Full Length Research Paper
ABSTRACT
In the early 20th Century, Guibourtia copallifera Benn. was extensively exploited as a source of gum copal. Its geographic distribution in Sierra Leone is now restricted to a few square kilometers in a single badly degraded forest reserve. We conducted a series of seed rain experiments to quantify its seed dispersal potential. Transects of seed traps were laid out and a total of 1,164,880 seeds were collected over the two months when G. copallifera sheds its? seeds. Seeds from a further 85 species were collected. The majority of species were wind dispersed followed by terrestrial animals, bats and birds. Leguminosae-Caesalpiniaceae had the highest number of species, followed by Euphorbiaceae, Apocynaceae, Rubiaceae and Sterculiaceae. There is no statistically significant difference between the transects (p>0.05, df=79, F=0.023) or the types of traps (p>0.05, df=118, t=0.089). Median dispersal distances of G. copallifera seeds was 41.22 m from the forest edge. The reserve still possesses a high natural regeneration potential for G. copallifera but increasing human disturbance is opening the canopy allowing pioneering species to dominate and reducing the ability of G. copallifera seedlings to establish.
Key words: Guibourtia copallifera, seed rain, seed dispersal, forest reserve, Sierra Leone.
INTRODUCTION
Seed production and dispersal are important functional attributes for the maintenance of plant populations, influencing the spatial distribution and composition of the plant community, in addition to affecting gene flow within and between populations and enabling the colonization of new sites and habitat restoration (McConkey et al., 2012; Kroiss and Hillers-Lambers, 2015).
Changes in the composition and abundance of seeds in the seed rain will have strong effects on the vegetation community and seed bank composition (Pearse et al., 2017; Barnes and Chapman, 2014). Strong disturbances lead to seed bank depletion either by massive germination of the seeds, or by the loss of a large number of seeds (Travlos et al., 2020; Ndor et al., 2012). Since 2002, Sierra Leone has lost 29,500 hectares of humid primary forest, a decrease of 11% (Global Forest Watch, 2020). At present, these resources are confronted with increasing deforestation due to growing population and demand for more agricultural land, timber, charcoal, fuel wood, mining and recurrent bush fires.
Research addressing community wide and species-specific seed rain patterns of forests in Sierra Leone is lacking. Most approaches to understanding tropical forest dynamics and plant species availability have focused on patterns of vegetation structure, composition, diversity and distribution (Sesay, 2019; Fayiah et al., 2020; Mattia and Sesay, 2020). Kasewe forest reserve has been through series of human exploitations since it was first gazetted in 1919 (Munro and Hiemstra-van der Horst, 2012a; Munro et al., 2017) for resources like gum copal, timber, poles, and other non-timber forest products (NTFPs) and bush meat. During the Ebola crisis of 2014 - 2016 the population within the forest increased as people fled their communities and found the forest as a source of livelihood. The resulting exploitations have expanded and increased over the years to levels which are no longer sustainable. Exploitation rates increased through artificial deforestation the use of power saws offered by affluent merchants in return for charcoal and related products to their benefactors in cities where these products are in high demand.
The most preferred species for charcoal production by the exploiters is G. copalliofera, a species of tree that was heavily exploited for gum copal during the colonial period but no longer being done at present. The high quality of charcoal produced from this species and the increased exploitation it currently faces in this unprotected reserve could pre-dispose this limited geographical range species to extirpation.
This study therefore aimed to characterize and evaluate the dynamics of seed dispersal by G. copallifera; specifically, to quantify the seed rain along the different land uses in the forest reserve, as well as analyze for differences in total abundance, species richness, diversity and composition in the seed rain.
MATERIALS AND METHODS
Study site
Kasewe Forest Reserve (8°18′53″N, 12°15′43″W?? / 8.31472; -12.26194) occupies a small range of steep sided hills of volcanic origin rising up to 500 m above the surrounding plain (Bowden, 1997; Lytwyna et al., 2006). The reserve covers 2,331 hectares, and the dominant vegetation is tropical forest containing a mosaic of moist semi-deciduous forest, evergreen forests and savanna that give way to medium altitude forest on the upper slopes (UNEP, 2008). The reserve is located in Moyamba District in the south-central part of Sierra Leone about 170 km east of the capital city of Freetown. The Bo-Freetown highway is one of the busiest provincial roads in the country, it forms one discernible boundary and allows easy access for exploitation and transport of products such as charcoal and timber from the reserve.
