ABSTRACT
Water is a scarce resource, and Okra (Abelmoschus esculentus) is grown in wet and dry seasons. For its dry season cultivation, alternative water sources are used for watering, but these sources are not sustainable in Burkina Faso. The aim of this study was to determine water deficit effect on okra’s behavior. Five genotypes of okra were subjected to three water regimes: (i) T1, watering at 100% of soil field capacity (SFC); (ii) T2, watering at 50% SFC; and (iii) T3, watering at 25% SFC. Results showed that water restrictions at 50% SFC and 25% SFC caused a reduction in growth’s parameters. This reduction was very pronounced under watering at 25% SFC. In addition, the restrictive water supply at 25% SFC significantly reduced the number of capsules and the number of seeds. Results also revealed a large inter-genotypes variation on agro-physiological parameters under effects of water stress. The genotype G259 had a better tolerance under water regime at 25% SFC while, under watering at 50% SFC, genotypes O2 and L2 have been the least sensitive for capsules and seed yield. For irrigated okra, it would be better to bring water to plants at 50% SFC, if the soil is sandy-loamy.
Key words: Abelmoschus esculentus, ecotype, water regime, effect, yield.
Water is the main environmental factor limiting agricultural production, especially in the least-watered regions. Thus, in the context of recurrent climatic changes, the irregular nature and uneven distribution of rains are factors that seriously compromise agricultural production. According to Niang (2009), agriculture is negatively affected by climate change, especially in developing countries (Nelson et al., 2009). This causes selective pressure on plant genetic resources, which could be a source of genetic erosion. According to Trinchant et al. (2004), about 20 million hectares of land are lost worldwide each year due to water and salt stress. Irrigated agriculture is from a distance the largest water use in the world, accounting for 69% of global removals (FAO, 2002).
In Burkina Faso, agriculture is essentially rain-fed and production remains dependent on weather conditions. Drought is one of the main environmental constraints that causes the most damage to agricultural production globally and in Burkina Faso in particular. Indeed, Burkina Faso's agricultural sector sustains the brunt of drastic effects of climate variability, particularly of rainfall and temperatures. Studies on agromorphological characterization of okra [Abelmoschus esculentus (L.) Moench] genetic resources in Burkina Faso have shown the traditional character of cultivated varieties (Ouédraogo, 2016). In addition, other studies on the genetic and molecular diversity of okra accessions have identified genotypes of interest that could help boost okra production in Burkina Faso. Other studies have already discussed water stress in okra in Burkina Faso, but these studies have focused on development stages the most vulnerable to water deficit (Nana et al., 2009a, b). However, ignorance of their adaptation to water stress limits their appropriate use especially in areas subject to periodic water deficits.
The success of agricultural production is also based on the possibility to use plant material that has an adaptive potential for water deficit conditions. Indeed, the availability of soil water is one of the factors limiting the growth of plants (Reynolds et al., 2004; Otieno et al., 2005). Water stress is reflected in the plant by a series of changes that affect morphological, physiological and biochemical characters, when the water needs of plant exceed the available quantities (Mefti et al., 2001). As a result, the physiological mechanism adopted in case of water stress can be an effective tool for differentiating between varieties (Radhouane, 2013). Indeed, tolerance to different stresses depends on species, varieties and even genotypes (Ullah et al., 2008). Understanding the response of okra accessions to water stress and identification of water deficit tolerance characters would strengthen the process of varietal selection of okra in Burkina Faso. It is in this perspective that this study was initiated with the general objective of identifying genotypes presenting the characters of tolerance in water deficit conditions. Specifically, it is about:
(i) Evaluating physiological and agronomic characteristics of Okra genotypes cultivated under water deficit
conditions;
(ii) Identifying the best water treatments for the production of okra in cultivation in irrigation.
Plant material used
The plant material used for this study was represented by five genotypes of okra, collected in three climatic zones of Burkina Faso, from a participatory selection (Ouédraogo, 2016). They were selected on the basis of their agromorphological performance and consumer preferences. Characteristics and provenance of the five genotypes are presented in Table 1.
