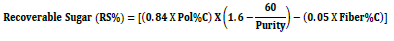ABSTRACT
Sugarcane chemical ripening is aimed at accelerating the sucrose accumulation in the stems for harvesting when the climate conditions of crop year are hardly optimal for natural ripening. The study aims at determining the best response of sugarcane varieties to glyphosate ripening effect and the harvest delay after its application in Ferké 2 Sugar Bowl, Northern Côte d’Ivoire, in order to improve the technological qualities of canes at the early harvest season. Twenty plantations (700 ha) hosting two commercial varieties NCo376 and SP711406 were treated with glyphosate (360 emulsion concentrated) at 0.8 L/ha. For each plantation, two sample plots of 1 ha control and treated were determined. Those samples were analyzed to determine the sucrose gradient all along the stalks and monitor their technological qualities after 10, 15 and 20 days. As results, glyphosate improved sucrose content and the recoverable sugar of treated varieties. SP71-1406 was more sensitive than NCo376 with uniform qualities all along stalks after 20 days. Gains of 1.6% sucrose content and 1.5% recoverable sugar were obtained, compared to the control. So, the uppermost parts preservation of harvested stalks is justified and a sugar gain of 0.13 t/ha except those generated by the ripener.
Key words: Glyphosate, ripener, technological quality, gradient, sucrose, recoverable sugar.
Glyphosate¦ (¦N-phosphonométhyl glycine, C3H8NO5P) is a glycine analogue¦¦. It is considered to be the most used herbicide worldwide for its biological efficiency as total weed-killer, its affordable cost and low toxicity (Goscinny and Hanot, 2012; Guimaraes et al., 2005).
It is used at low dose for cereal or oleaginous cropping, like desiccant for pre-harvest (Steinmann et al., 2012). The diversified exploitation of the glyphosate properties made it a multiform uses pesticide in agriculture.
In sugarcane cropping, the application of glyphosate at the end of crop cycle before tillage has helped to develop practices of zero tillage and minimum tillage in view of reducing production costs (Almeida et al., 2005). At low dose (0.8 to 1 L/ha), glyphosate has long been used as sugarcane ripener in order to carry out the harvest when climatic conditions are unfavorable to the natural ripening process (low daily thermal difference, soil moisture, high relative humidity of the air) (Meschede et al., 2010)¦. Glyphosate once absorbed by the leaves of the cane, is the only herbicide that can block the activity of the enolpyruvylshikimate-3-Phosphate synthase (EPSPS). This enzyme is located at the beginning of shikimic acid path and that of pentose phosphates involved in the conversion of carbohydrate precursors derived from glycolysis into aromatic amino acids. The enzyme is a priori in the chloroplasts where it catalyzes the combination of shikimate-3-phosphate (S3P) with phosphoenol pyruvate to form 5-enolpyruvylshikimate-3-phosphate (ESP). The latter is a precursor of aromatic amino acids (tryptophan, phenylalanine and tyrosine), hormones, vitamins and other essential metabolites in plants. Structural similarities with phosphoenol pyruvate enable glyphosate to be attached to the fixation site of the EPSPS substrate, to inhibit its activity and thereby block its translocation into the chloroplast. By blocking the activity of the EPSPS, glyphosate therefore prevents the degradation of sugars synthesized and stored in the sugarcane stalks into aromatic amino acids. The constant presence of the active site of the EPSPS enzyme in plants enables glyphosate to act on a wide range of weeds. The inhibition of the functioning of the shikimic acid pathway causes a deficiency in aromatic amino acids, and eventually, the death of the plant by nutritional deficiency (Geiger and Fuchs, 2002; Zablotowicz and Reddy, 2004).
In Côte d’Ivoire, the use of glyphosate as ripener is mainly practiced in sugarcane cropping, at the beginning of the harvest season where it has a beneficial effect on sucrose content. In this country, sugarcane harvest season spreads over 5 to 6 months from November to April, with a period of severe drought in December and January, which is very favorable to the accumulation of sucrose in the stalks (ripening). It is especially during the first month of the harvest season that climatic conditions are highly unfavorable to the natural maturation of many sugarcane varieties (usually with abundant flowering) intended to be harvested at that time. This explains the resort to chemical ripening of these varieties with weed-killer like glyphosate so as to accelerate the sucrose accumulation process in the stalks and the time of harvest. The second challenge is to avoid over-ripening of plots treated beyond a certain time which may depend on the variety cultivated (Péné et al., 2016).
The study aims at determining, on the one hand, the sugarcane variety that has the best response to the glyphosate ripening treatment and, on the other hand, the efficiency period of the treatment between the date of application and the date of harvest.
