation between primordia initiation and fruiting bodies (r = 0.69***), although all are statistically significant (P < 0.05) (Table 4).
DISCUSSION
Oyster mushroom growth response to spawn, substrates and cropping shelters
Pleurotus ostreatus is well known for its degradation ability of lignocellulosic residuals (Ritota and Manzi, 2019; Bellettini et al., 2019). Assessment of spawn use success is based on mushroom growth and yield. In this study, grain spawn, substrates and shelter structure treatments affected time from full colonization to primordia initiation, length of mushroom stalk, diameter of mushroom caps, the average number of primordia, average number of fruiting body, and average of individual weight of fruiting bodies. Musanze (2013)and Muswati et al. (2021) evaluated the suitability of locally available substrates for oyster mushroom and found that substrates had affected significantly the number of pinning, stalk length and caps diameter. The findings, however, were not in agreement with Tavarwisa et al. (2021) who reported that wheat straw demonstrated significantly (p≤ 0.05) higher mycelial colonization rate than uncomposted grevillea sawdust and maize cobs. Kimenju et al. (2009)performed the study on relative performance of Pleurotus florida on agro-industrial and agricultural substrate and found that popcorn cobs influenced greatly stem circumference (49.0%), mushroom height 69% and cap diameter (16.6%) compared to the control (elephant grass). The findings were in agreement to the results from Hoa et al. (2015) who found that Substrates with 100% CC were the most suitable substrate formulas for cultivation of oyster mushrooms Pleurotus ostreatus and PC in which they gave the highest values of cap diameter, stipe thickness, mushroom weight, yield, BE, protein, fibre, ash, mineral content (Ca, K and Mg) and short stipe length. Materials with high quality of lignin, hemicelluloses and cellulose contents make mycelia to remain vegetative for a long time, which results in vigorous growth, late pinning and fruit bodies formation (Kimenju et al., 20009) when compared to substrates with low content of carbohydrate, which influence the primordium and fruiting body formation. Hence, we can conclude that popcorn cobs and other combinations of popcorn cobs had poor nutritional value compared to kikuyu grass and saw dust.
Furthermore, other factors have been reported to influence the delay of pinning and fruiting bodies formation, such as high moisture content in substrate (Kimenju et al., 2009). This study also revealed that rice spawn performed better compared to popcorn spawn on growth and yield of P. ostreatus. The findings are in agreement with Hoa et al. (2015) and Jayachandran et al. (2017) who reported that Brown rice was found to be the most favorable for mycelium growth of two oyster mushroom species and they found that corn cobs and acacia saw dust were selected as favorable lignocellulosic substrate sources for mycelium growth, pinning, number of fruit body of both oyster mushrooms in their study for evaluating the effects of temperature and nutritional conditions on mycelium growth of two oyster Mushrooms (P. ostreatus and Pleurotus cystidiosus). The presence of the right proportion of α-cellulose, hemicellulose, pectin, and lignin in the popcorn cobs, wheat straws and their mixtures to other substrates were the probable cause of the higher rate of mycelium in the corn cobs substrate. Naraian et al. (2009) reported that the carbon and nitrogen ratio for 100% of corn cobs, 100% of saw dust, 50% of corn cobs+ 50% of saw dust were 34.5, 51.7 and 42.55, respectively; They concluded that mycelium growth and primordial development of Pleurotus florida were dependent on the lignocellulosic materials, especially the C/N ratio. These finding were in agreement to the results reported by Kim et al. (2010) that higher C/N ratio favoured the mycelium growth, and lower C/N ratio favoured the fruiting body growth. The capacity of mushrooms to grow on lignocellulosic substrates is related to the vigour of their mycelium (Kortei et al., 2014).
