Full Length Research Paper
ABSTRACT
Nutrition plays a vital role in growth, development and the prevention of possible nutrient-deficiency diseases that may cause permanent health risks in the later stages of life. Due to this, the concentrations of iron, zinc, copper, manganese and β-carotene in four varieties of plantain were investigated in this study. The relationship between the micro-minerals in the fruit, the sampling location and soil were also studied. Plantain fruit samples were collected from farms in the Ashanti Region of Ghana. The samples were oven-dried and digested using the Kjeldahl method for micro-minerals analysis with atomic absorption spectrophotometer. The β-carotene content was determined using HPLC. The data obtained was subjected to analysis of variance to determine differences in the concentrations. The micro-minerals and β-carotene concentrations in the varieties of plantain did not vary significantly. Cluster analysis showed that some of the samples had almost the same nutrient concentrations though they were not of the same variety.
Key words: Anaemia, β-carotene, hidden hunger, micronutrients, nutrition, plantain, food security.
INTRODUCTION
The quest to fight malnutrition is a global concern which needs all hands to resolve. Malnutrition results from the inability to obtain the correct nutrients in the right proportion in diet. This may have a number of implications especially in developing countries where poor nutrition is predominant (Stein, 2010; Kalu and Etim, 2018; Mantadakis et al., 2020). It is important to note that inadequate intake of iron (Fe) is the prevailing nutritional deficiency in the world and the main cause of anaemia (Akhtar, 2013; Biesalski, 2013; Mantadakis et al., 2020; Mattiello et al., 2020). Likewise, zinc (Zn) deficiency in diet leads to various growth and reproduction disorders and often results in death, especially in developing countries (Oliver and Gregory, 2015; Wessels, 2021).
Copper (Cu), is another micro-mineral that maintains nerve cells, immune system and make red blood cells in the body (Scheiber et al., 2014; Greenough et al., 2016; Morrell, et al., 2017; Sigdel and Janaswamy, 2020; Espinosa and Stein, 2021). It also helps in the formation of collagen and Fe absorption in the body and also plays a role in energy production (Ware, 2017; Moustarah and Mohiuddin, 2019). Although high level of Cu can affect brain function, its deficiency can also lead to Menker’s, Wilson’s and Alzheimer’s disease (Morrell et al., 2017). Manganese (Mn) is also another micro-mineral needed for digestion, bone growth, immune system functioning, cellular energy regulation, reproduction and blood clotting (Aschner and Aschner, 2005; Sigdel and Janaswamy, 2020). It is a co-actor for the activation of a number of enzymatic reactions in the body such as amino acid, lipid and carbohydrate metabolism (Yoon et al., 2011; Ge et al., 2022). Studies have shown that the element is good for pregnant women but excessive accumulation of manganese in the nervous system may cause Parkinson’s type syndrome called Manganism (Aschner and Aschner, 2005).
It is established that the visual and immune systems are particularly dependent on vitamin A to function (Kennedy et al., 2003; Biesalski and Nohr, 2004; Huang et al., 2018) and a deficiency may lead to blindness in humans. Although this vitamin is freely available in animal products like milk, egg and fish, over 40% of children under age five years suffer from vitamin A deficiency (Black et al., 2013). This could be due to the fact that animal foods are expensive and therefore not affordable to the poor in developing countries. However, there is a precursor of vitamin A in plant products which may help improve the situation of vitamin A deficiency in poor countries and also create jobs for local farmers if they cultivate the plants that have precursors of vitamin A. According to Biesalski (2013), 80% of vitamin A in developing countries is from provitamin A which is found in plants. Vitamin A deficiency is considered as a major global health problem (Akhtar, 2013; Biesalski, 2013; Timoneda et al., 2018). Lack of vitamin A in a diet leads to an increased risk of eye disease and death from serious infections causing increased mortality rate among children and women in under-developed countries (Biesalski, 2013). This deficiency, if not corrected could lead to blindness (Lewallen and Courtright, 2001; Biesalski, 2013; Timoneda et al., 2018).