Experimental site
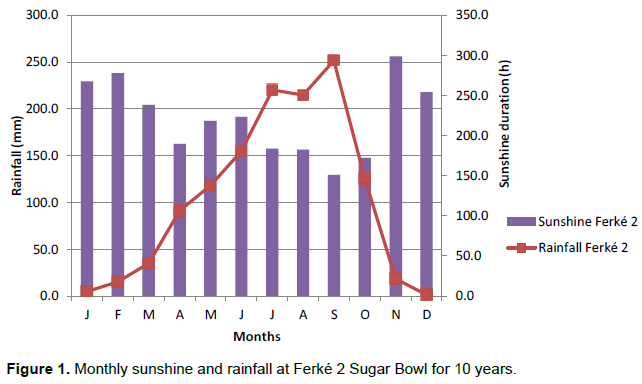
The sugar bowl of Ferké 2, where the study was conducted, is located at Ferkessédougou in northern Côte d’Ivoire (9°14’- 9°35’N and 5°15’- 5 °24’W and 323 m altitude). The prevailing climate is humid sub-tropical, with a dry season from November to March and a rainy season from April to October (Figure 1). The average annual rainfall is 1200 mm and there is a diversity of soils whose majority is ferralitic and shallow (40 to 60 cm) because of induration (Bigot et al., 2005; Brou, 2005).
Plant material
The effect of glyphosate was assessed on commercial varieties of sugarcane NCo376 originating from South Africa and SP71-1406, Brazilian variety, which were introduced in Côte d’Ivoire, in May 1960 and January 1987 respectively. The first one was the most cultivated in 2007 with 15% of cultivated surface areas at the time of the study and the second one was developing then.
Experimental design
Twenty plots in ripening phase, a total of approximately 700 ha were treated in late September-early October by aerial spraying with glyphosate (Roundup® ) applied at a dose of 0.8 L/ha through a slurry dose of 15 L/ha.
Each plot was divided into two experimental sample plots of 1 ha from which 12 canes were sampled 10, 15 and 20 days after treatment and analyzed in the laboratory in order to determine the technological qualities of sugarcane.
Saccharimetric analyses
In the laboratory, each sampled stalk was cut into four pieces or quarters, a base (Q1), two middles (Q2 and Q3), and a top (Q4). Each set of 12 quarters was individually ground using an electric grinder (“Jeffco” food and fodder cutter grinder, model 265B size 10, L1710 series). The pulp resulting from each set of cane was submitted separately to a hydraulic press (Pinette Emidecau Ind.125). Saccharimetry analyses were carried out separately on the collected extract from each pulp. The brix juice (total sugar) was measured using a refractometer (SCHMIDT+HAENSCH, model DURSW, 29129 series) at 20°C. A part of the juice was clarified according to the basic lead acetate method of Horne (lead acetate hydroxide (II) or Horne salt) at 2.5 g per 250 ml of undiluted juice (ICUMSA GS5/7-1, 1994 quoted by Hoareau et al., 2008 and Kouamé et al., 2010). The juice was then filtered through WHATMAN paper 91, and the Pol was read out by polarimeter (SACCHAROMAT Z, 29305 series). The juice Pol was determined from the Brix and Pol read out by Schmidt table for saccharimeter. The juice purity (Pol rate in Brix) was then calculated. The fiber rate was determined using a correspondence table from the weight of the fiber obtained after pressing the ground material.
The sucrose content (SC% or Pol%C) was determined by multiplying the juice Pol by an n factor read out on a second table for a weight of 500 g cane pulp cake (Hoarau, 1970). The recoverable sugar (RS%) was determined as follows (Fauconnier, 1991):
Measurement of cane losses in the top parts of the stalks
The cane losses on farm in the top parts (white tips) were collected in five experimental sample plots of 10 m² each spread across each of the 11 treated plots before harvest and sampled (350 ha). For each sampled plot, the stuffed top parts from each of the five plots were collected separately and weighed in order to determine the average weight of cane per hectare.
Statistical data analysis
An analysis of variance was applied to data collected using the Statistica 7.1 software on Windows 7. The Newman-Keuls post-hoc test was used in case of significant differences between treatments for each agronomic or technological criterion considered (Newman, 1939; Keuls, 1952; Shaffer, 2007).