Seedrain sampling
Construction of seed traps and placement
In order to assess the nature of the seed rain and dispersal capability of G. copallifera in the reserve, seed traps were set up along pre-cut transects in degraded areas adjacent to the forest edge and in the interior of forest patches. Transects were up to 300 m long with equally spaced pairs of sampling units (traps). One trap consisted of a 1.5 m2 plastic mesh (1mm mesh) with the corners lifted 1.2 m above ground level on poles, while the other consisted of swept ground demarcated by four wooden stakes driven into the ground but with ends sticking out at about two meters from the mesh trap. Forty-five transects were set out in the forest and five at right angles to the forest edge into the degraded forest. Traps were monitored on a weekly interval for 12 weeks. In the degraded areas, focal trees of G. copallifera were selected from which transects were located with the aim of observing the dispersal dynamics of this species. Transects in the degraded area had traps installed at 10 m intervals out to 300 m from the forest edge.
Seed collection and identification
Traps were emptied on a weekly basis with standard data sheet for each type of trap that was installed. Contents of each trap within the trap line was emptied into a separate labelled bag or container and leaves and twigs removed. Physical states of the fruits and seeds were noted: rotten, germinated, partly eaten, insect infested and animal feeding. Seeds were grouped in two classes namely G. copallifera (Kobo) and others. Trap contents were taken into the laboratory and sorted by hand. All potentially viable G. copallifera seeds (>1.5 mm diameter) were extracted and identified (Cottrell, 2004). Other species were identified by comparison to the seed collections held at the National Herbarium of Sierra Leone in the Department of Biological Sciences, Njala University.
Statistical analyses
Majority of the analyses were done in R version 3.6.2 (R Core 2020). ANOVA single-factor analysis, paired t-tests and linear regression were used to determine the effectiveness of the different types of traps, dispersal in the different environments, changes over time. Variability with seed collected in the different traps was visualized using box plots.
RESULTS
Types of traps and layout of the experiment
Trap types were installed at 45 transect locations to determine if there was a significant difference in capture efficiency. A total of 1,164,880 seeds were collected. Among these 44.94% (523,467) of seeds collected were from stations with both ground and mesh traps; while 22.88% (266,518) from ground traps and 20.97% (244,244) from mesh traps. The effectiveness of the traps types was determined by a t-test. This showed there was no statistical difference between the two types of traps (p>0.05, df=118, t=0.089).
Floristic composition, species richness and diversity of seed rain
A total of 1,164,880 seeds were collected resulting in a final density of 2,107 seeds m2 (300 seeds m2 week-1). Eighty-five species from forty-one genera were collected; 65 species were identified to species level, 9 to genera, 6 to family and 5 remained unidentified. The large majority of species were dispersed by wind (50 species), followed by animals (20) including birds and bats (15).
Guibourtia copallifera was the most abundant species, followed by Nesogordonia papaveriefera (A.Chev.), Hymenocardia lyrata Tul. and Memecylon normandii Jacq. Leguminosae-Caesalpiniaceae had the highest number of species, followed by Euphorbiaceae, Apocynaceae. Rubiaceae and Sterculiaceae. Species belonging to Rhizophoraceae, Chrysobalanaceae, Ixonanthaceae and Ulmaceae were present in very low numbers. Seeds of G. copallifera made up 58.43% of all seeds and were slightly more likely to be captured by the mesh traps (61% of seeds) compared to the ground traps (54%). Species richness ranged from 3 to 12 species per trap during the collection period.
Status of seeds in traps
Seed conditions were classified as intact (80 to 85%), germinated (8 to 12%), insect infested (1 to 2%), decayed (rotten) (2 to 3%) or partly eaten (2 to 4%). Intact seeds germinated in 3 to 7 days but the majority of intact seeds failed to germinate.
Seed quality and production are main considerations overseeing the regeneration, structure, also progression of trees in natural forests (Galipeau et al., 1997; Viglas et al., 2013). Harsh and Joshi (1993) have announced 70% harm to Albizia seeds because of insects and diseases out of which 40% was because of the insects, Bruchus bilineatopygus and B. sparsemaculatus. Diminished viability and germination disappointment of these damaged seeds have been accounted for (Ponnuswami et al., 1990). Singh and Bhandari (1986) have detailed scope obliteration of chilgoza pine seeds in India at Kalpa, Himachal Pradesh, by Dioryctria abeitella Schiff. causing up to 50% seed damage. Essentially, seeds of one more significant species, teak (Tectona grandis) are damaged by Dichocrosis (Conogethes) punctiferalis (Guenee) (Pyralidae) causing up to 70% seed destruction in storage. Locally, decomposition on forest floor can be constrained by the chemical quality of litter (Krishna and Mohan, 2017; Prescott et al., 2017), microenvironmental conditions (Rodríguez-Paredes et al 2012; Tymen et al., 2012) and soil properties (Li et al., 2018; Kravkaz-Kuscu et al., 2018).
Weekly seed collection
Weekly seed collection was virtually the same for both trap types; median average 1217.4, while that of mesh is 1227.4 (Figure 1).