Pin-head formation (primordium initiation) was observed following the invasion of substrates by mycelia growth. In this study, the mixture of popcorn cobs and rice spawn under semi-controlled condition (mikeka) structure showed the shortest time to the primordia initiation with only 3±1 days after full colonization of substrates. In general, the results showed that the primordia were initiated in the range of 3 to 8 days after full colonization for all substrates. The time required for the formation of pin-heads is comparable with reports by other similar studies elsewhere; Girmay ET AL (2016) reported pin-head formation of oyster mushrooms cultivated in different substrates to be between 23 and 27 days from spawning, while Fan et al. (2000) reported it to be 20 to 23 days. On the other hand, the findings were in agreement to Shah et al. (2004) who found that pin-heads appeared in about 6 days. Such variations in mycelia growth rate, colonization and primordial initiation have been observed when mushroom species were grown on a range of substrates including grevillea sawdust, wheat straw, corn cobs, bagasse, and banana leaves (Vetayasuporn, 2006; Islam et al. 2009; Gizaw, 2015). These results differed with Iqbal et al. (2016) who reported 46 ± 3 days after spawn inoculation. Pinhead formation is closely related to temperature and humidity. Temperatures below 17°C directly delay the pinhead formation (Pathmashini et al., 2008). Mikeka and shade net structure favoured the pin formation because the soil used to mulch the complete colonization substrates in trenches under shelters maintained the moisture content for primordia initiation. However, Ananbeh and Almomany (2005) and Shah et al. (2004) working on wheat straw and wheat straw mixed with saw dust reported somewhat shorter periods of 31 ± 4 and 28 ± 1 days after inoculation, respectively. The time from the pinhead formation to the first harvest for Pleurotus ostreatus was around 4 ± 1 days for popcorn cobs and it combines with other substrates, being in agreement with those of Iqbal et al. (2005) who conducted similar research. Shah et al. (2004) reported 24 days for pinhead formation on uncomposted grevillea sawdust medium. The days for pinhead formation and days for flush (fruiting bodies) formation recorded in this study were longer than previous findings. This may probably be associated with the temperature and humidity.
Oyster mushroom yield (Total biological yield, biological efficiency, economic yield) response to spawn, substrates and cropping shelters
Grain spawn, substrate and cropping condition treatments also had an effect on average weight of the individual fruiting body, the number of fruiting body, total biological yield, biological efficiency and economic yield of Pleurotus ostreatus. The results showed that the two treatments of popcorn cobs × rice grain spawn and (popcorn cobs+ wheat straw+ saw dust) × rice grain spawn were the best performer in all three cropping environmental conditions (controlled, semi- controlled and uncontrolled conditions). These two treatments influenced significantly either the average weight of an individual fruiting body, number of fruiting bodies, total biological yield, biological efficiency and economic yield of oyster mushrooms. Though there was an increase of 24, 27 and 28% of total biological yield, biological efficiency and economic yield, respectively, over positive control wheat straw under a controlled condition, this small ratio of carbon to nitrogen might have been responsible for the higher biological efficiency and economic yield of oyster mushroom as reported by kim et al. (2010) and Alborés et al. (2006) who revealed that higher C/N ratio favoured the mycelium growth, and lower C/N ratio favoured the fruiting body growth. The findings are in full agreement toHoa et al. (2015)who reported that substrate formula of 100% of corn cobs gave the highest yield and biological yield compared to other substrates such as sawdust, banana leaves and wheat straw. The results of the study showed that the uncomposted grevillea sawdust substrates under Mikeka shelter condition decreased 27% below positive control wheat straw for total biological yield, biological efficiency and economic yield. The findings differ with Vetayasuporn (2006)who reported that grevillea sawdust gave the maximum mushroom yield (536.85 g per 1 kg substrate) and this yield was significantly different to those found from bagasse (360.84 g), peat of coconut husk (278.78 g) at a confidence level of 95% for the study of oyster mushroom cultivation on different cellulosic substrates.