Effect of glyphosate on the variety NCo376 ripening
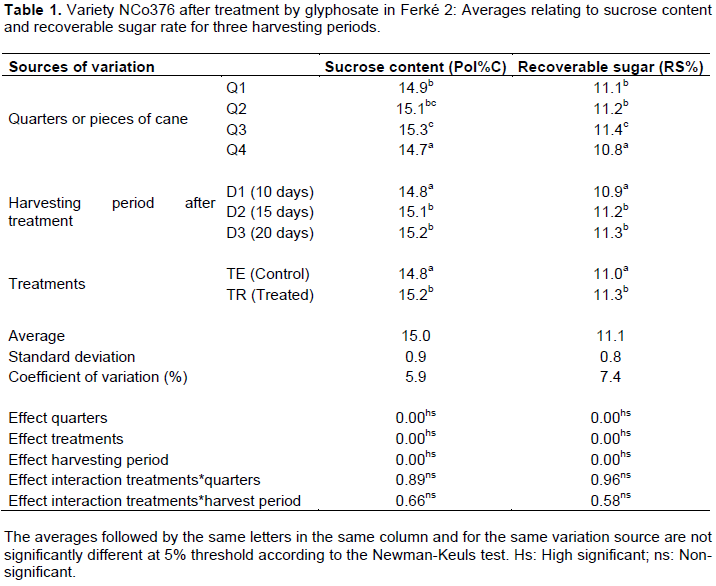
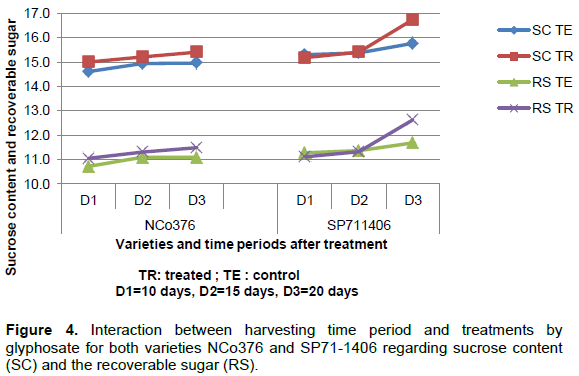
The effects of the ripening treatment on variety NCo376 related to the gradient of sucrose content in the cane stems and the period of time after treatment proved significant at 5% threshold (Table 1). However, the lack of interaction between the different treatments of the study for this variety showed that the gradients of sucrose content and recoverable sugar in the stalks, particularly between the top part and the other part of the stalk, were not attenuated despite the application of glyphosate and the period observed after the treatment (Figures 2 and 4).
Effect of glyphosate on the ripening of variety SP71-1406
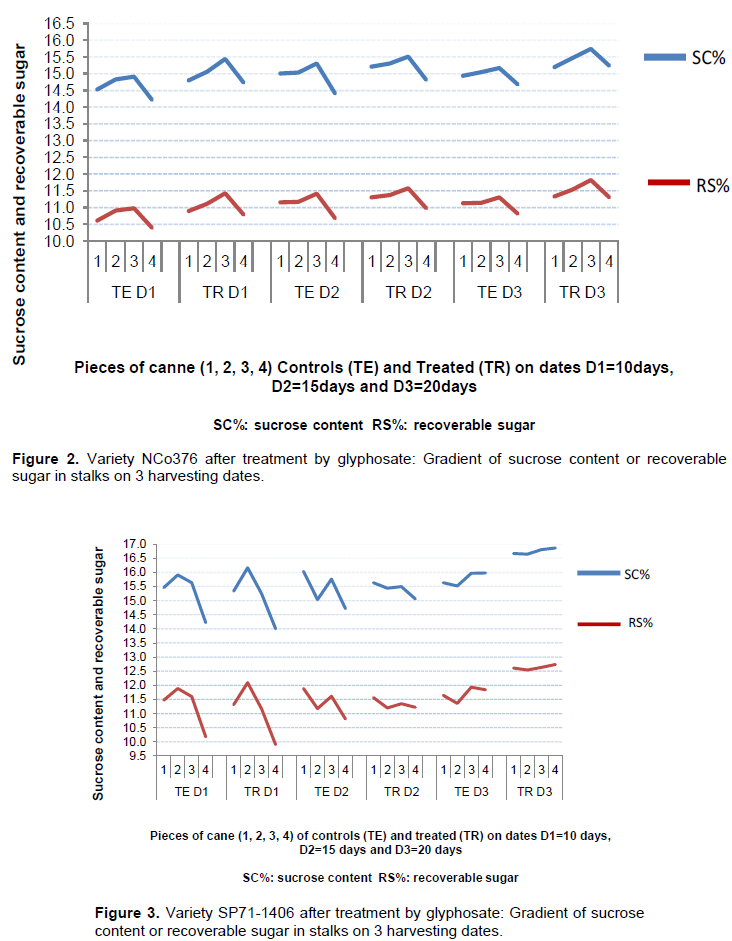
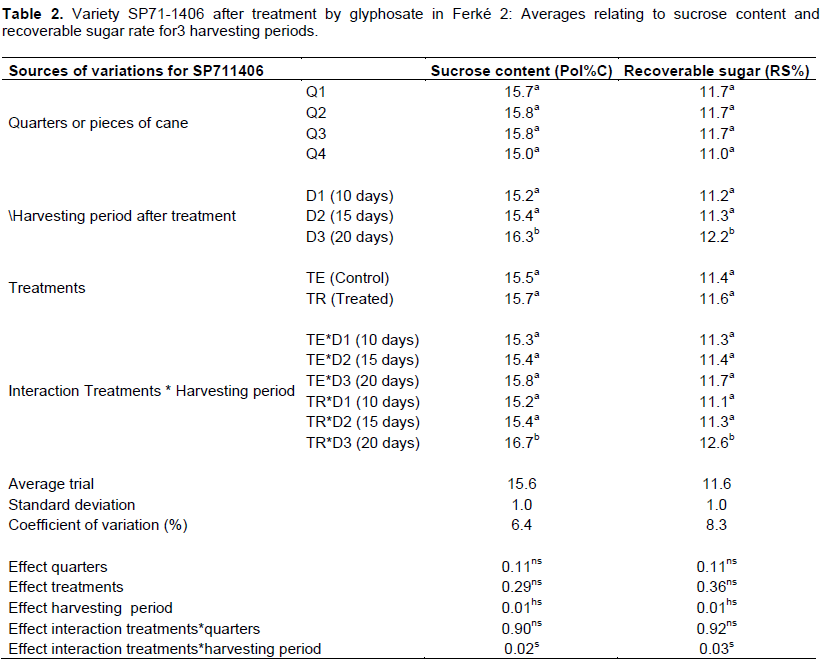
Unlike NCo376, the effects of the ripening treatment on SP71-1406 related to the gradient of sucrose content in cane stalks was proved not significant (Table 2). The significant interaction effect between the different treatments of the study for SP71-1406 showed that the gradients of sucrose content and recoverable sugar in the stalks, particularly between the top part and the basal and middle parts of the stalk, were mitigated due to the application of glyphosate and time periods of 20 d observed there after (Figures 3 and 4).