No significant linear trend was observed for the first 8 weeks the transect traps were set out, but there was then a rapid decline close to zero by week 12. An ANOVA single-factor analysis was performed to confirm that the results were highly significant (p<0.001). A “broken stick” regression was then conducted to show the effective termination of dispersal at week 8 (Figure 2).
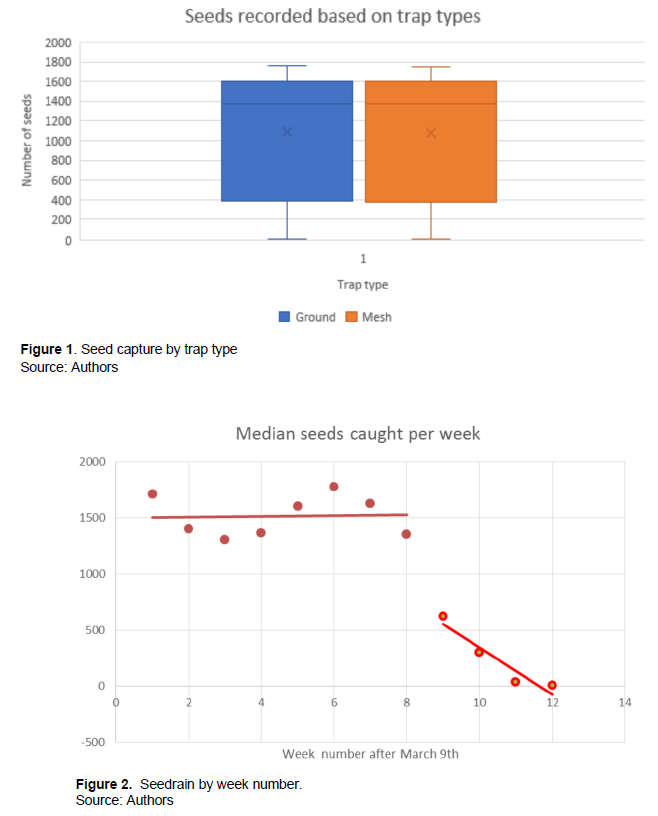
A linear regression of median seed rain per trap per week for weeks 1 to 8 showed an r2 value of only 0.002, which was not significant (p>0.05, df=8, F=0.017).
Seed rain by habitat
Total seed rain density was higher in the forest plots. Deposition of seeds in the interior of the forest is 3 times greater than that recorded in the degraded area or forest edge. Variability in patterns of seed deposition was high among sites and transects.
Seed species richness at the forest edge was less in terms of species and numbers. More Species were recorded in the interior of the forest than at the edge, with the numbers of G. copllifera and other species higher in the interior of the forest and lower at the edges.
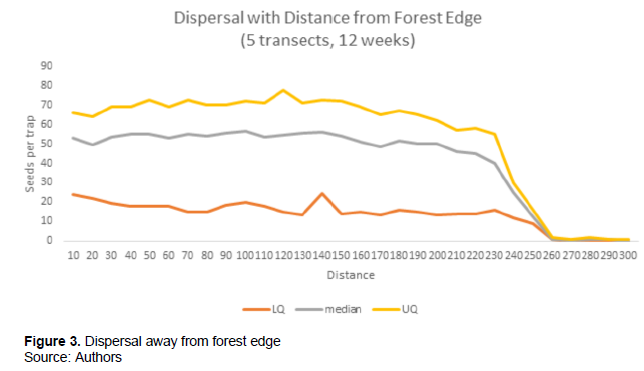
Seed numbers are fewer under the parent trees and increases as the distance increases from the trees. In the degraded and forested patches, seeds deposition is observed to be similar. Less of the seeds are found under the parent plant and more seeds recorded at distance. Distances are shorter and reduced in undegraded patches while seeds are carried by wind to long distances in degraded areas. Seeds were successful in dispersing away from the forest edge but that the rate (seeds per trap) was similar (linear regression r2 = 0.9702).
Seed dispersal in relation to the forest edge
A one-way ANOVA was used to express the number of seeds caught in relation to distance from the forest edge. No statistically significant difference observed between the 5 transects (p>0.05, df=79, F=0.023). The number of seeds that lands in the traps varies with the distance from the parent tree. An irregular pattern was observed with several peaks due to rain fall, wind intensity and duration, forest disturbances and canopy cover. Overall median dispersal distance of all species was 41.22 m. But a seed rain of greater than 1 per meter square of G. copllifera was observed out to 280 m. At 230 m the number of seeds in the traps fell rapidly. Regarding this species, dispersal distances were narrow and limited within the forest especially in areas where canopy was thick and dense. Whilst the opposite is true for the degraded patches and open areas. Seeds were carried over long distances in days where wind intensity and duration prolonged over a considerable period. Based on this study, wind action and intensity could carry seeds up to 280 m on very windy days but very few were recorded beyond this distance.