Plantain is among staple foods that are grown in many parts of the world (Tenkouano et al., 2019). It is an important source of nutrients in diets of people from Latin America, Africa and South-east Asia (Abiodun-Solanke and Falade, 2010). Plantain is a popular dietary staple food due to its versatility and good nutritional value. The fruit has high carbohydrate content (31 g/100 g) and low-fat content (0.4 g/100 g). They are good sources of vitamins and minerals, particularly iron (24 mg/kg), potassium (9.5 mg/ kg), calcium (715 mg/kg), vitamin A, phosphorus, zinc, sodium, and magnesium (Okareh et al., 2015). It has significant quantities of ascorbic acid, thiamin, pyridoxine, riboflavin and niacin, dietary fiber and resistant starch which helps to reduce the blood sugar level (Ayodele and Erema, 2011). The potassium in plantain plays a major role in regulating blood pressure (Fernandes and Rodrigues, 2007; Houston, 2011).
It had been established that, soil structure and physicochemical properties have great impact on plant growth and development (Wolkowski, 1990; Oldfield et al., 2019) as well as nutrient stored for consumption by consumers. The need to ensure high crop production and food security has intensified cropland management and food production which consequently results in soil degradation (Oldfield et al., 2019). The situation in Ghana is not different from the global picture where the government is embarking on “planting for Food and Jobs” to ensure food security. In so doing, “planting for Food and Jobs” uses fertilizer to enhance soil fertility. Potential contribution of building soil organic matter (SOM) as a means to increase crop production and minimize the environmental impact on agriculture has not yet been broadly quantified (Adhikari and Hartemink, 2016; Chabbi et al., 2017; Oldfield et al., 2019).
In Ghana where this study was conducted, plantain plays an important role in national food security. It also provides income to local farmers. Although farmers cultivate different varieties, they do not consider the nutritional content of the varieties cultivated. They also do not pay attention to planting location, cropland management, soil nutrient and nutrient quality of the crop consumed. Therefore, this study sought to investigate the concentrations of Fe, Zn, Cu, Mn and β-carotene in four varieties of plantain cultivated and consumed in Ghana. It also sought to investigate the relationship between trace and macro-elements in soil and plantain crop from different communities.
MATERIALS AND METHODS
Sampling of plantain
Four varieties of plantain, Musa paradisiaca var. “Apem”, M. paradisiaca var. “Apantu”, M. paradisiaca var. “Asamienu” and M. paradisiaca var. “Oniaba” and the soils in which they were planted were collected from different fields in Ashanti Region using purposive sampling for analysis of nutrients. These crops were sampled from Adudwan, Atonsuagya, Benim, Juaben, Kofiase, Krobo, Mampong, Nintin, New Koforidua and Obuasi in the Ashanti Region of Ghana.
Analysis of micronutrients
To analyse the micro-minerals (that is, Fe, Zn, Mn, Cu), the plantain samples were peeled, cut into pieces (ca. 1 cm × 1 cm), packaged in brown envelopes and oven dried at 60°C to remove moisture until a constant weight was obtained. The dried samples were then milled using a Kenwood blender, packaged and labeled for analysis. The samples were digested using the sulphuric acid-hydrogen peroxide method (Allen et al., 1974; Lowther, 1980). Thus, 0.10 g of oven-dried samples was placed into a 100 ml Kjeldahl flask. A volume of 4.4 ml of digestion reagent comprising of a mixture of 350 ml hydrogen peroxide, 0.42 g selenium powder, 14 g lithium sulphate and 420 ml sulphuric acid was added and heated gently at 80 to 90°C. The temperature was gradually increased to 150 to 200°C and held for 2 h until the digest was clear. The samples were then left on the plate for 30 min to cool. The digest was topped up to 50 ml with distilled water for further analysis. The filtrate from the digestion was used for Fe, Zn, Mn and Cu analysis using a 200 series Atomic Absorption Spectrophotometer (AAS) (Agilent Technologies, Santa Clara, CA). The digestion and analysis were repeated three times per sample and the mean value used for statistical analysis.