Characteristics of the culture substrate
The substrate used was soil taken at 20 cm deep and submitted to National Office of Soils for granulometric and physicochemical analyzes. Analytical results (Table 2) show that textural class was sandy-loamy (62.75% sand, 23.52% silt and 13.30% clay). In addition, this soil had fairly good chemical characteristics because it contained the necessary nutrients (organic matter, total carbon, assimilable phosphorus, assimilable potassium, Ca2+, Mg2+, etc.) to allow a good growth of plants under a sufficient water supply. The choice of this type of substrate was guided by its saturation rate, its ability to good soil water retention capacity and its pH, okra preferring slightly acidic to neutral soils (Hamon and Charrier, 1997). In addition, a soil of similar texture was used by Nana (2010) because the okra's roots manage to collect easily the water it contains.
Determination of the soil capacity of the soil
The soil field capacity will be determined with a PVC pipe 2 cm in diameter and 18 cm high approximately, with one of its ends closed with a nylon mesh screen having mesh smaller than 2 mm. The pipe will then be filled with soil up to 2 cm from the edge. The dry weight (X) of the earth will be determined and thereafter saturated with water. After draining for 48 h, the wet weight of the earth (Y) is determined. The soil field capacity (SFC) is calculated by the following formula:
Experimental device and treatments
Each genotype was subjected to three water regimes in a greenhouse using a split-plot design and 5 replications. Plants were grown in pots arranged in three randomized blocks. Each pot received 17 kg of homogenized dry soil as a growing substrate. Seedlings were sown on 5th August, 2017 at three seeds per pot, and thinned to one per pot two weeks after sowing (19 August, 2017). Plants were subjected to three water regimes beginning three weeks after sowing (vegetative stage); combining two factors: three-levels of water regime versus soil field capacity (100% SFC, 50% SFC and 25% SFC) and one-level watering frequency (every three days). Plants in pots receiving 100% SFC (control) were watered every three days with 3 L of water, while plants in treatments T1 and T2 were watered at 50% or 25% SFC with 1.5 or 0.75 L per pot, respectively. A sample of three plants (pots) of each genotype was selected for physiological evaluations.
Temperature and relative humidity inside the greenhouse
Given the influence of environmental factors, especially temperature and relative humidity on the effects of water treatments on plants, these two parameters were recorded daily from the induction of water stress at different times of day (7 am, 12 pm and 5 pm) using a thermohygrometer. Thus, during the induction period of water stress, temperature and relative humidity in the greenhouse oscillated respectively between 25.45 and 33.43°C and between 70 and 84.33%. The maximum temperature was recorded at 12 o'clock when the relative humidity was lower (Figure 1). On the other hand, humidity was higher at 7 o'clock when the temperature was relatively low.
Evaluation of growth parameters
Height of plants
The height of plants was measured at the vegetative stage, at the fructification and at the end of the cycle; respectively on 44th day after sowing (DAS), 65th DAS and 104th DAS using a measuring tape.
Number of leaves, length and width of leaves
Foliar performances of plants cultivated under the three hydric regimes were evaluated at the vegetative stage (44th DAS) and at the fruiting stage (65th DAS). These performances were the number of leaves per plant (NLea/P), leaves length (LLe) and leaves width (LWi).
(i) The number of leaves per plant (NLea/P) was determined in situ by counting leaves of each plant.
(ii) The length and width of the leaves (LLe and LWi) expressed in centimeters, were determined by in-situ measurements, using a graduated ruler.
Yield estimation
Yield evaluation consisted of the measurement of quantitative aspects such as capsule yield; seed yield and its components. The studied parameters were the following:
(i) Number of capsules per plant (NCap/P);
(ii) Number of seeds per capsule (NSe/Cap);
(iii) Weight of seeds per capsule (PSe/Cap) in grams;
iv) Weight of 100 seeds (W100Se) in grams.
The number was determined by counting and weighing was carried out using a precision balance of 0.01 g.