Girmay et al. (2016) performed a study to evaluate the growth and yield performance of P. ostreatus on different substrates and found that the lowest biological and economic yield, as well as the lowest percentage of biological efficiency of oyster mushroom, was from uncomposted grevillea sawdust. The performance of oyster growth and yield in uncomposted grevillea sawdust substrate was minimal. Similarly, the biological efficiency (BE) also varied significantly among the different substrates used. Variable ranges of BE have been reported when different lignocellulosic materials were used as substrates for cultivation of oyster (Liang et al., 2009). This could be attributed to the fact that the lignocellulosic materials in uncomposted grevillea sawdust are generally low in protein content and thus insufficient for the cultivation of mushrooms (Obodai et al., 2003; Rambey et al., 2019). Therefore, grevillea sawdust substrate for mushroom production should undergo a period of composting to break down the cellulose and lignin components of the wood to release the essential materials for the establishment of mushroom mycelia. It may also require additional nitrogen, phosphate and potassium. Shah et al. (2004)in a comparative study on cultivation and yield performance of oyster mushroom on different substrates (wheat straw, leaves, saw dust) reported that as a substrate, saw dust showed best biological efficiency( 64.69 %) followed by saw dust + leaves (62.9 %), wheat straw + leaves (57.85 %), wheat straw (44.72 %), sawdust + wheat straw (43.59 %) and leaves (21.05 %). The results showed that kikuyu grass alone and in combination and interaction were the least mainly in the interaction of popcorn spawn under controlled environment with 329 g, 23 % and 290 g for total biological yield, biological efficiency and economic yield, respectively. This was in agreement with Onyango et al. (2011)who reported that grass straw produced the least number of the fruiting body and least biological yield of 23%, while corn cobs and wheat straw had 67 and 40.8%, respectively. Onyango et al. (2011) reported that the wheat brans used to increase proteins in grass straw might alleviate the biological yields.
Kumari and Achal (2008) and Musanze (2013) conducted a study on effect of different substrates on the production and non-enzymatic antioxidant activity of Pleurotus ostreatus and found that small and tiny fruit bodies were found in case of lawn grass as substrate. Generally, the Growth, development, productivity and post-harvest quality of any crop largely depend on the interaction between the plant genetics and the environmental conditions under which they are grown (Rajasekar et al., 2013). Rajasekar et al (2013) reported that vegetables grown under shade net produced better yields than that under open fields; that may be the reason why the oyster mushroom under shade net and Mikeka structures expressed better yields than that of inside the house condition. The water holding capacity of substrates caused by top soils influenced the relative humidity inside the shelters and high yields of oyster mushroom, though, the dark house shelter condition indicated the least of yields due to the low substrates moisture contents.
Pearson correlation coefficiency analysis between vegetative growth, pin head formation and number of fruiting body
Fungi are similar to plants, but unlike plants, they lack chlorophyll; thus, fungi cannot carry out photosynthesis. For plants, vegetative growth determines the yields to be produced, contrary, for oyster mushrooms as fungi; there is no relationship between vegetative growth (mycelia growth), primordia initiation and a number of the fruiting body. The linear Pearson correlation coefficient analysis showed that, mycelium running rate or extension for mycelium was not correlated with the number of fruiting bodies produced. This means that, the higher mycelia growth for substrates the less the number of fruiting bodies and less number of primordia initiation. Contrary, the more primordia initiation, the more fruiting bodies were obtained. These results are supported by Bilal et al. (2014) who revealed that, materials with high-quality lignin and cellulose contents take a longer time to start pinning and initiate fruiting body as compared to the substrates with low contents of the lignin and cellulose. This may be the reason why kikuyu grass and wheat straw took only 2-3 weeks to be fully colonised. While corn cobs, saw dust and their mixtures took more than 3 weeks to complete the colonization. As compared to the substrates with low nutrition values, the substrates with high nutrition values take a short time for full colonization and ramification. This is because the mycelia remain vegetative for a longer period hence the vigorous growth and late pinning. In turn, the highly colonized substrates exhibited low mycelia densities. The primordia initiation, number of fruiting bodies and average weight of individual fruiting body of oyster mushroom was not associated with a high rate of mycelia. The result was similar to the finding of Alborés et al. (2006) who reported that there was a positive correlation between the C/N ratio of substrate and mycelium growth rate but not correlated to primordia.