The micro-minerals in soil were extracted using diethylene triamine pentaacetic acid (DTPA) following Lindsay and Norvell (1978). An amount of soil sample (10 g) was placed in a 50 ml graduated conical centrifuge tube and 20 ml of DTPA extracting solution was added to it. The centrifuge tube and its content were shaken for 2 h on a shaker. The contents were then filtered. The extract obtained was used for estimation of different micronutrients using the AAS following (Motsara and Roy, 2008). To estimate the concentration of a specific micro-element, the element-specific hollow cathode lamp was selected and mounted on the AAS. When the flame was started, the instrument was set at zero using a blank solution. The standard solutions of different concentrations were aspirated and a standard concentration curve was plotted for the element. The samples were then aspirated and the concentration of the samples were read and recorded.
β-carotene analysis
The carotenoids in the plantain samples were extracted following Rodriguez-Amaya and Kimura (2004). Five grams (5 g) of the sample was weighed into a mortar (Fisher Scientific, Leicestershire, England) and 0.5 g of pyrogallol (Merck, Darmstadt, Germany) was added and mixed. Furthermore, 20 ml of ice-cold acetone (VWR, Leicestershire, England) was added to the mixture and left for 5 min. The extract was filtered using Whatman® filter paper (GE Healthcare Bio-Sciences, Pittsburgh, PA) after which the residue was washed twice with acetone till it became colourless. A total of 50 ml of acetone was used for the extraction. The residue was discarded and the filtrate washed in 15 ml of petroleum ether (Merck) in a 500 ml-separating funnel (Fisher Scientific). Deionized water was added slowly (by the side of the funnel to avoid emulsification of the carotenoid) to wash the acetone used for the extraction. The washing was repeated 6 to 9 times until all the acetone was washed out. The carotenoid extract dissolved in the petroleum ether was filtered through 2 g of anhydrous sodium sulphate (Merck). The volume of the filtrate was then recorded and the extract dried on nitrogen gas.
The dried sample was reconstituted with 1 ml hexane, vortexed and transferred into a 20 ml vail (Agilent) for HPLC analysis using an Agilent 1100 series HPLC system. The analyses were performed on a tracer excel 120 ODS-A column (25 cm × 0.46 cm, 5μm particle size, Teknokroma, Barcelona, Spain) at a temperature of 25°C. The mobile phase was a mixture of methanol, hexane and methyl ether amine (Merck) in a ratio of (90: 10: 0.01 v/v/v). The flow rate of the mobile phase was 1 ml/min. The total analysis time per sample was 35 min. Hexane was analysed as a blank in order to identify chromatograms coming from the samples. The chromatogram of β -carotene was identified by comparing the retention times of chromatograms from the samples with that of a beta-carotene standard (Merck).
For the purposes of quantification, calibration curve was obtained using solutions containing a carrot standard at a concentration of 0.17 µg/ml in hexane, 3 injections per concentration. The concentration of beta-carotene in each sample was calculated based on the linear relationship between the peak area of the standard and that of the sample.
Determination of macro-elements
Determination of phosphorous (P), potassium (K) and sodium (Na)
Digest from the sulphuric acid–hydrogen peroxide method was used for the determination of potassium, phosphorus and sodium. Potassium and Sodium concentrations were determined using the flame photometer whiles Phosphoros was determined using the spectrophotometer.
Phosphorus
Phosphorus was determined using the ascorbic acid method
Colour forming reagent referred to as reagents ‘A’ and ‘B’ were used for the test. Twelve grams (12 g) of ammonium molybdate in 250 ml distilled water, 0.2908 g of potassium antimony tartarate in 100 ml distilled water and I L of 2.5 M H2SO4 constituted Reagent A. The three solutions were mixed together in a 2 L volumetric flask and made up to volume with distilled water. Reagent B was prepared by dissolving 1.056 g of ascorbic acid to every 200 ml of reagent A. Ten milliliters (10 ml) aliquot of the samples were pipetted into 25 ml volumetric flasks. 10 ml of distilled water was pipetted into each of the working standards to give sample and standard the same background solution.