Data analysis
Collected data were subjected to an analysis of variance (ANOVA) using the software XLSTAT 7.5.8. The Fisher test at the 5% threshold was used for averaging discrimination. Graphics were made using the Excel 2016 spreadsheet.
Influence of water treatments on growth parameters
Height of plants
The height of plants subjected to watering 100% SFC (T1); 50% SFC (T2) and 25% SFC (T3) was measured at the vegetative and fruiting stages and at harvest.
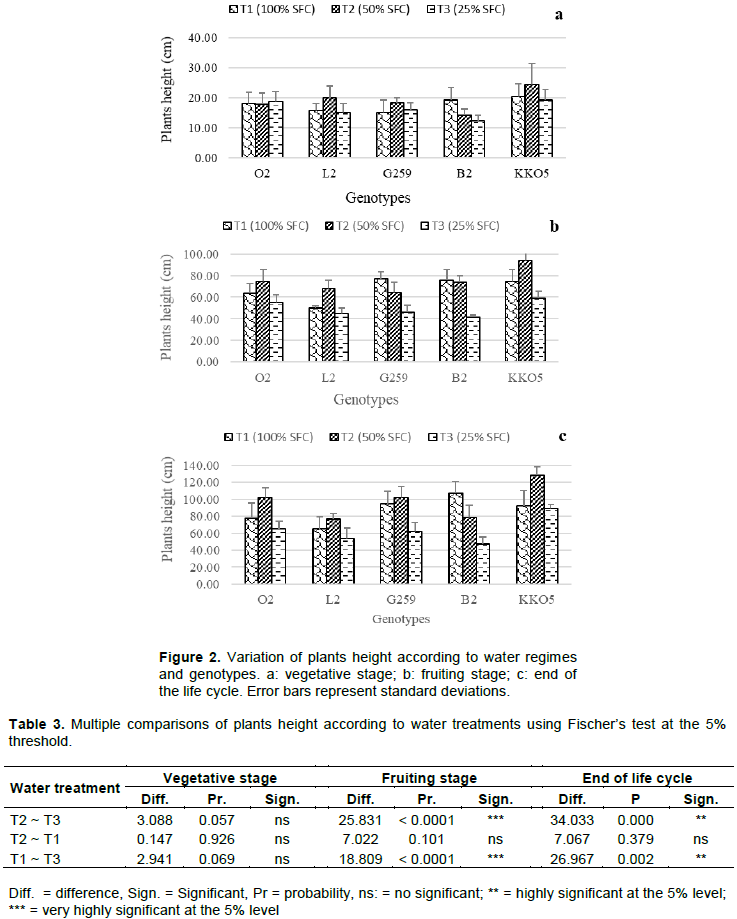
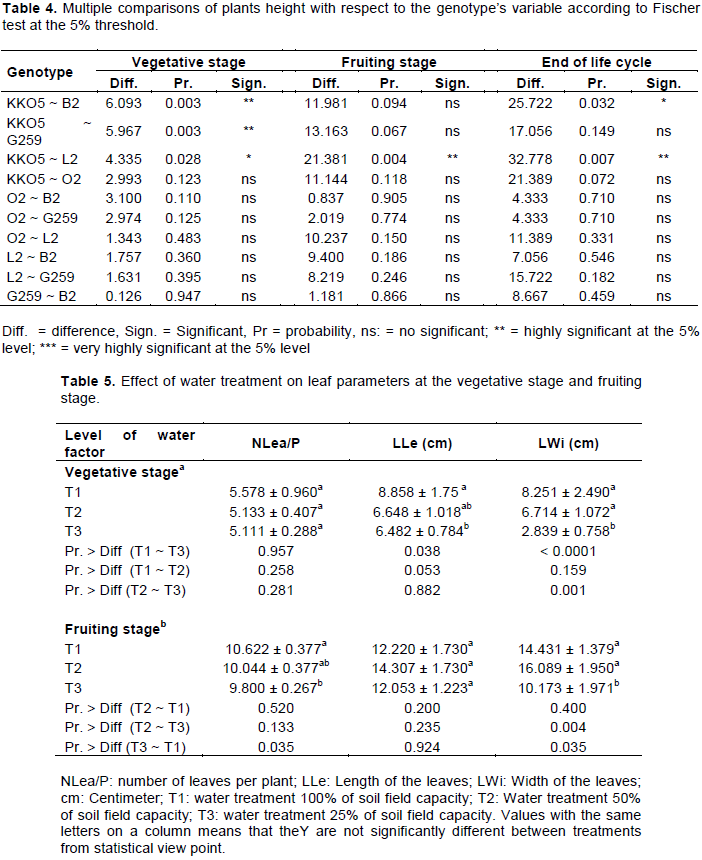
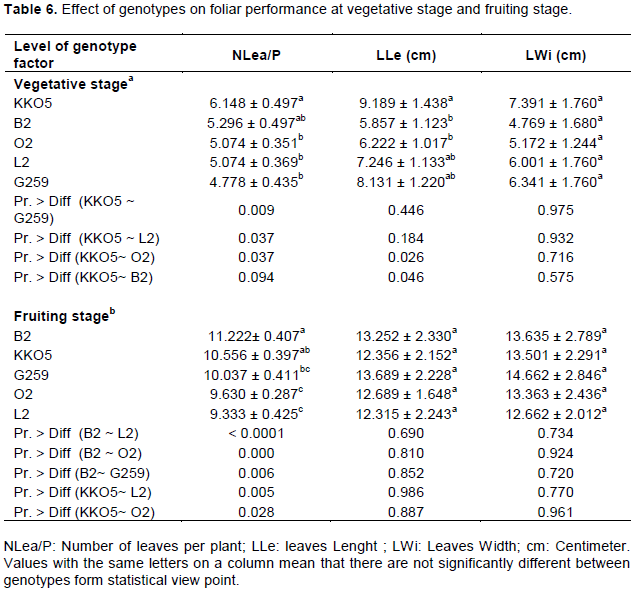
Results showed that at the vegetative stage, the decrease in water supply at 50 and 25% of the soil field capacity does not have a negative effect on the height of plants (Figure 2a). At this stage of development, plants of genotypes L2, G259 and KKO5 watered at 50% SFC, presented better performance in terms of height (Figure 2a).
At the fruiting stage and at harvest, plants of all five genotypes watered at 25% of the soil field capacity were the lowest (Figures 2b and 2c). Reducing the amount of water supplied to plants impacts negatively on their growth and normal development, compared to the non-limiting water regime (Son, 2010). However, the height of plants of genotypes O2, L2 and KKO5 watering at 50% SFC, was higher than that of plants of T1 and T3 treatments. Statistical analysis reveals a very highly significant difference between T2 and T3 water regimes (P < 0.0001) and between T1 and T2 treatments (P < 0.0001) for plants height to fruiting (Table 3); on the other hand, there is no significant difference between T1 and T2 water treatments for plant height at this stage of development. In addition, the comparison of plants height at the end of the life cycle shows the same trend as at the fruiting stage. Indeed, T2 and T3 water treatments had significantly different effects (P = 0.000) on height; as well as T1 and T3 treatments (P = 0.002).
Comparison of plants height based on the genotype factor (Table 4) showed that genotype KKO5 was distinguishable from genotypes B2, G259 and L2, but this depended on the stage of development of the plants. Indeed, at the vegetative stage there was a highly significant difference between plants height of KKO5 and that of B2 (P = 0.003); and between the height of KKO5 and that of G259 (P = 0.003). In addition, plants height of KKO5 and L2 were significantly different (P = 0.028) at the vegetative stage, very significantly different at the fruiting stage (P = 0.004) and at the end of the life cycle (P = 0.007). A significant difference was also revealed between plants height of KKO5 and plants height of B2 at the end of the life cycle (P = 0.032).