Seed dispersal in the forest
The seed rain of the forest was highly variable but with an average of 297 seeds m-2 week−1, but with considerable spatial and temporal patchiness. More than 60 species were represented in the seed rain. These were dominated by a small number of species producing a large seed crop, with most species represented by few seeds during the period. Total seed rain from wind-dispersed versus animal-dispersed tree species did not match their proportions in the forest. Wind dispersed seeds were relatively common compared to others. Additionally, no previous studies have assessed the proportion of seed rain of this species and this forest patch in general.
DISCUSSION
Types of traps and layout of the experiment
The study shows no statistically significant difference between ground traps and net traps in terms of total seeds collected. Although the total number of seeds is highest in traps with large area, seed density does not vary significantly across traps with different areas which is consistent with Morris et al. (2011). In forest seed rain studies, large seed traps are usually used to capture large seeds of fleshy-fruited and tree species (Vergara-Tabares et al., 2021). However, our values seem consistent with records from other locations such as Carrillo-Arreola et al. (2020) who collected 8,500 seeds m-2 forest matrix. Kitamura et al. (2005) observed 22 species of birds and six species of mammals foraging in 15 different species of fruit-bearing plants in Khao Yai National Park in Thailand.
Floristic composition, species richness and diversity
Results on the species richness and diversity indicate that despite the disturbed nature of the forest there is still a high amount of diversity. G. copallifera seeds were the most abundant, followed by Nesogordonia papaveriefera (A.Chev.), Hymenocardia lyrata Tul. and Memecylon normandii Jac.-Fél. The abundance and seed density values in this study (85 species from 41 plant families) are higher than those in several other studies of primary and secondary tropical forests, 16 species (Chalermsri et al., 2020), 26 species (Wang et al., 2019), 15 species (Ce´sar et al., 2017), 40 species (Sritongchuay et al., 2014). Similar levels of diversity are the 65 species recorded in the Serra da Bodoquena National Park (Brachtvogel et al., 2020), 56 species (Dhillon et al., 2003) and 46 species by Villicana- Hernandez et al., (2020) in Yucatan, Mexico.
Seed density was found to be higher in this study relative to two similar studies in semi-deciduous tropical forest for Cameroon (≅297 seeds.m-². year-1; Hardesty and Parker, 2002 Nuñez et al., 2021;), and the southeast of Brazil (≅442 seeds.m-². Year-1; Grombone-Guaratini and Rodrigues, 2002). The higher number of seeds found in this study reflect that the large number of mature trees producing a high number of seeds produced by G. copllifera (Peili et al., 2019; Wickert et al., 2017). Peak abundance of tree seeds coincides with the start of the rainy season, while seeds of other herbaceous species peak at other times.
Human disturbances in this forest also strongly affect the plant community structure; more specifically, G. copallifera species are gaining dominance in non-degraded areas and losing dominance in degraded areas. The seed dispersal distance for this species was strongly correlated with the plant than with seed traits (Augspurger et al., 2017; Thomson et al., 2011). Indeed, the dispersal of G. copallifera seeds in our study was largely due to plant character more than the seed size. The parent plant crown and the height positively influenced the seed dispersal of this species.
Status of seeds in traps
Conditions of collected seeds in traps varied depending on the several prevailing factors like rainfall, wind, humidity, sunlight, germination rate and volume of leaf litters. Damage was less in the mesh traps as a result of the fact that ground trap is being affected by run off, ground animals and insect prey which reduces the evidence on the ground compared to mesh trap which drains water and is more difficult to access by terrestrial seed predators.
Germinated seeds were recorded even in the absence of soil especially for G. coppallifera. Regarding seed decay, the principal factors thought to control paces of litter decay include barometrical relative humidity (Gregorich et al., 2017; Beyaert and Voroney, 2011), the herbaceous plant layer (Ossola et al., 2016; Zirbel et al., 2017), and soil temperature and dampness (Cortez 1998; Mao et al. 2018; Sun and Zhao, 2016). Song et al. (2021) observed that decomposition rate of leaves increased under mats of exotic weed Tradescantia fluminensis Vell.. This is attributed to a positive microclimate and living space for the decomposers. Studies on seed insect pests, conducted at the Pakistan Forest Institute, Peshawar, revealed that seeds of 30 out of 70 tree species were infested with 24 insect species. Among them 86% were Coleoptera, and 5.6% were Lepidoptera, while 8.4 % was a hymenopteran parasite (Rehman, 1993).