The averages followed by the same letters in the same column and for the same variation source are not significantly different at 5% threshold according to the Newman-Keuls test. Hs: High significant; ns: Non-significant; s: Significant.
Regarding variety SP711406, statistical analyzes showed that the effect of the ripening treatment had canceled the gradients of sucrose content and existing recoverable sugar in cane stalks between the basal and middle parts, on the one hand, and between the basal and the top parts, on the other hand (Table 2).
For variety SP71-1406, the time period of 20 days enabled to obtain a gain of 1.6% of sucrose content and 1.5% of recoverable sugar compared to the control.
These results show that the treatment with glyphosate helps to obtain gains in sucrose content and sugar in both sugarcane varieties considered, but with a shorter treatment response time period for SP71-1406 (more sensitive) compared to NCo376.
Cane weight and sugar losses on-farm
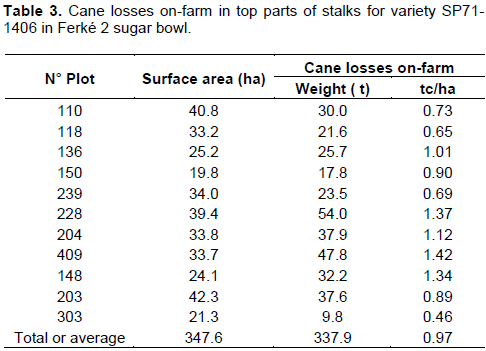
The cane losses on-farm relating to the top parts were estimated to 1 t per ha (Table 3). With 16.7% sucrose content and 12.6% recoverable sugar observed 20 days after treatment on variety SP71-1406, losses could be estimated at about 0.13 t of recoverable sugar per ha, that is, nearly 43 tons of sugar over the 350 ha of sampled plots.
Sugar yield gain related to the treatment
The results of the study showed that the ripening treat-ment by glyphosate helped significantly improve the sucrose content, the recoverable sugar and therefore sugar yield among the tested varieties. They confirm those obtained in many previous studies (Villegas and Torres, 1993; Bennett and Montes, 2003; Viator et al.,
2003).
The treatment of the variety SP71-1406 has significantly improved the technological qualities of top parts so much so as to cancel the gradient of sucrose content in the stalks.
The good response of SP71-1406 to ripening treatment compared to NCo376 using glyphosate reinforces earlier observations made by Silva and Caputo (2012) on varietal differences in sugarcane vis-à-vis this treatment.
It was observed that the harvesting period of time after ripening treatment by glyphosate was generally 25 to 35 days (Silva and Caputo, 2012), while the one observed for SP71-1406 in this study is shorter (15 to 20 days). Moreover, the varieties respond differently to ripening treatment depending on climatic parameters such as temperature and solar radiation. The particularly upright shape of SP71-1406 (including the active leaf apparatus) enables it to capture more efficiently light energy compared to varieties more sensitive to pouring down or having plagiotropic leaves such as NCo376. This helps explain in part the differences in response to the ripening effect in both varieties (Hopkins, 1995).
Effect of glyphysate on the gradient of sucrose content in cane stalks
In conditions of natural ripening in sugarcane, sucrose accumulation in stalks occurs first in the basal parts before moving progressively towards the top parts. As the ripening goes on, the sucrose content tends to be uniform along the cane stalks (McCormick et al., 2008; Silva and Caputo, 2012). In most cases, the tops are poor in sucrose and very rich in starch unlike to the lower parts of the stalk. This is why in most countries where manual harvesting of sugarcane is practiced, the top parts, immature and very poor in sucrose, are eliminated. These are characterized by an active cell growth that causes quick hydrolysis of part of the sucrose accumulated in the stalks by the vacuolar invertase acid cells. Hexoses stemming from this hydrolysis migrate into the cytoplasm of cells to be used for the benefit of growth (Lingle, 1999). One of the effects of glyphosate is to cause the death of the apical bud of cane stalk or inhibit the synthesis of indole acetic acid (IAA) which is a growth hormone. This results in an increase in ethylene synthesis by the action of 1-aminocyclopropane-1-carboxylic acid (ACC) synthase (Liang et al., 1992).