Foliar performance of plants
The number of leaves per plant (NLea/P), the length and width of the leaves (LLe and LWi) have varied according to water treatments (Table 5) and genotypes (Table 6). In the vegetative stage, the best performance of leaves was observed at the control treatment (T1). However, the effect of water stress was more pronounced on NLea/P, LLe and LWi, with low values when watering was done at 25% SFC (Table 5a and 5b). Analysis of variance revealed no significant difference between water treatments for the number of leaves per plant (NLea/P). On the other hand, for the length of the leaves (LLe), there was a significant difference between treatments T1 (control) and T3. In addition, water stress negatively affected width of the leaves. The reduction was of 65.59% under water regime at 25% SFC compared to 18.62% under water regime at 50% SFC. Fisher’s test reveals a very highly significant water regime effect between T1 and T3 (P < 0.0001) for leaf width (Table 5a).
At the fruiting stage, the same trend was observed for the three leaf parameters. However, analysis of variance did not reveal any significant difference between water treatments for number of leaves and leaf length. The width of the leaves of plants subjected to water treatment T2 was very significantly different (P = 0.004) from that of leaves of plants to treatment T3 (Table 5b). It also appears that the width of leaves of treatment T1 is significantly different (P = 0.035) from that of leaves of treatment T3.
Inter-genotypes comparison shows that at the vegetative stage, genotype KKO5 had a relatively larger number of leaves with relatively longer and wider leaves (Table 6a). On the other hand, genotype G259 had the low number of leaves per plant; and genotype B2 had shorter leaves. Analysis of variance shows a significant difference between genotypes KKO5 and L2 (P = 0.037); between KKO5 and O2 (P = 0.037) and highly significant (P = 0.009) between genotype KKO5 and genotype G259 for number of leaves per plant (NLea/P). At the fruiting stage, genotypes B2 and KKO5 had more leaves per plant, but the low number of leaves per plant was observed at genotypes O2 and L2 (Table 5b). Analysis of variance revealed a significant difference between KKO5 and O2 (P = 0.028); highly significant between B2 and O2 (P = 0.000), between B2 and G259 (P = 0.006), between KKO5 and L2 (P = 0.005); and highly significant between B2 and L2 (P <0.0001) for NLea/P. At the fruiting stage, for the length and width of the leaf, no significant difference was observed between genotypes.
Effect of water deficit on yields
Number of capsules per plant (NCap/P)
The number of capsules per plant (NCap/P) ranged from 4.20 to 6.00 in treatment T1 (100% SFC), with the most capsules recorded in genotype G259 (6 capsules on average). In plants subjected to watering 50% SFC, the number of capsules per plant ranged from 4.00 to 6.32. However, in plants subject to 25% SFC, the number of capsules per plant ranged from 3.33 to 5.00. The effect of water stress induced by watering 25% SFC, resulted in a decrease in the number of capsules per plant of four genotypes (Figure 3) compared to the control water regime (100% SFC). This reduction was 47% for each of genotypes L2 and KKO5; 17 and 5% respectively for genotypes O2 and G259. However, under water regime of 50% SFC, three genotypes (B2; L2 and KKO5) recorded a decrease in the number of capsules per plant of the order of 4, 5 and 33% respectively. In general, genotype G259 produced fewer capsules per plant, but the number of capsules per plant was less influenced by deficient water regimes (Figure 3). Analysis of variance shows that this genotype G259 is significantly different from genotypes O2 (P = 0.017) and L2 (P = 0.024).
Number of seeds per capsule and its components
Plants watered at 25% SFC had a reduced number of seed per capsule regardless of genotypes (Figure 4).
This reduction was approximately 30% for O2, L2 and G259; and about 60% each for both B2 and KKO5.
Regardless of water regime, KKO5 had the lowest number of seeds per capsule compared to O2 which had the highest number of seeds. Analysis of variance reveals a significant difference (P = 0.03) between these two genotypes (O2 and KKO5).
For seed yield components, water regime T3 (25% SFC) decreased seed weight per capsule (WSe/Cap) and weight of 100 seeds (W100Se) of all genotypes (Figures 5 and 6). On the other hand, with T2 water treatment (50% SFC), four genotypes (O2, G259, B2 and KKO5) had reduced WSe/Cap and W100Se compared to plants receiving the control treatment (100% SFC). In addition, genotype effect showed that the WSe/Cap and W100Se of genotypes L2 and KKO5 were lower than those of other genotypes. However, genotype effect was not significant for these two parameters.