Weekly seed collection
The number of seeds captured weekly on the interior and edges of the forest seem to coincide with the frequency of monthly wind, sun and rain data for that area. More seeds are captured in weeks with more frequent rainy days and less in less windy and raining days in the weeks. When the rate and duration of precipitation increased in September, the data showed a significant decrease in the number of seeds captured particularly for G. copallifera, since this was at the end of its fruiting season. The temporal variability of seed rain related to rainfall has been reported by other studies (Gummadi et al., 2017; Perini et al., 2019).
Seed rain was observed to be greater in the interior of the forest than at the edges. Studies on the interior and edges observed that seed rain on both sites was higher along the forest edge, also observed by other studies (Diogo et al., 2015; Dunham et al., 2018; de Melo et al., 2006; Magrach et al., 2014; Razafindratsima et al., 2021). The low abundance and diversity of seed captured in the degraded area shows a limitation on seed dispersal either due to felling of trees, and or increasing human activities. Similar results by other studies for seed dispersal from forests adjacent to pasture found that seeds were mostly anemochory and limited to 10m distance (Aide and Cavelier, 1994), 5 m (Holl,1999), and 4 m (Zimmerman et al., 2000; Cubiña and Aide, 2001) from the forest edge.
The fact that the seed rain dynamics in both forest edge and interior was affected by the surrounding tree heights, number of trees, species and intensity of human activities, shows that matured and taller trees produce more seeds and have a higher chance to disperse more seeds (Thomson et al., 2011).
Seed rain numbers
Over sixty species were shedding seed at the same time as G. copallifera, however, it is possible that some species have been missed due to human error (misclassification or identification), low density or being physically restricted to small areas in the forest; additional species disperse at other times of the year. G. copallifera seeds dominated seed rain traps. Numbers of seed dispersed by this species is plentiful on the forest floor.
Relationship between distance and dispersal frequency
The most general and perhaps obvious dispersal pattern found in the study was the fairly uniform seed rain out to about quarter of a kilometer from the seed source (Figure 3). The results illustrate how an analysis of a species seed dynamics can contribute to a better understanding of the general ecological phenomena, including species dispersal and spread. This is an indication to the fact that this species has the ability to establish itself in distant locations in the absence of the parent plant. The proportion of seed that is randomly dispersed over relatively long distances from the source plants of G. copallifera ranged from 5 to 50 % of the total seed set. Despite this long-distance dispersal advantage of G. copallifera seeds, its’ establishment in the new area is opposed by predation, soil conditions, depth of seed in soil / leaflitters and human activities.
CONCLUSION
G. copallifera has an extremely restricted geographical distribution in Sierra Leone with the majority of the population in a single small and degraded forest reserve. Disturbance to the forest through charcoal burning allows the pioneer species to be dominant and in some grass patches which form fire-climax vegetation communities are establishing. Our results showed that G. copallifera produces a lot of wind dispersed seeds and densities of up to 70 to 100 per sq meters were observed 250 meters from the forest edge. Only 0.8% of the seeds were found to be empty. Dispersal of G. copallifera seeds would seem to be sufficient to allow it to maintain its population in the forest but not to disperse to other similar forest patches which are many kilometers away.
The results obtained demonstrate that Kasewe Forest Reserve still has a high natural regeneration potential for G. copallifera; nevertheless, there are several causes for concern which will be critical for continued natural regeneration; the on-going disturbances have reduced tree cover, increased the number of clearings and increased the availability of light, thereby facilitating the dominance of pioneer species. Thus, the national and district authorities must be careful for the implementation of the conservation measures and enhance effective co-management and protection program involving the local people to ensure fruitful conservation of this species in its most abundant site. Protection and aided natural regeneration may be another alternative option for effective natural regeneration and conservation of this species especially in degraded habitats within the reserve.
CONFLICT OF INTERESTS
The authors have not declared any conflict of interests.
ACKNOWLEDGEMENTS
We thank the staff of the Sierra Leone National Herbarium in the Department of Biological Sciences for their technical support. We are grateful to the Ministry of Forestry and Food Security for granting us permission to conduct this research in Kasewe Forest Reserve. We would like to thank Andrew Kamanda, Josaphus Junan, Joseph Longha, Janet Saidu, Emmanuel kamanda Kinnie Jarawallay for their assistance with fieldwork and data collection. Special thanks to Maxwell Williams and Emmanuel Saidu for data compilation and input.