Ten milliliters (10 ml) of distilled water was added to the standards as well as the samples after which 4 ml of reagent B was added and their volumes made up to 25 ml with distilled water and mixed thoroughly. The flasks were allowed to stand for 15 min for colour development after which the absorbance of the standards and samples were determined using a spectrophotometer at a wavelength of 882 nm.
Determination sulphate (SO4)
The turbidimetric method was used. 5 ml of the sample was measured out of 100 ml extracted sample from the volumetric flask. 10 ml of sodium acetate buffer solution was added to the sample and 1.0 g of barium chloride (BaCl2) was added to the extract and diluted with distilled water to 25 ml. The absorbent was read using AAS (Shimaduze, UV-1800 Series, Japan) at a wavelength 440 nm.
Statistical analysis
The data obtained from the micronutrient and β-carotene analysis were compared using analysis of variance followed by Turkey's test where statistical differences were obtained (Minitab version 17; Minitab Inc., State College, PA). The cluster analysis using Euclidean distance was performed with a pulled data of β-carotene concentration, total carotene concentration in 100 g, wet weight of the plantain samples; dry weight of the plantain samples, percentage moisture content (%), ash content and the concentrations of phosphorus, potassium, sodium, iron, copper and zinc in the samples.
RESULTS AND DISCUSSION
Concentration of micronutrient in plantain
Micronutrient deficiency is a major health challenge to humans due to the damage associated with it in the later ages of individuals. This is so because Fe is needed for blood formation while Zn serves as a co-enzyme for a number of biochemical reactions in the body. β-carotene is also said to be one of the provitamin A percussors obtained from plants. The consumption of β-carotene helps to improve eye sight. A deficiency in β-carotene may lead to eye disease or blindness (Lewallen and Courtright, 2001; Biesalski, 2013; Dewett et al., 2021).
The concentration of each of the micro-minerals, Fe, Zn, Mn, Cu studied was not significantly different in all four varieties of plantain (Fe: F = 0.95, d.f. = 3, P = 0.428; Zn: F = 0.15, d.f. = 3, P = 0.927; Mn: F = 0.35, d.f. = 3, P = 0.789; Cu: F = 0.28, d.f. = 3, P = 0.841; Figure 1).
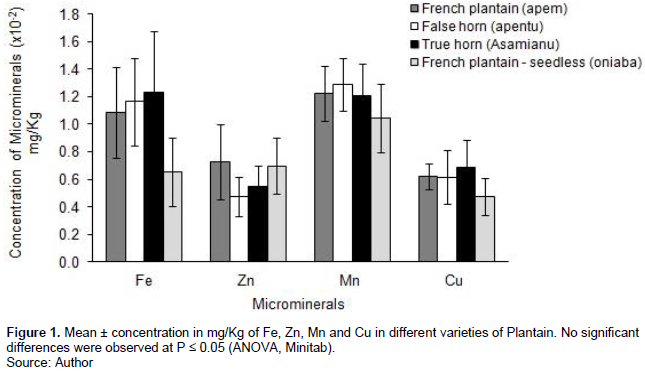
Plantains do well in a wide geographical area such as the tropics and serve as a staple food for some developing and tropical countries in the world (Englberger et al., 2006; Adepoju et al., 2012). The results from the analyses of the micronutrient concentrations of four varieties showed that French plantain, false horn, and true horn had higher concentrations of Fe than the French plantain-seedless although not statistically significant. This implies that the variety does not have any effect on the micronutrients obtained by the individual and that all four varieties can be used to manage iron deficiency. Findings from this study support the report by Okareh et al. (2015) that plantain is rich in nutrients such as Fe. The Mn concentrations in the four varieties were also high. The findings of the study were similar to the results obtained by Odenigbo et al. (2013) that reported that plantains are a rich source of Fe and vitamin A. Iron and Mn are micronutrients that help improve blood hemoglobin level for proper metabolic functioning of the body and enhance growth. This implies that the addition of plantain in the diet of pregnant women and nursing mother helps to reduce the potential risk of micronutrient deficiency. This explains why in Ghana the Ashantis give plantain-rich foods such as ‘fufu’, ‘ampesi’ and mashed plantain popularly called ‘et?’ in the twi language to pregnant women and breastfeeding mothers.