Comparison for four flushes under different cropping environment conditions
The yield per flush and percentage yield per flush for the first four flushes varied with the substrates. In all treatments, the yields were highest in the first flush, and then declined gradually in the second, the third and the fourth. According to the results presented in the Tables 1 to 6, the variation among the flushes is very high under all environmental conditions (controlled, semi-controlled and uncontrolled). The yields declined from the first flush to the second, ranging between 1-20%, from the 2nd flush to the 3rd flush, ranging 13-34%, while form 3rd flush to the 4th they were 34- 65%. The large variation found at 4th flush was due to the overuse of nutrients by the substrates. Among the cropping environmental conditions, the controlled condition was the best in diminishing the variation between flushes. The mixture of uncomposted grevillea sawdust + kikuyu grass + wheat straw substrate showed declines from the 2nd flush to the 3rd, and from 3rd to the 4th flush, with 54 and 35, respectively of variation under semi-controlled conditions (mikeka shelter); both conditions, semi-controlled and uncontrolled, used trenches and soils as casing to maintain moisture. The biological efficiency of substrates cultivated under mikeka and shade net were high at 1st and 2nd flushes, while the BE started to decline at 3rd and 4th flushes; this due to over degradation of carbohydrates in substrates by oyster mushroom. The study indicates that yields per flush decreased as the flushes advanced from first to fourth. This finding was slightly in agreement with Kimenju et al. (2009), who recorded 32.2 g per flush from first flush, second 17.1 g and third 5.5. He also gave corresponding percentages as 69.6, 23.6 and 6.8%. The overall yields for the three flushes were 4051 g, giving an average of 202.6 g per bag of 2 kg. This finding differs from Kivaisi et al (2003), who reported 4 to 6 flushes, with a yield of 643.4 g per bag when using varied substrates.
Oei (2005) reported that the yield is expected to be 20% of the weight of the wet substrate. Kimenju et al. (2009) and (Sharma et al., 2013) recorded only 3 flushes on wheat straw, sugar bagasse, and rice straw. Thus, the lack of nitrogen may be one of the factors affecting the overall yield values in uncomposted grevillea sawdust, kikuyu grass and their combinations cropping in different shelters. Corn cobs and the mixtures of other substrates also contain high amount of lignin. Low degradation of lignocellulosic substances of uncomposted grevillea sawdust and kikuyu grass by P. ostreatus might be another factor affecting the overall low yield values of oyster mushrooms.
CONCLUSION
Mushroom farming is a short duration, high yielder, which requires intensive care for better production. In Kenya, farmers normally use dark or semi-dark house shelters to grow oyster mushrooms and this may be challenging to small farmers due to the requirements for controlling the production in such environmental conditions. In the present study, popcorn cobs substrates spawned by rice grain spawn mulch with soil under Mikeka cropping shelter showed promising results in terms of biological and economic yields. Mikeka and shade-net cropping shelters showed a potential to influence higher yields than dark house shelters. Generally, these findings provide the easiest way to cultivate oyster mushrooms by using crop residuals rather than those normally used that pose environmental nuisances. This study recommends the use of rice grain spawn with corn cobs residuals as substrates under Mikeka cropping shelters to obtain better growth and yields.
ACKNOWLEDGMENTS
The authors are grateful for the funding by World Bank through Centre of Excellent for Sustainable Agriculture and Agribusiness Management (CESAAM) at Egerton University.
CONFLICT OF INTERESTS
The authors have not declared any conflict of interests.
REFERENCES
|