This study showed that after the artificial maturation, it was not necessary to carry out the elimination of the top parts of stalks (except leaf apexes and flower scapes) in varieties like SP71-1406 whose good response to ripening has enabled uniformity of sugar along the stems. So, production losses on-farm through the top parts on treated plots, planted with this variety were assessed at 1 t of cane/ha or 0.13 t of sugar/ha.
The differences in chemical ripening, observed between both varieties could be partly explained by their contrasting skills at flowering. NCo376 is a variety with a very high flowering rate (80 to 100%) in cropping conditions on the perimeter of Ferké unlike SP711406 that flowers very little there. When the treatment does not occur before flowering in varieties highly sensitive to photoperiod, inflorescences intercept a portion of the chemical ripener to the detriment of the leaf apparatus resulting less beneficial effect of the treatment. Previous studies have shown that abundant flowering could cause in the event of over-ripening the phenomenon of pith process (formation of a marrow in the center of the cane stem). This phenomenon, whose magnitude depends on the variety cultivated, causes the reduction of tech-nological qualities. The pith process induces the drying of inner stem from the top parts with weight loss through dehydration, significant drop in cane yield and difficulty in extracting sugar (Caputo et al., 2007; Silva and Caputo, 2012; Leite et al., 2011).
Although artificial ripening by glyphosate is an alternative to natural ripening of sugarcane when climatic conditions are unfavorable (Cardozo and Sentelhas, 2013), some drawbacks associated with its use have been reported. Glyphosate induces senescence of the apical bud and development of lateral buds that are detrimental to the technological qualities of stalks. The growth inhibition by glyphosate causes the reduction in the average number of internodes and the average weight per cane stem. In particular, the use of glyphosate as ripener negatively affects ratoon and number of stalks after harvesting. The height and number of cane stalks per unit surface area is reduced and undulations are observed in the plots (Dalley and Richard-Junior, 2010).
Other cane ripening substances
According to Azania et al. (2013), there are two types of sugarcane ripeners of which one causes lethal stress and the other non-lethal stress for the plant. Glyphosate is considered as lethal stress ripener for sugarcane. It is a growth inhibitor that causes sucrose accumulation in sugarcane while preventing it from being used as an energy source for developing meristems. This ability to reduce the growth rate forces the cane to ripen. Richard et al. (2006) reported a better sugar yield in sugarcane varieties treated with trinexapac-ethyl, imazapyr, or nicosulfuron compared to glyphosate related to the reduction of cane yield caused by the latter. These include non-lethal stress ripeners for sugarcane whose action does not cause permanent growth failure or death of the apical bud as in the case of glyphosate, but which induce the production of ethylene which is responsible for sucrose accumulation in stems (Bueno et al., 2011).
Fluazifop-p-butyl (aryloxyphenoxypropionate group) has a systemic foliar action. Applied at low doses (0.1 to 0.3 l/ha), it is quickly absorbed and migrates into the growth points by inhibiting acetyl coenzyme Acarboxylase (ACCase) which is an enzyme responsible for the biosynthesis of fatty acids (Hugh, 2000). It thus limits the formation of membrane lipids necessary for cell growth. It causes mortality of apical bud and necrosis as in the case of glyphosate, but more slowly by maintaining photosynthesis always active with sucrose accumulation in stems. The treated areas can be harvested between 28 to 35 days with a risk of loss in cane technological qualities beyond that period (Silva and Caputo, 2012). Fluazifop-p-butyl inhibits flowering thus avoiding the risk of pith process. It has no depressive effect on ratoon unlike glyphosate. However, previous studies conducted in Louisiana showed that fluazifop-p-butyl was less efficient than glyphosate because of its depressive effect on cane yield in the treated areas (Watson and Stefano, 1986; Dalley and Richard-Junior, 2010).
Maleic hydrazide (1.2-dihydro-3.6-pyridazinedione) is a growth regulator which favors suppression of apical dominance in plants. It is considered as potential ripener in sugarcane inducing sucrose accumulation in the stalks with growth reduction (Silva and Caputo, 2012).
The chemical compound Imazapyr (groups of imidazolinones) is absorbed through the leaves and rapidly migrates into the meristematic zones where it accumulates. By inhibiting acetolactate synthase (ALS), it blocks the synthesis of amino acids with branched chains (valine, leucine, isoleucine), thereby stopping protein synthesis (including DNA) and cane growth. Imazapyr does not control flowering in cane according to Lavanholi et al. (2002), but rather favors accumulation of sucrose in stalks.