Analysis of beta-carotene content in plantain
The concentrations of beta-carotene in all four varieties of plantain were not significantly different (F = 1.17, d.f. = 3, P = 0.33; Figure 2).

β-carotene is one of the antioxidants obtained from plant food that helps in the prevention and management of eye problems. Plantain could contribute to the nutritional quality of the people in Ghana and help manage micro nutrient deficiency problems considering the micronutrients in the fruit. This is because carotenoids in the plant help in the production of provitamin A which is needed for biochemical processes in humans. Provitamin A carotenoids, most importantly beta-carotene, followed by alpha-carotene, are those which are converted into vitamin A in the body and help protect humans against infection, night blindness and eye disease (Newilah et al., 2009; Biesalski, 2013; Huang, 2018). The addition of these carotenoids in diet will help reduce the risk of degenerative diseases such as cancer, cardiovascular disease, cataracts and muscular degeneration (Silalahi, 2002; Bhatt and Patel, 2020; Chaudhary et al., 2020).
There was higher concentration of β-carotene in “Apem” (French plantain), “Apantu” (False orn), and “Oniaba” (seedless French plantain) varieties of Musa paradisiaca than “Asamianu” (True horn) although the variations in concentrations were not significantly different. This implies that the variety of plantain eaten by an individual does not affect the amount of vitamin A obtained from the food.
Cluster analysis
The results of the cluster analysis showed four major clusters at 66.67% similarity in nutrient concentration.
The clusters with the four major groups from the dendrogram had samples from the four different varieties of plantain fruits used for the study. Samples AM 3, ON 2, and EM 17 clustered at 99% similarity while AAU 1, EM8, EM 10 and EM3 clustered with a similarity of 99.51%. In another cluster, AU 11, EM 5 and ON 3 clustered at a similarity of 99.81 closeness in nutrient concentration. On the other hand, sample ON 11 did not cluster with any other sample from the same or different variety after the 61% similarity (Figure 3).
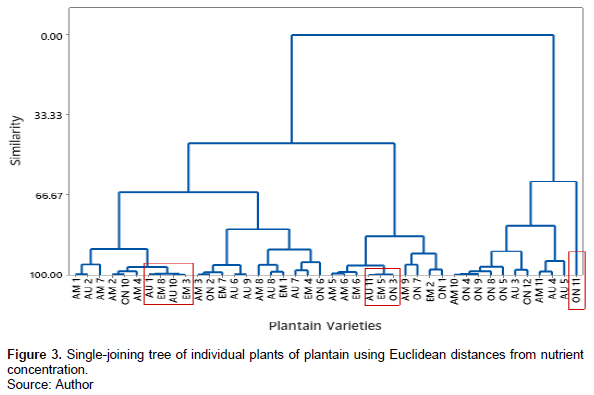
Cluster analysis showed almost perfect similarity between AU11, EM 5 and ON3. This implies that these samples from the different varieties picked from different locations contained almost the same nutrient. It can also be deduced from the results that AG-AM-1 and AG-ON-1 which were different varieties had similar nutrient profile. AG-AM-1 clustered in group 2 and AG-ON-1 clustered in group 4 from the dendrogram produced.
The significant differences observed in the concentrations of Fe, Cu, Zn, P, Na and S in the crop samples from the different locations where the plantain crops were sampled shows that the place of planting has an influence on the concentration of nutrient obtained by the plant. The results support the report of the soil structure. Moreover, physicochemical characters determine the soil nutrients made available to plant (Wolkowski, 1990; Oldfield et al., 2019). There was also a significant difference between the soil pH and electrical conductivity. This eventually influences the nutrient quality of the crop produced by plants.