Alborés S, Pianzzola MJ, Soubes M, Cerdeiras MP (2006). Biodegradation of agro industrial wastes by Pleurotus spp. for its use as ruminant feed. Electronic Journal of Biotechnology 9(3):4-9. |
|
|
Amuneke EH, Dike KS, Ogbulie JN (2011). Cultivation of Pleurotus ostreatus: An edible mushroom from agro base waste products. Journal of Microbiology and Biotechnology Research 1(3):1-14. |
|
|
Ananbeh KM, Almomany AR (2005). Production of oyster mushroom Pleurotus ostreatus on olive cake agro waste. Dirasat Agricultural Sciences 32(1):64-70. |
|
|
Anike FN, Yusuf M, Isikhuemhen OS (2016). Co-substrating of peanut shells with cornstalks enhances biodegradation by Pleurotus ostreatus. Journal of Bioremediation and Biodegradation 7(1):1-7. |
|
|
Baysal E, Yigitbasi ON, Colak M, Toker, H, Simsek H, Yilmaz F (2007). Cultivation of Agaricus bisporus on some compost formulas and locally available casing materials Part I, Wheat straw based compost formulas and locally available casing materials. African Journal of Biotechnology 6(19):2225-2230. |
|
|
Bellettini MB, Fiords FA, Maieves HA, Teixeira GL, Ávila S, Hornung PS, Ribani RH (2019). Factors affecting mushroom Pleurotus spp. Saudi Journal of Biological Sciences 26(4):633-646. |
|
|
Bilal S, Mushtaq A, Moinuddin K (2014). Effect of different grains and alternate substrates on oyster mushroom (Pleurotus ostreatus) production. African Journal of Microbiology Research 8(14):1474-1479. |
|
|
Carrasco J, Zied DC, Pardo JE, Preston GM, Pardo-Giménez A (2018). Supplementation in mushroom crops and its impact on yield and quality. AMB Express 8(1):1-9. |
|
|
Chang ST, Lau OW, Cho KY (1981). The cultivation and nutritional value of Pleurotus sajor-caju. European Journal of Applied Microbiology and Biotechnology 12(1):58-62. |
|
|
Fan L, Pandey A, Mohan R, Soccol CR (2000). Use of various coffee industry residues for the cultivation of Pleurotus ostreatus in solid state fermentation. Acta Biotechnologica 20(1):41-52. |
|
|
Girmay Z, Gorems W, Birhanu G, Zewdie S (2016). Growth and yield performance of Pleurotus ostreatus (oyster mushroom ) on different substrates. AMB Express 6(87):1-7. |
|
|
Gizaw B (2015). Cultivation and yield performance of Pholiota nameko on different agro industrial wastes. Academia Journal of Food Research 3(3):032-042. |
|
|
Hoa HT, Wang CL, Wang CH (2015). The effects of different substrates on the growth, yield, and nutritional composition of two Oyster mushrooms (Pleurotus ostreatus and Pleurotus cystidiosus). Mycobiology 43(4):423-434. |
|
|
Iqbal SM, Rauf CA, Sheik MI (2005). Yield performance of oyster mushroom on different substrates. International Journal of Agriculture and Biology 7:900-903. |
|
|
Iqbal B, Khan I, Shan B, Naeem A, Ullah W, Khan N, Adnan M, Shah RA, Junaid K, Ahmed N, Iqbal M (2016). Substrates evaluation for quality, production and growth of oyster mushroom. Journal of Entomology and Zoology Studies 4(3):98-107. |
|
|
Jaetzold R, Schimdt H, Hornetz B, Shisnaya C (2007). Farm management handbook of Kenya. Natural conditions and farm management information. Volume II B. Nairobi Kenya. pp. 379-413. |
|
|
Jayachandran A, Nadesan S, Murugaiyan K (2017). Comparative study of different grains on spawn development of Pleurotus florida. International Journal of Science Invention Today 6(6):728-735. |
|
|
Kim SS, Lee JS, Cho JY, Kim YE, Hong EK (2010). Effects of C/N ratio and trace elements on mycelial growth and exo-polysaccharide production of Tricholoma mistake. Biotechnology and Bioprocess Engineering 15(2):293-298. |
|
|
Kortei NK, Odamtten GT, Appiah V, Obodai M, Adu-Gyamfi A, Annan T, Akonor PT, Annan SN, Acquah SA, Armah JO, Mills SW (2014). Microbiological quality assessment of gamma irradiated fresh and dried mushrooms (Pleurotus ostreatus) and determination of D10 values of Bacillus cereus in storage packs. European Journal of Biotechnology and Bioscience 2(1):28-34. |
|
|