Ethephon (2-chloro-ethylphosphonic acid) is a growth regulator with systemic action which penetrates the tissues of the plant and decomposes into ethylene, a compound highly soluble in water and stable in aqueous solution at a pH below 3.5 and temperatures above 75°C. It reduces growth but is widely used as a flowering inhibitor, stimulating the emergence and tillering of ratoon until six months after harvesting and as ripener in sugarcane having flowered or not. A differential response of sugarcane varieties to ethephon applications as ripener has been reported (Silva et al., 2007; Castro et al., 2001; Gururaja Rao et al., 1996; Tomlin, 1994). Its inhibitory action on flowering helps avoids the risk of pith process of cane stalks which has the effect of significantly reducing impaired cane and sugar productivity. Furthermore, ethephonhelps anticipate the harvest by at least 21 days and its effect persists for 60 to 90 days after application, which enables to exploit the treated plots for a relatively long period from the beginning until the middle of crop harvest (Caputo et al., 2007; Dalley and Richard-Junior, 2010).
Sulfometuron-methyl (sulfonylurea) is characterized by its systemic action on meristematic zones after foliar uptake inhibiting thus growth and cell division without directly interfering with mitosis and DNA synthesis. It inhibits the synthesis of amino acids with non-cyclic carbon chains such as valine, leucine, and isoleucine by affecting the acetolactate synthase enzyme (ALS) from the precursor alpha-ketobutyrate. Herbicides of this group do not directly block the action of growth activators that are auxin, gibberellins or cytokynins but strongly stimulate the production of ethylene, which is a response of the plant to the phyto-toxicity of the product. This causes paralysis and inhibition of apical meristem development causing in cane the reduction of internodes length formed after application of the herbicide as ripener. Leaf formation is thus inhibited in favor of sucrose accumulation in stems. After application, the treated areas can be harvested after 25 to 45 days according to Silva and Caputo (2012), while Almeida et al. (2005) showed that harvesting could be anticipated by15 days. It is a ripener which does not act on the apical bud so that the stems recover their normal growth even if the treated areas are not harvested or are harvested late (Leite et al., 2011).
Trinexapac-ethyl (cyclohexanedione group) is a chemical compound that induces a large accumulation of sucrose in cane stems. It is preferentially absorbed by the leaves and roots and then passes in meristematic zones where it inhibits the synthesis of gibberellic acid which is involved in cell growth and division, inhibiting thus the development of the plant while favoring sucrose accumulation in cane stems without adversely affecting cane yield as in the case of glyphosate (Van Heerden et al., 2015). The other benefits of trinexapac-ethyl as sugarcane ripener lie in flowering reduction, brix increase and cane juice purity, and the absence of depressive effect on subsequent ratoon. The recommended dose as ripener ranges from 0.8 to 1.2 l/ha and the areas treated with this compound can be harvested after 35 to 55 days (Guimaraes et al., 2005; Rainbolt, 2005; Richard et al., 2006; Dalley and Richard-Junior, 2010; Leite et al., 2011; Silva and Caputo, 2012).
Impact of chemical treatments on biodiversity
The advantages of the application of glyphosate and other ripening products have been proved. However, the impact of these treatments on the status of populations of certain sugarcane pests must be emphasized. Thus, in recent years, increased attacks of Eldana saccharina, sugarcane stem borer in the sugar bowl northern Côte d’Ivoire have been reported (Péné et al., 2016). This intensification of stem borer attacks could be explained by the destruction of resources and shelter for natural enemies (parasitoids) of stem borers that are Trichogramma, preventing their maintenance and survival in nature (Goebel et al., 2010). Chemical treatments, especially by air, destroy the natural hosts of parasitoids and thus have the effect of reducing the natural parasitism of Trichogramma. The diversity of trichograms is important especially as the host plant diversity is large (Lamy et al., 2013). For this purpose a study is underway in Côte d’Ivoire in order to determine the parasitism rate and identify the natural enemies of the tropical stem borer with a view of biological control of E. saccharina.
The study shows that glyphosate, applied as ripener in the early crop harvest season at the dose of 0.8 L/ha, proved efficient on varieties NCo376 and SP71-1406, with a significant improvement of their sucrose content and recoverable sugar, that is, respectively 1.6 and 1.5%, at 20 days after treatment. SP71-1406 proved particularly sensitive to the treatment with induced ripening in 20 days and a deletion of the gradients of sucrose content and recoverable sugar between the top and basal parts of stalks, unlike NCo376. Taking into account cane losses on-farm across the top parts estimated at 1 t/ha, this corresponds to sugar losses of 0.13 t/ha for variety SP71-1406 when treated. It appears thus relevant, during the manual harvest, to cut the highest possible top parts of the stems so as to limit sugar losses on such varieties responding well to the ripening treatment. The application of glyphosate as chemical ripener causes lethal stress on sugarcane with a depressive effect on ratoons. The study exposes the existence of other substances with non- lethal stress, such as trinexapac-ethyl, ethephon and sulfometuron-methyl. However, all these chemical treatments, whatever their agro-technological benefits and their targets, destroy the habitats of natural enemies of stem borers and therefore result in reduction of their parasitism and change of the status of borers whose attacks and geographic areas have been increasing in recent years.
The authors have not declared any conflict of interests.