The results in Table 1 show the concentrations of micro-elements (Fe, Cu and Zn) in plantain crop sampled from different locations in Ashanti Region. There were significant differences in the concentrations of Fe, Cu and
Zn in plantain crops from the different locations (Table 1).
Table 2 show the mean concentration of macro elements (N, P. Na and S) in the plantain crops sampled from the different communities. There were significant differences in the concentrations of N, P, Na and S in the plantain crop from the different locations (Table 2).
The results of correlation of primary minerals in the soil and the plantain crops show significant negative correlation between potassium concentrations in the crop against nitrate, pH in the soil and electrical conductivity in the soil (Table 3). There was negative correlation between total nitrogen in the crop against nitrate, pH in the soil and electrical conductivity. pH and electrical conductivity in the soil against P in the crop also showed negative correlation. There was statistically significant correlation between total nitrogen in the crop and K concentration in the crop and pH in the soil and electrical conductivity. There was highly significant correlation between S in the soil and S in the crop (Table 4). The same was observed in pH in the soil and electrical conductivity. Sulphur in the soil showed insignificant negative correlation with sodium in the crop. Sodium in the crop also showed insignificant negative correlation with pH in the soil and electrical conductivity.

Table 5 shows the Pearson’s correlation of the micro-elements in the crop and soil. Iron in the soil showed negative non-significant correlation with iron in the crop, copper in both the soil and the crop, zinc in both the soil and the crop and pH. Though iron in the soil showed negative correlation with electrical conductivity in the soil, it was highly significant. Copper in the crop also showed negative non-significant correlation with Zn and pH in the soil. There was positive significant correlation between copper in the soil and pH and electrical conductivity and pH.

CONCLUSION
All the varieties of plantain studied were rich in Fe, Zn and β-carotene. It is important for people to diversify their diets to include plantain dishes to help improve nutrition and minimize future health challenges which could occur due to micronutrient deficiency. The variety consumed does not have significant effect on the nutrient obtained by consumers. The planting location and quality soil has significant effect on the quality of nutrient obtained by the crop. It is therefore important to consider the planting location and soil quality to ensure quality nutrition and food security.
ACKNOWLEDGEMENT
The author is grateful to NUFFIC for supporting this research under the NICHE-GHA-195-205 grant. Also grateful to all the field assistants who helped in the data collection.
CONFLICT OF INTERESTS
The author has not declared any conflict of interests.
REFERENCES
|
Abiodun-Solanke AO, Falade KO (2010). A review of the uses and methods of processing banana and plantain into storable food products. Journal of Agriculture and Social Science 9(2). |
|
|
Adepoju OT, Sunday BE, Folaranmi OA (2012). Nutrient composition and contribution of plantain (Musa paradisiacea) products to dietary diversity of Nigerian consumers. African Journal of Biotechnology 11(71):13601-13605. |
|
|
Adhikari K, Hartemink AE (2016). Linking soils to ecosystem services-A global review. Geoderma 262:101-111. |
|
|
Akhtar S (2013). Zinc status in South Asian populations-an update. Journal of Health, Population, and Nutrition 31(2):139. |
|
|