Results show that capsule and seed yields were significantly influenced by water regimes (Table 7). Water stress imposition by decreasing the amount of water supplied to plants caused a significant reduction in the number of capsules per plant (NCap/P), number of seeds per capsule (NSe/Cap), weight of seeds per capsule (WSe/Cap) and the 100-seeds weight (W100Se) in treatments 50 and 25% SFC compared to the control treatment (100% SFC). This reduction was much more accentuated in the 25% SFC treatment for which, the difference with the control treatment (100% SFC) was very highly significant. Our results are consistent with those of Son et al. (2011) who had found that in sesame (Sesamum indicum), water stress induced by reducing water supply to 80, 60 and 40% SFC caused a reduction in capsules production and weight.
The present results showed a highly significant effect of water regime on plants height at fruiting, but also at the end of the life cycle. Indeed, the stress induced by the water supply at 25% SFC caused a reduction in the height of plants by 28% at fruiting and 30% at the end of the life cycle. Our results corroborate those of Son (2010) who observed a reduction in the height of sesame plants (S. indicum) when they were watered to less than 50% SFC. On the other hand, at the vegetative stage, no significant difference between water regimes was observed. These results may be justified by the fact that at the vegetative phase, measurements were made at 23 days after the beginning of the imposition of deficit water regimes, that is, 8 deficient water supplies; while at fruiting and at the end of the life cycle, measurements were made respectively at 44 and 83 days after the onset of stress, respectively 15 and 28 deficient water supplies. This assumes that water deficit has gradually increased over time. In addition, water requirements of juvenile plants are lower compared to their needs at reproductive stage during which metabolic reactions requiring water are higher. Based on our results, water treatment T3 (25% SFC) does not seem to favor the height growth of okra genotypes after their vegetative stage. This depressive effect of water stress on growth follows the loss of turgor of cells. Water stress induced by water supply at 50% SFC or 25% SFC did not have a significantly reducing effect on the number of leaves per plant at the vegetative stage as well as with fruiting in okra genotypes studied. However, Meftah (2012) found a decrease in number of leaves in cowpea grown under water stress. Gorai et al. (2010) have noticed a significant decrease in the number of leaves on a Medicago sativa crop irrigated at 40% SFC compared to controls. These results may be due to severe water stress suffered by plants. The number of leaves is a genetic trait (Lorgeou and Martin, 2005); therefore, it is not influenced by water regime. Under restrictive water regime conditions, the yield and its components were negatively impacted compared to the control water regime. Indeed, our results showed that the number of capsules per plant (NCap/P), the number of seeds per capsule (NGr/Cap), the seed weight per capsule (WSe/Cap) and the weight of 100 seeds (W100Se) were significantly reduced under water conditions at 25% SFC. Ohashi et al. (2009) found a significant decrease in seed yield on a soybean crop under water stress. According to Farooq et al. (2009), the reduction of seeds replenishment is attributable to a decrease in the distribution of photo assimilates between different reserve organs. Any water stress negatively affects key physiological phenomena such as photosynthesis and translocation of photo assimilates, which are directly related to fruits and seeds formation. Indeed, the transport of photo assimilates across the phloem plays a key role in crop productivity and yield (Hopkins, 2014). The significant decrease in the yield of capsules and its different components noted in the treatment 25% SFC could be explained by the concomitant reduction in plant height at the fruiting stage and at the end of the cycle, but also by the decrease in the number of capsules, leaves and leaves dimensions at the time of fruiting.