REFERENCES
|
Almeida JCV de, Leite CRF, Souza JRP de (2005). The effect of maturators on technological characterists of sugar cane on soils with and without water stress. Semin: Ciênc. Agrár. 26(4):441-448.
|
|
|
|
Azania CAM, Pinto LR, Adriano RC, Perecin D, Azania AP (2013). The Use of Glyphosate in Sugarcane: A Brazilian Experience. In. Herbicides-Current Research and Case Studies in Use. 22 p.
|
|
|
|
|
Bennett PG, Montes G (2003). Effect of glyphosate formulation on sugarcane ripening. Sugar J. 66(1):22
|
|
|
|
|
Bigot S, Yao TB, Oszwald J, Diedhiou A (2005). Facteur de variabilité pluviométrique en Côte d'Ivoire et relations avec certaines modifications environnementales. Sécher 16(1):5-13.
|
|
|
|
|
Brou YT (2005). Climat, mutations socio-économiques et paysages en Côte d'Ivoire. Mémoire de synthèse des activités scientifiques. P. 212.
|
|
|
|
|
Bueno AF, Batistela MJ, Bueno RCOF, França-Neto JB, Nishikawa MAN, Filho AL (2011). Effects of integrated pest management, biological control and prophylactic use of insecticides on the management and sustainability of soybean. Crop Prot. 30:937-945.
Crossref
|
|
|
|
|
Caputo MM, Silva MA, Beauclair EGF, Gava GJC (2007). Acúmulo de sacarose, produtividade e florescimento de cana-de-açúcarsobreguladoresvegetais. Interciencia 32(12):834-840.
|
|
|
|
|
Cardozo NP, Sentelhas PC (2013). Climatic effects on sugarcane ripening under the influence of cultivars and crop age. Sci. Agric. 70(6): 449-456.
Crossref
|
|
|
|
|
Castro PRC, Miyasaki JM, Bemardi M, Marengo D, Nogueira MCS (2001). Efeito do ethephon na maturação e produtividade da cana-de-açúcar. Rev. Agricultura 76(2):277-290.
|
|
|
|
|
Dalley CD, Richard-Junior EP (2010). Herbicides as ripeners for sugarcane. Weed Sci. 58(3):329-333.
Crossref
|
|
|
|
|
Fauconnier R (1991). La canne à sucre. Collection Le technicien d'agriculture tropicale. Maisonneuve et Larose pp. 44-61.
|
|
|
|
|
Geiger DR, Fuchs MA. (2002). Inhibitors of Aromatic Amino Acid Biosynthesis (Glyphosate). In. Herbicide Classes in Development. pp. 59-85.
|
|
|
|
|
Goebel FR, Tabone E, Do Thi Khanh H, Roux E, Marquier M, Frandon J (2010). Biocontrol of Chilo sacchariphagus (Lepidoptera: Crambidae) a key pest of sugarcane: lessons from the past and future prospects. Sugar Cane Int. 28:128-132.
|
|
|
|
|
Goscinny S, Hanot V (2012). Le glyphosate dans tous ses états. Institut Scientifique de Santé Publique, Unité Pesticides. Labinfo 7fr :12-16
|
|
|
|
|
Guimaraes ER, MA, Mutton JM, Pizauro Jr, Mutton MJR (2005). Sugarcane growth, sucrose accumulation and invertase activities under trinexapac-ethyl treatment. Cientifica 33:20-26.
|
|
|
|
|
Gururaja Rao PN, Singh S, Mohan NK (1996). Flowering suppression by ethephon in sugarcane and its effect on yield and juice quality. Indian J. Plant Physiol. 1(4):307-309.
|
|
|
|
|
Hoarau M (1970). Utilisation de la presse hydraulique pour la détermination de la richesse saccharine de la canne à sucre. In : La canne à sucre, par Fauconnier et Bassereau. IRAT, Maisonneuve et Larose. Pp. 387-419.
|
|
|
|
|
Hoareau S, Hoareau W, Petit A, Corcodel L (2008). Etat des lieux de la polarisation proche infrarouge sur les différents produits de l'industrie sucrière réunionnaise. In. 4ème Rencontre Internationale de l'AFCAS. Guadeloupe. Pp. 1-15.
|
|
|
|
|
Hopkins WG (1995). Les facteurs qui influencent la photosynthèse et la productivité. In. Physiologie végétale. Pp.261-266
|
|
|
|
|
Hugh M (2000). Classes de modes d'action des herbicides. FICHE TECHNIQUE. Imprimeur de la Reine pour l'Ontario. 17 p.
|
|
|
|
|
Keuls M (1952). The use of the "studentized range" in connection with an analysis of variance. Euphytica 1:112-122.
Crossref
|
|
|
|
|
Kouamé DK, Péné BC, Zouzou M (2010). Criblage de variétés commerciales de canne à sucre prometteuses dans le périmètre sucrier de ferké 2 au nord de la Côte d'Ivoire : optimisation de la durée de sélection. Sci. Nat. 7(1):97-106.