Allen SE, Grimshaw HM, Parkinson JA, Quarmby C (1974). Chemical Analysis of Ecological Materials, (ed. SE Allen) Blackwell Scientific Publications. |
|
|
Aschner JL, Aschner M (2005). Nutritional aspects of manganese homeostasis. Molecular Aspects of Medicine 26(4-5):353-362. |
|
|
Ayodele OH, Erema VG (2011). Glycemic indices of processed unripe plantain meals. African Journal of Food Science 4:514-521. |
|
|
Biesalski HK (2013). Hidden hunger. In Hidden hunger (pp. 25-50). Springer, Berlin, Heidelberg. |
|
|
Biesalski HK, Nohr D (2004). New aspects in vitamin A metabolism: the role of retinyl esters as systemic and local sources for retinol in mucous epithelia. Journal of Nutrition 134(12):3453S-3457S. |
|
|
Bhatt T, Patel K (2020). Carotenoids: potent to prevent diseases review. Natural Products and Bioprospecting 10(3):109-117. |
|
|
Black RE, Victora CG, Walker SP, Bhutta ZA, Christian P, De Onis M, Ezzati M, Grantham-McGregor S, Katz J, Martorell R, Uauy R (2013). Maternal and child under nutrition and overweight in low-income and middle-income countries. The Lancet 382(9890):427-451. |
|
|
Chabbi A, Lehmann J, Ciais P, Loescher HW, Cotrufo MF, Don A, SanClements M, Schipper L, Six J, Smith P, Rumpel C (2017). Aligning agriculture and climate policy. Nature Climate Change 7(5):307-309. |
|
|
Chaudhary B, Chaudhary P, Chauhan A (2020). Review on Importance of Carotenoids in Health and Medicine. |
|
|
Dewett D, Lam-Kamath K, Poupault C, Khurana H, Rister J (2021). Mechanisms of vitamin A metabolism and deficiency in the mammalian and fly visual system. Developmental Biology 476:68-78. |
|
|
Englberger L, Wills RB, Blades B, Dufficy L, Daniells JW, Coyne T (2006). Carotenoid content and flesh color of selected banana cultivars growing in Australia. Food and Nutrition Bulletin 27(4):281-291. |
|
|
Espinosa CD, Stein HH (2021). Digestibility and metabolism of copper in diets for pigs and influence of dietary copper on growth performance, intestinal health, and overall immune status: a review. Journal of Animal Science and Biotechnology 12(1):1-12. |
|
|
Fernandes FA, Rodrigues S (2007). Ultrasound as pre-treatment for drying of fruits: dehydration of banana. Journal of Food Engineering 82(2):261-267. |
|
|
Ge EJ, Bush AI, Casini A, Cobine PA, Cross JR, DeNicola GM, Dou QP, Franz KJ, Gohil VM, Gupta S, Kaler SG (2022). Connecting copper and cancer: from transition metal signalling to metalloplasia. Nature Reviews Cancer 22(2):102-113. |
|
|
Greenough MA, Munoz AR, Bush AI, Opazo CM (2016). Metallo-pathways to Alzheimer's disease: lessons from genetic disorders of copper trafficking. Metallomics 8(9):831-839. |
|
|
Houston MC (2011). The importance of potassium in managing hypertension. Current hypertension reports 13(4):309-317. |
|
|
Huang Z, Liu Y, Qi G, Brand D, Zheng SG (2018). Role of Vitamin A in the Immune System. Journal of Clinical Medicine 7(9):258. |
|
|
Kalu RE, Etim KD (2018). Factors associated with malnutrition among underfive children in developing countries: A review. Global Journal of Pure and Applied Sciences 24(1):69-74. |
|
|
Kennedy G, Nantel G, Shetty P (2003). The scourge of "hidden hunger": global dimensions of micronutrient deficiencies. Food Nutrition and Agriculture (32):8-16. |
|
|
Lewallen S, Courtright P (2001). Blindness in Africa: present situation and future needs. British Journal of Ophthalmology 85(8):897-903. |
|
|
Lowther JR (1980). Use of a single sulphuric acid?hydrogen peroxide digest for the analysis of Pinus radiata needles. Communications in Soil Science and Plant Analysis 11(2):175-188. |
|
|