Inter-genotypes comparison showed a reduction in the number of capsules per plant by 47% for each of genotypes L2 and KKO5, 17 and 5% respectively for genotypes O2 and G259 under restrictive water regimes 25% SFC. On the other hand, under water regime 50% SFC, these are genotypes B2, L2, and KKO5 recorded a decrease in the number of capsules per plant of the order of 3, 5 and 33% respectively. In general, genotypes B2 and G259 were tolerant of water restriction while genotype KKO5 was the most sensitive to water deficit for capsule yield. In addition, genotypes O2 and L2 were less sensitive to water supply 50% SFC. This diversity in sensitivity to water deficit can be attributed to climatic zones where these genotypes are from. Indeed, genotype KKO5 comes from the Sudan's worst-weathered climate zone (> 900 mm rainfall/year) while genotypes G259 and B2 come from respectively the medium-watered areas (600 - 900 mm/year) and less watered (<600 mm/year). These last two genotypes seem already to be adapted to medium or low water regimes. In addition, independently of water regime, genotype G259 produces fewer capsules per plant compared to other genotypes. This result could be justified by an influence of genetic factors rather than by the water factor which is environmental. Processes involved in crop yield development are influenced by both genetic factors that are intrinsic to the plant and environmental factors (Radhouane et al., 2014).
From the results obtained, it appears that the restriction of water supply to 25% SFC negatively affects not only the height of plants at the fruiting stage and at the end of the life cycle, the length and the width of the leave but also, the yield in capsules, seeds yield and its components. In case of irrigated cultivation, restrictive water supply can be applied at the vegetative stage of okra, but these restrictive contributions should be avoided during the reproductive stage, especially during flowering-fruiting until full maturity. With regard to agronomic parameters, under the water deficit induced by water supply at 25% SFC, genotype KKO5 was the most sensitive; however, genotype that has been less sensitive was G259. In addition, genotypes O2, L2 and B2 showed some tolerance to water restriction at 50% SFC for capsules and seeds yields.
The authors have not declared any conflict of interests.
The authors are grateful to members of "Laboratoire Biosciences” for manuscript correction.
REFERENCES
|
FAO (2002). Eau et Agriculture : division de la mise en valeurs des terres et des eaux; 28 p.
|
|
Farooq M, Wahid A, Kobayashi N, Fujita D, Basra SMA (2009). Plant drought stress: Effects mechanisms and management effects of drought on plants. Agronomy for sustainable Development 29(1):185-212.
Crossref
|
|
|
|
|
Gorai M, Haceh A, Neffati M (2010). Differential responses in growth and water relationship of Medicago sativa (L.) cv Gabès and Astragalus gombiformis (Pom) under water limited conditions. Emirates Journal of Food and Agriculture 22:1-12.
Crossref
|
|
|
|
|
Hamon S, Charrier A (1997). Les gombos. In: Charrier, A., Jacquot, M., Hamon, S. & Nicolas, D. (Eds).: Amélioration des plantes tropicales. CIRAD et ORSTOM, Montpellier 1374-7:313-333.
|
|
|
|
|
Hopkins WG (2014). Physiologie végétale.
View
|
|
|
|
|
Lorgeou J, Martin B (2005). Précocité des variétés de maïs grain. Perspectives Agricoles 309 p.
|
|
|
|
|
Meftah Y (2012). Effet du stress hydrique sur le comportement de deux populations de niébé (Vigna unguiculata L.) inoculées par quatre souches rhizobia autochtones. Mémoire Magistère Agronomie, Ecole Nationale Supérieure Agronomique El-Harrach-Alger P 96.
|
|
|
|
|
Mefti M, Abdelguerfi A, Chebouti A (2001). Etude de la tolérance à la sécheresse chez quelques populations de Médicago truncatula (L.) Gaertn: In Delgado I, Lloveras J, (Eds) : Qualtity in lucerne and medics for animals production; Option Méditerranéennes: Séria A. Séminaires Méditerranéens 45:173-176.
|
|
|
|
|
Nana R (2010). Evaluation de la réponse au stress hydrique de cinq variétés de gombo [Abelmoschus esculentus (L.) Moench]. Thèse de Doctorat Unique, Université de Ouagadougou, Burkina Faso, 143 p.
|
|
|
|
|
Nana R, Zombre G, Tamini Z, Sawadogo M (2009a). Effet du régime hydrique sur les rendements du gombo en culture de contre-saison. Sciences and Nature 6(2):107-116.