Kimenju JW, Odero GOM, Mutitu EW, Wachira PM, Narla RD, Muiru WM (2009). Suitability of locally available substrates for oyster mushroom (Pleurotus ostreatus) cultivation in Kenya. Asian Journal of Plant Sciences 8(7):510-514. |
|
|
Kivaisi AK, Magingo FSS, Mamiro B (2003). Performance of Pleurotus flabellatus on water hyacinth (Eichhornia crassipes) shoots at two different temperature and relative humidity regimes. Tanzania Journal of Science 29(2):11-18. |
|
|
Kumari D, Achal V (2008). Effect of different substrates on the production and non-enzymatic antioxidant activity of Pleurotus ostreatus ( Oyster mushroom ). Life Science Journal 5(3):73-76. |
|
|
Kumla J, Suwannarach N, Sujarit K, Penkhrue W, Kakumyan P, Jatuwong K, Vadthanarat S, Lumyong S (2020). Cultivation of mushrooms and their lignocellulolytic enzyme production through the utilization of agro-industrial waste. Molecules 25(12):2811. |
|
|
Liang CH, Lee YL, Kuo HC, Wu TP, Jian, SY, Huang WL (2009). Antioxidant properties of novel solid-state fermented culinary-medicinal mushroom and fungi products. International Journal of Medicinal Mushrooms 11(3):259-268. |
|
|
Muswati C, Simango K,Tapfumaneyi L, Mutetwa M, Ngezimana W (2021). The effects of different substrate combinations on growth and yield of oyster mushroom (Pleurotus ostreatus). International Journal of Agronomy 32(4):23-25. |
|
|
Nadir H, Ali A, Muhammed GAR (2016). Determination of yield and Quality of Oyster Mushroom (Pleurotus florida) using Different Substrates in Halabja, Kurdistan Reign-Iraq. Journal of Plant Production 7(7):787-790. |
|
|
Naraian R, Sahu RK, Kumar S, Garg SK, Singh CS, Kanaujia RS (2009). Influence of different nitrogen rich supplements during cultivation of Pleurotus florida on corn cob substrate. The Environmentalist 29(1):1-7. |
|
|
National Farmers Information Service (NAFIS) 2014. Mushroom production in kenya. Copeel Agrisience. |
|
|
Musanze R (2013). Relative performance of oyster mushroom (Pleurotus florida) on agro-industrial and agricultural substrate. International Journal of Agronomy and Plant Production 4(1):109-116. |
|
|
Nongthombam J, Kumar A, Ladli B, Madhushekhar M, Patidar S (2021). A review on study of growth and cultivation of oyster mushroom. Plant Cell Biotechnology and Molecular Biology 22(5&6):55-65. |
|
|
Obodai M, Cleland-Okine J, Vowotor KA (2003). Comparative study on the growth and yield of Pleurotus ostreatus mushroom on different lignocellulosic by-products. Journal of Industrial Microbiology and Biotechnology 30(3):146-149. |
|
|
Odendo M, Kirigua V, Kimenju JW, Wasilwa L, Musieba F, Orina M (2009). Analysis of Mushroom Value Chain in Kenya. East African Agricultural and Forestry Journal 76(3&4): |
|
|
Oei P (2005). Mushroom Cultivation: Appropriate Technology for Mushroom Growers. The Netherlands Backhuys Publishing: Leiden. No.Ed.3 pp. xii + 429 pp. |
|
|
Ojwang DO (2014). Molecular characterization of the wild edible mushrooms of the Pleurotus species in Kenya (Doctoral dissertation). |
|
|
Onyango BO, Palapala VA, Arama PF, Wagai SO, Gichimu BM (2011). Morphological characterization of Kenyan native wood ear mushroom [Auricularia auricula and the effect of supplemented millet and sorghum grains in spawn production. Agriculture and Biology Journal of North America 2(3):407-414. |
|
|
Oseni TO, Dlamini SO, Earnshaw DMT, Masarirambi M (2012). Effect of substrate pre-treatment methods on oyster mushroom (Pleurotus ostreatus) production. International Journal of Agriculture and Biology 14(2):32-36. |
|
|
Pardo-Giménez A, Picornell Buendia MR, de Juan Valero JA, Pardo-González JE, Cunha Zied D (2010). Cultivation of Pleurotus ostreatus using supplemented spent oyster mushroom substrate. In XXVIII International Horticultural Congress on Science and Horticulture for People (IHC2010): International Symposium on 933:267-272. |
|
|
Pathmashini L, Arulnandhy V, Wijeratnam RW (2008). Cultivation of oyster mushroom (Pleurotus ostreatus) on sawdust. Ceylon Journal of Science (Biological Science) 37(2):177-182. |
|
|