REFERENCES
|
Aide TM, Cavelier J (1994). Barriers to Lowland Tropical Forest Restoration in the Sierra Nevada de Santa Marta, Colombia. Restoration Ecology 2(4):219-229. |
|
|
Augspurger CK, Franson SE, Cushman KC (2017). Wind dispersal is predicted by tree, not diaspore, traits in comparisons of Neotropical species. Functional Ecology 31(4):808-820. |
|
|
Barnes AD, Chapman HM (2014). Dispersal traits determine passive restoration trajectory of a Nigerian montane forest. Acta Oecologia 56:32-40. |
|
|
Beyaert RP, Voroney RP (2011). Estimation of decay constants for crop residues measured over 15 years in conventional and reduced tillage systems in a coarse-textured soil in southern Ontario. Canadian Journal of Soil Science 91(6):985-995. |
|
|
Bowden DJ (1997). The geochemistry and development of lateritized footslope benches: The Kasewe Hills, Sierra Leone, Geological Society, London, Special Publications 120(1):295-305. |
|
|
Brachtvogel C, Pereira ZV, Silva SM (2020). Spatial variation of seed rain in deciduous tropical forest. Research, Society and Development 9(9):2525-3409. |
|
|
Carrillo AF, Quintana-Ascencio PF, Ramírez- Marcial N, González-Espinosa M (2020). Seed rain and establishment in successional forests in Chiapas, Mexico. Acta Botanica Mexicana 127:1-28. |
|
|
Ce'sar RG, Rother DC, Brancalion PHS (2017). Early Response of Tree Seed Arrival After Liana Cutting in a Disturbed Tropical Forest. Tropical Conservation Science 10:1-7. |
|
|
Chalermsri A, Ampornpan L and Purahong W (2020). Seed Rain, Soil Seed Bank, and Seedling Emergence Indicate Limited Potential for Self-Recovery in a Highly Disturbed, Tropical, Mixed Deciduous Forest. Plants 9(1391):1-14. |
|
|
Cortez J (1998). Field decomposition of leaf litters: relationships between decomposition rates and soil moisture, soil temperature and earthworm activity. Soil Biology and Biochemistry 30(6):783-793. |
|
|
Cubiña A, Aide TM (2001). The effect of distance from forest edge on seed rain and soil seed bank in a tropical pasture. Biotropica 33(2):260-267. |
|
|
De Melo FP, Dirzo R, Tabarelli M (2006). Biased seed rain in forest edges: Evidence from the Brazilian Atlantic Forest. Biological Conservation 132(1):50-60. |
|
|
Dhillon MK, Sharma HC, Singh R, Naresh JS (2003). Mechanisms of resistance to shoot fly, Atherigona soccata in sorghum. Euphytica 144(3):301-312. |
|
|
Diogo IJS, Fortunato MEM, Costa IR (2015). Seed deposition in the edge-interior gradient of a degraded fragment of tropical semideciduous forest, Northeastern Brazil. Revista de Biología Tropical 63(4):981-994. |
|
|
Dunham AE, Razafindratsima OH, Rakotonirina P, Wright PC (2018). Fruiting phenology is linked to rainfall variability in a tropical rain forest. Biotropica 50(3):396-404. |
|
|
Fayiah M, Dong S, Singh S, Tulcan RXS, Sheriff K, Kargbo IR, Barrie A (2020). Plant diversity and regeneration potentials in Protected Area Forests of Sierra Leone. Current Research in Agricultural Sciences 7(2):64-83. |
|
|
Galipeau C, Kneeshaw D, Bergeron Y (1997). White Spruce and Balsam Fir Colonisation of a Site in the Southeastern Boreal Forest as Observed 68 Years after Fire. Canadian Journal of Forest Reseach 27(2):139-151. |
|
|
Global Forest Watch (2020). Forests of Sierra Leone: Global Forest watch Newsletter. Available at: View |
|
|
Gnoumou A, Bognounou F, Hahn K, Thiombiano A (2011). Woody plant diversity and stand structure in the Comoe -Lerba Reserve, Southwestern Burkina Faso (West Africa). Journal of Biological Sciences 11(2):111-123. |
|
|
Gregorich EG, Janzen H, Ellert BH, Helgason BL, Qian B, Zebarth BJ, Angers DA, Beyaert RP, Drury CF, Duguid SD, May WE, Mcconkey BG, Dyck MF (2017). Litter decay Controlled by temperature, not soil properties, affecting future soil carbon. Global Change Biology 23(4):1725-1734. |
|
|
Gummadi S, Rao KPC, Seid J, Legesse G, Kadiyala MDM, Takele R, Amede T and Whitbread A (2017). Spatio-temporal variability and trends of precipitation and extreme rainfall events in Ethiopia in 1980-2010. Theoretical and Applied Climatology pp. 1-15. |
|
|
Hardesty BD, Parker VT (2002). Community seed rain patterns and a comparison to adult community structure in a West African tropical forest. Plant Ecology 164(1):49-64. |