Crossref
|
|
|
|
|
Nana R, Tamini Z, Sawadogo M (2009b). Effets d'un stress hydrique intervenu pendant le stade végétatif et la phase de floraison chez le gombo. International Journal of Biological and Chemical Sciences 3(5):1161-1170.
Crossref
|
|
|
|
|
Nelson GC, Rosegrant MW, Koo J, Robertson R, Sulser T Zhu T (2009). Climate change: Impact on Agriculture and costs of Adaptation. Washington: IFPRI, P 19.
|
|
|
|
|
Niang I (2009). Le changement climatique et ses impacts : les prévisions au niveau mondial. In adaptation au changement climatique; IEPF (ed). Liaison Energie- Francophonie 85:13-19.
|
|
|
|
|
Ohashi O, Nakayama N, Saneoka H, Mohapatra P, Fujita K (2009). Differences in the responses of stem diameter and pod thickness to drought stress during the filling stage in soybean plants. Acta Physiology Plant 31:271-277.
Crossref
|
|
|
|
|
Otieno DO, Schmildt MWT, Adiku S, Tenhunen J (2005). Physiological and morphological responses to water stress in two Acacia from contrasting habitats. Tree Physiology 25(3):361-371.
Crossref
|
|
|
|
|
Ouédraogo MH (2016). Etude de la diversité génétique des gombos [Abelmoschus. esculentus (L.) Moench] cultivés au Burkina Faso. Thèse de Doctorat Unique, Université Ouaga I Pr Joseph KI-ZERBO, 140 p.
|
|
|
|
|
Radhouane L, Aissa N, Romdhane L (2014). Effets d'un stress hydrique appliqué à différents stades de développement sur l'aspect quantitatif et qualitatif des semences chez un écotype autochtone de sorgho grain (Sorghum bicolor). Journal of Applied Biosciences 74:6149-6156.
Crossref
|
|
|
|
|
Radhouane L (2013). Comparaison de la nutrition minérale du mil (Pennisetum glaucum L. R. Br.) en présence de stress hydrique et salin. Journal of Applied Biosciences 6:5114-5129.
Crossref
|
|
|
|
|
Reynolds JF, Kemp PR, Ogle K, Fernandez RJ (2004). Modifying the "pulse-reserve" paradigm for deserts of the North America: precipitation pules, soil water and plant responses. Oecolozia 141(2):194-210.
Crossref
|
|
|
|
|
Son D, Compaoré E, Bonkoungou S, Sangaré S (2011). Effet du stress hydrique sur la croissance et la production du sésame (Sesamum indicum), Journal of Applied Biosciences 37:2460-2467.
|
|
|
|
|
Son D (2010). Effet du stress hydrique sur la croissance et la production du sésame [Sesamum indicum (L.)]. Mémoire de DEA, Université Polytechnique de Bobo-Dioulasso 40 p.
|
|
|
|
|
Trinchant JC, Boscari A, Spennato G, Van De Syppe C, Le Rudulier D (2004). Prolin betaine accumulation and metabolism in alfalfa plants under sodium chloride; exploring its compartmentalization in nodules. Plant Physiology 135(3):1583-1594.
Crossref
|
|
|
|
|
Ullah I, Ur-Rahman M, Ashraf M, Yusuf Zafar Y (2008). Genotypic variation for drought tolerance in cotton (Gossypium hirsutum L.): leaf gas exchange and productivity. Flora 203:105-115.
Crossref
|
|
|
|
|
Züllich G, Kopainsky B, Pedercini M (2012). Analyse de la vulnérabilité multisectorielle en vue de la formulation d'une stratégie nationale d'adaptations aux changements climatiques à moyen et à long terme à l'horizon de 2025 et 2050 du Burkina Faso. Programme d'Action National d'Adaptation à la variabilité et aux changements climatiques du SP/CONNED 70 p.
|
|
|
|