Crossref
|
|
|
|
|
Lamy F, Bolland P, Viannay D, Pintureau B (2013). Influence du paysage sur les populations de Trichogrammes (Hymenoptera, Trichogrammatidae). Bull. Soc. Entomol. France 118(2):197-206.
|
|
|
|
|
Lavanholi MGDP, Casagrande AA, Oliveira LAF de, Fernandes GA, Rosa RF da (2002). Aplicação de etefon e imazapyrem cana-de-açúcaremdiferentesépocas e sua influência no florescimento, acidez do coldo e teores de açúcares nos colmos – variedade SP70-1143. STAB. Açúcar, Álcool e Subprodutos. 20(5):42-45.
|
|
|
|
|
Leite GHP, Crusciol CAC, Silva MA (2011). Desenvolvimento e produtividade da cana-de-açúcarapósaplicação de reguladoresvegetaisemmeio de safra. Semin: Ciênc. Agrár. 32(1): 129-138
Crossref
|
|
|
|
|
Liang X, Abel S, Keller JA, Shen NF, Theologis A (1992). The 1-aminocyclopropane-l-carboxylate synthase gene family of Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 89:11046-11050.
Crossref
|
|
|
|
|
Lingle SE (1999). Sugar metabolism during growth and development in sugarcane internodes. Crop Sci. 39(2):480-486.
Crossref
|
|
|
|
|
McCormick AJ, Cramer MD, Watt DA (2008). Culm sucrose accumulation promotes physiological decline of mature leaves in ripening sugarcane. Field Crops Res. 108(3):250-258.
Crossref
|
|
|
|
|
Meschede DK, Veliniv ED, Carbonari CA (2010). Effect of Glyphosate and Sulfometuron-Methyl on the Growth and Technological Quality of Sugarcane. Planta Daninha 28(SPE):1135-1141.
|
|
|
|
|
Newman D (1939). The distribution of range in samples from a normal population, expressed in terms of an independent estimate of standard deviation. Biometrika 31(1):20-30.
Crossref
|
|
|
|
|
Péné BC, Kouamé KD, Dove H, Boua BM (2016). Incidence des infestations du foreur de tiges Eldana saccharina (Lepidoptera, Pyralidae) en culture irriguée de canne à sucre selon la variété et la période de récolte en Côte d'Ivoire. J. Appl. Biosci. 102:9687-9698.
|
|
|
|
|
Rainbolt C (2005). Evaluation of trinexapac-ethyl for use as a ripener in Florida sugarcane. Proc. Am. Soc. Sugar Cane Technol. 25:118.
|
|
|
|
|
Richard EP, Dalley Jr CD, Viator RP (2006). Ripener influences on sugarcane yield in Louisiana. Proc. Am. Soc. Sugar Cane Technol. 26:54-55.
|
|
|
|
|
Shaffer JP (2007). Controlling the false discovery rate with constraints: The Newman–Keuls test revisited. Biom. J. 49:136-143.
Crossref
|
|
|
|
|
Silva MA, Caputo MM (2012). Ripening and the Use of Ripeners for Better Sugarcane Management. In. Crop Management-Cases and Tools for Higher Yield and Sustainability. Edited by Fabio R. Marin. Published by InTech Janeza Trdine 9, 51000 Rijeka, Croatia Copyright © 2012 InTech. 1-32.
|
|
|
|
|
Silva MA, Gava GJC, Caputo MM, Pincelli RP, Jerônimo EM, Cruz JCS (2007). Uso de reguladores de crescimentocomopotencializadores do perfilhamento e da produtividadeemcana-soca. Bragantia 66(4):545-552.
Crossref
|
|
|
|
|
Steinmann HH, Dickeduisberg M, Theuvsen L (2012) Uses and benefits of glyphosate in German arable farming. Crop Prot. 42:164-169.
Crossref
|
|
|
|
|
Tomlin C (1994). The pesticide manual. (10th. Edition), Blackwell Scientific Publications, Cambridge.
|
|
|
|
|
Van Heerden PDR, Mbatha TP, Ngxaliwe S (2015). Chemical ripening of sugarcane with trinexapac ethyl (Moddus). Mode of action and comparative efficacy. Field Crops Res. 181:69-75.
Crossref
|
|
|
|
|
Viator BJ, Viator C, Jackson W, Waguespack H, Richard Junior EP (2003). Evaluation of potassium-based ripeners as an alternative to glyphosate and the effects of 2,4-D on herbicidal cane ripening. Sugar J. 66(1):21
|
|
|
|
|
Villegas FT, Torres JSA (1993). Efecto del Roundup usado como madurante en la producción de ca-a de azúcar. Int. Sugar J. 95(1130):59-64.
|
|
|
|
|
Watson EC, DeStefano RP (1986). The use of fluazifop-butyl and sethoxydim as sugarcane ripeners. Proc. Am. Soc. Sugar Cane Technol. 6:56-58.
|
|
|
|
|
Zablotowicz RM, Reddy KN (2004). Impact of glyphosate on the Bradyrhizobium japonicum symbiosis with glyphosate-resistant transgenic soybean: a mini review. J. Environ Qual. 33(3):825-831.
Crossref
|
|