|
|
Harsh NSK, Joshi KC (1993). Loss assessment of Albizia lebbek seeds due to insects and fungal damage. Indian Forester 119 (11):932-935. |
|
|
Holl KD (1999). Factors limiting tropical rain forest regeneration in abandoned pasture: seedrain, seed germination, microclimate, and soil. Biotropica 31:229-242. |
|
|
Kitamura S, Yumoto T, Poonswad P, Chuailua P, Plongmai K, Noma N, Maruhashi T, Wohandee P (2005). Fruit-frugivore interactions in a moist evergreen forest of Khao Yai National Park in Thailand. Tropics 14(4):344-355. |
|
|
Kravkaz-Kuscu IS, Sariyildiz T, Cetin M, Yigit N, Sevik H, Savaci G (2018) Evaluation of the soil properties and primary forest tree species in Taskopru (Kastamonu) District. Fresenius Environmental Bulletin 27(3):1613-1617. |
|
|
Krishna MP, Mohan M (2017). Litter decomposition in forest ecosystems:a review Energy, Ecology and Environment 2(4):236-249. |
|
|
Kroiss SJ, Hillers-lambers J (2015). Recruitment limitation of long-lived conifers: implications for climate change responses. Ecology 96(5):1286-1297. |
|
|
Li Y, Tian D, Yang H, Niu S (2018). Size-dependent nutrient limitation of tree growth from subtropical to cold temperate forests. Functional Ecology 32:95-105. |
|
|
Lytwyna J, Burkea K, Culver S (2006). The nature and location of the suture zone in the Rokelide orogen, Sierra Leone: Geochemical evidence. Journal of African Earth Sciences 46(5):439-454. |
|
|
Magrach A, Laurance WF, Larrinaga AR, Santamaria L (2014). Meta analysis of the effects of forest fragmentation on interspecific interactions. Conservation Biology 28:1342-1348. |
|
|
Mao R, Zhang XH, Song CC, Wang XW, Finnegan PM (2018). Plant functional group controls litter decomposition rate and its temperature sensitivity: An incubation experiment on litters from a boreal peatland in northeast China. Science of the Total Environment 626:678-683. |
|
|
Mattia SB, Sesay S (2020). Ground Forest inventory and assessment of carbon stocks in Sierra Leone West Africa. Natural Resources Management and Biological Sciences, Edward R. Rhodes and Humood Naser, IntechOpen, pp. 1-22. |
|
|
McConkey KR, prasad S, Corlett RT, Campos-Arceiz A, Brodie JF, Rogers H, Santamaria L (2012). Seed dispersal in changing landscapes. Biological Conservation 146:1-13. |
|
|
Morris K, Raulings EJ, Melbourne WH, Nally RM, Thompson RM (2011). A novel trap for quantifying the dispersal of seeds by wind. Journal of Vegetation Science 22(5):807-817. |
|
|
Munro P, van der Horst G, Healy S (2017). Energy justice for all? Rethinking Sustainable Development Goal 7 through struggles over traditional energy practices in Sierra Leone. Energy Policy 105(C):635-641. |
|
|
Munro PG, van der Horst GA (2012a). The Domestic Trade in Timber and Fuelwood Products in Sierra Leone: Current Dynamics and Issues. Freetown: FAO/EU. |
|
|
Ndor E, Dauda NS, Chammang HB (2012). Effect of germination media and seed size on germination and seedling vigour of fluted pumpkin (Telferia occidentalis) Hook. F. International Journal of Agricultural Sciences 2(3):113-115. |
|
|
Nuñez NH, Chazdon RL, Russo SE (2021). Seed Rain-Successional Feedbacks in Wet Tropical Forests. Ecology 102(7):1-30. |
|
|
Ossola A, Hahs AK, Nash MA, Livesley SJ (2016). Habitat complexity enhances comminution and decomposition processes in urban ecosystems. Ecosystems 19(5):927-941. |
|
|
Peili M, Guo L, Gao Y, Qi L, Cao B (2019). Effects of Seed Size and Sand Burial on Germination and Early Growth of Seedlings for Coastal Pinus thunbergii Parl. in the Northern Shandong Peninsula, China. Forests 10(281):1-14. |
|
|
Perini M, Dias HM, Kunz SH (2019). The role of environmental heterogeneity in the seed rain pattern. Floresta e Ambiente 26(SPE1):1-10. |
|
|
Pearse IS, Lamontagne JM, Koenig WD (2017). Inter-annual variation in seed production has increased over time (1900-2014). Proceedings of the Royal Society 284:1666. |
|
|
Ponnuswamy AS, Surendran C, Parameshwaran S (1990). Effect of insect damage on seed germination in Albizia lebbek. Indian Forester 116(6):509-510. |
|
|
Prescott CE, Reid A, Wu SY, Nilsson MC (2017). Decomposition rates of surface and buried forest-floor material. Canadian Journal of Forest Research pp. 1-6. |