Porselvi A, Vijayakumar R (2019). Evaluation of paddy straw varieties on the cultivation and nutritional value of two oyster mushroom species. International Journal of Research in Advent Technology 7(5):556-563. |
|
|
Rajasekar M, Arumugam T, Kumar SR (2013). Influence of weather and growing environment on vegetable growth and yield. Journal of Horticulture and Forestry 5(10):160-167. |
|
|
Rambey R, Sitepu IDB, Siregar EBM (2019). Productivity of oyster mushrooms (Pleurotus ostreatus) on media corncobs mixed with sawdust. In IOP Conference Series: Earth and Environmental Science 260(1):012076. |
|
|
Randive S (2012). Cultivation and Study of growth of oyster mushroom on different agricultural waste substrate and its nutrient Analysis. Advances in Applied Science Research 3(4):1938-1949. |
|
|
Ritota M, Manzi P (2019). Pleurotus spp. cultivation on different agri-food by-products: Example of biotechnological application. Sustainability (Switzerland) 11(18):5049. |
|
|
Sánchez C (2010). Cultivation of Pleurotus ostreatus and other edible mushrooms. Applied Microbiology and Biotechnology 85(5):1321-1337. |
|
|
SAS Institute Inc. (2017). SAS® 9.4 System Options: Reference, Fifth Edition. Cary, NC: SAS Institute Incorporated. AS® pp27513-2414. |
|
|
Shah ZA, Ashraf M, Ch MI (2004). Comparative study on cultivation and yield performance of oyster mushroom (Pleurotus ostreatus) on different substrates (Wheat Straw, Leaves, Saw Dust). Pakistan Journal of Nutrition 3(3):158-160. |
|
|
Sharma S, Yadav RKP, Pokhrel CP (2013). Growth and Yield of Oyster mushroom (Pleurotus ostreatus) on different substrates. Journal on New Biological Reports 2(1):3-8. |
|
|
Singh R, Yadav DB, Ravisankar N, Yadav A, Singh H (2020). Crop residue management in rice-wheat cropping system for resource conservation and environmental protection in north-western India. Environment, Development and Sustainability 22(5):3871-3896. |
|
|
Stanley OH, Awi-Waadu G D (2010). Effect of substrates of spawn production on mycelial growth of oyster mushroom species. Agriculture and Biology Journal of North America 1(5):817-820. |
|
|
Tavarwisa DM, Govera C, Mutetwa M, Ngezimana W (2021). Evaluating the suitability of baobab fruit shells as substrate for growing oyster mushroom (Pleurotus ostreatus). International Journal of Agronomy 45:24-32. |
|
|
Tekeste N, Dessie K, Taddesse K, Ebrahim A (2020). Evaluation of different substrates for yield and yield attributes of oyster mushroom (Pleurotus ostreatus) in crop-livestock farming system of Northern Ethiopia. The Open Agriculture Journal 14(31):30-35. |
|
|
Tesfay T, Godifey T, Mesfin R, Kalayu G (2020). Evaluation of waste paper for cultivation of oyster mushroom (Pleurotus ostreatus) with some added supplementary materials. AMB Express 10(1):1-8. |
|
|
Thakur MP (2020). Advances in mushroom production: key to food, nutritional and employment security: A review. Indian Phytopathology 73(3):377-395. |
|
|
Vetayasuporn S (2006). Oyster Mushroom Cultivation on Different Cellulosic Substrates. Research Journal of Agricultural and Biological Science 2(6):548-551. |
|
|
Waiganjo MW, Ngeli P, Gateri MW, Muriuki AW (2008). Cultivation and commercialization of edible mushrooms in Kenya: A review of prospects and challenges for smallholder production. In International Symposium on Underutilized Plants for Food Security, Nutrition, Income and Sustainable Development 806:473-480. |
|
|
Yang D, Liang J, Wang Y, Sun F, Tao H, Xu Q, Wan X (2016). Tea waste: an effective and economic substrate for oyster mushroom cultivation. Journal of the Science of Food and Agriculture 96(2):680-684. |
|
|
Zakil FA, Xuan LH, Zaman N, Alan NI, Salahutheen NAA, Sueb MS, Isha R (2022). Growth performance and mineral analysis of Pleurotus ostreatus from various agricultural wastes mixed with rubber tree sawdust in Malaysia. Bioresource Technology Reports 17:100873. |
|
Copyright © 2024 Author(s) retain the copyright of this article.
This article is published under the terms of the Creative Commons Attribution License 4.0