Mantadakis E, Chatzimichael E, Zikidou P (2020). Iron deficiency anemia in children residing in high and low-income countries: risk factors, prevention, diagnosis and therapy. Mediterranean Journal of Hematology and Infectious Diseases 12(1). |
|
|
Mattiello V, Schmugge M, Hengartner H, von der Weid N, Renella R (2020). Diagnosis and management of iron deficiency in children with or without anemia: consensus recommendations of the SPOG Pediatric Hematology Working Group. European Journal of Pediatrics 179(4): 527-545. |
|
|
Morrell A, Tallino S, Yu L, Burkhead JL (2017). The role of insufficient copper in lipid synthesis and fatty?liver disease. IUBMB life 69(4): 263-270. |
|
|
Motsara MR, Roy RN (2008). Guide to laboratory establishment for plant nutrient analysis. Food and agriculture organization of the United Nations, 204. FAO Fertilizer and Plant Nutrition Bulletin No. 19. ISBN 978-92-5-105981-4. |
|
|
Moustarah F, Mohiuddin SS (2019). Dietary iron. |
|
|
Newilah GN, Dhuique-Mayer C, Rojas-Gonzalez J, Tomekpe K, Fokou E, Etoa FX (2009). Carotenoid contents during ripening of banana hybrids and cultivars grown in Cameroon. Fruits 64(4):197-206. |
|
|
Odenigbo AM, Asumugha VU, Ubbor S, Ngadi M (2013). In vitro starch digestibility of plantain and cooking-banana at ripe and unripe stages. International Food Research Journal 20(6):3027. |
|
|
Okareh OT, Adeolu AT, Adepoju OT (2015). Proximate and mineral composition of plantain (Musa Paradisiaca) wastes flour; a potential nutrients source in the formulation of animal feeds. African Journal of Food Science and Technology 6(2): 53-57. |
|
|
Oldfield EE, Bradford MA, Wood SA (2019). Global meta-analysis of the relationship between soil organic matter and crop yields. Soil 5(1):15-32. |
|
|
Oliver MA, Gregory PJ (2015). Soil, food security and human health: a review. European Journal of Soil Science 66(2):257-276. |
|
|
Scheiber IF, Mercer JF, Dringen R (2014). Metabolism and functions of copper in brain. Progress in Neurobiology 116:33-57. |
|
|
Sigdel A, Janaswamy S (2020). Micro minerals. Scholarly Journal of Food and Nutrition 2(5)-2020 272-277. |
|
|
Silalahi J (2002). Anticancer and health protective properties of citrus fruit components. Asia Pacific Journal of Clinical Nutrition 11(1):79-84. |
|
|
Stein AJ (2010). Global impacts of human mineral malnutrition. Plant and Soil 335(1-2):133-154. |
|
|
Tenkouano A, Lamien N, Agogbua J, Amah D, Swennen R, Traoré S, Thiemele D, Aby N, Kobenan K, Gnonhouri G, Yao N, Astin G, Sawadogo-Kabore S, Tarpaga V, Issa W, Lokossou B, Adjanohoun A, Amadji GL, Adangnitode S, Igue KAD, Ortiz R (2019). Promising high-yielding tetraploid plantain-bred hybrids in West Africa. International Journal of Agronomy. Article ID 3873198, 8 p. |
|
|
Timoneda J, Rodríguez-Fernández L, Zaragozá R, Marín MP, Cabezuelo MT, Torres L, Juan RV, Barber T (2018). Vitamin A deficiency and the lung. Nutrients 10(9):1132. |
|
|
Ware M (2017). Health benefits and risks of copper. Medical News Today. Available at: |
|
|
Wessels I (2021). Metal Homeostasis during Development, Maturation, and Aging: Impact on Infectious Diseases. race Metals and Infectious Diseases. |
|
|
Wolkowski RP (1990). Relationship between wheel?traffic?induced soil compaction, nutrient availability, and crop growth: A review. Journal of Production Agriculture 3(4):460-469. |
|
|
Yoon M, Schroeter JD, Nong A, Taylor MD, Dorman DC, Andersen ME, Clewell III HJ (2011). Physiologically based pharmacokinetic modeling of fetal and neonatal manganese exposure in humans: describing manganese homeostasis during development. Toxicological Sciences 122(2):297-316. |
|
Copyright © 2024 Author(s) retain the copyright of this article.
This article is published under the terms of the Creative Commons Attribution License 4.0