|
|
Razafindratsima OH, Raoelinjanakolona NN, Heriniaina RR, Nantenaina RH, Ratolojanahary TH, Dunham AE (2021) Simplified Communities of Seed-Dispersers Limit the Composition and Flow of Seeds in Edge Habitats. Frontiers in Ecology and Evolution pp. 1-13. |
|
|
Rehman WU (1993). Studies on seed insect pests of forest trees. Pakistan Journal of Forestry 43:127-138. |
|
|
Rodríguez-Paredes D, Rommel Montúfar-Galárraga R, Henrik Meilby H (2012). Effects of micro-environmental conditions and forest disturbance on the establishment of two Andean palms in Ecuador. Open Journal of Ecology 2(4):233-243. |
|
|
Sesay AUB (2019). Assessment of tree species diversity and composition at Kasewe Forest Reserve Moyamba district, Sierra Leone. Undergraduate dissertation submitted to the Department of Wildlife Management and Conservation, School of Natural Resources Management, Njala University. |
|
|
Singh P, Bhandari RS (1986). Insect pests of forest tree seeds and their control. pp. 155-171 in Proceedings of the National Seminar on Forest Tree Seeds. Indo-Danish Project on Seed Production and Tree Improvement. Andhra Pradesh Forest Department. Hyderabad, India. |
|
|
Song S, Hu X, Zhu J, Zheng T, Zhang F, Ji C, Zhu J (2021). The decomposition rates of leaf litter and fine root and their temperature sensitivities are influenced differently by biotic factors. Plant Soil 461(1):603-616. |
|
|
Sritongchuay T, Gale GA, Stewart A, Kerdkaew T, Bumrungsri S (2014). Seed Rain in Abandoned Clearings in a Lowland Evergreen Rain Forest in Southern Thailand. Tropical Conservation Science 7(3):572-585. |
|
|
Sun Y, Zhao S (2016). Leaf litter decomposition in urban forests: test of the home-field advantage hypothesis. Annals of Forest Science 73(4):1063-1072. |
|
|
Thomson FJ, Moles AT, Auld TD, Kingsford RT (2011). Seed dispersal distance is more strongly correlated with plant height than with seed mass. Journal of Ecology 99(6):1299-1307. |
|
|
Travlos I, Gazoulis I, Kanatas P, Tsekoura A, Zannopoulos S, Papastylianou P (2020) Key Factors Affecting Weed Seeds' Germination, Weed Emergence, and Their Possible Role for the Efficacy of False Seedbed Technique as Weed Management Practice. Frontiers in Agronomy 2(1):1-9. |
|
|
Tymen B, Vincent G, Courtois EA, Heurtebize J, Dauzat J, Marechaux I, Chave J (2012). Quantifying micro-environmental variation in tropical rainforest understory at landscape scale by combining airborne LiDAR scanning and a sensor network. Annals of Forest Science 74(32):1- 13. |
|
|
UNEP (2008). World Database on Protected Areas: Site Information, UN Environment Programme World Conservation Monitoring Centre. |
|
|
Vergara-Tabares DL, Blendinger PG, Tello A, Peluc SI, Tecco PA (2021). Fleshy-fruited invasive shrubs indirectly increase native tree seed dispersal. Oikos 1(9):1-10. |
|
|
Viglas JN, Brown CD, Johnstone JF (2013). Age and Size Effects on Seed Productivity of Northern Black Spruce. Canadian Journal Forestry Research 43:534-543. |
|
|
Villicana-Hernandez GJ, Mart_?nez-Nataren DA. Alvarez-Espino RX, Mungu?a-Rosas MA (2020). Seed Rain in a Tropical Dry Forest and Adjacent Home Gardens in the Yucatan. Tropical Conservation Science 13:1-9. |
|
|
Wang Y, LaMontagne JM, Lin F, Yuan Z, Ye J, Wang X, Hao Z (2019). Similarity between seed rain and neighbouring mature tree communities in an old?growth temperate forest. Journal of Forest Research 31(2):1-10. |
|
|
Wickert KL, O'Neal ES, Davis DD, Kasson MT (2017). Seed Production, Viability, and Reproductive Limits of the Invasive Ailanthus altissima (Tree-of-Heaven) within Invaded Environments. Forests 8(226):1-12. |
|
|
Zimmerman JK, Pascare JB, Mitchell AT (2000). Barriers to forest regeneration in an abandoned pasture in Puerto Rico. Restoration Ecology 8(4):350-360. |
|
|
Zirbel CR, Bassett T, Grman E, Brudvig LA (2017). Plant functional traits and environmental conditions shape community assembly and ecosystem functioning during restoration. Journal of Applied Ecology 54(4):1070-1079. |
|
Copyright © 2024 Author(s) retain the copyright of this article.
This article is published under the terms of the Creative Commons Attribution License 4.0