Full Length Research Paper
ABSTRACT
The study aimed at understanding the spatial distribution of Fusarium wilt (FW) in different banana growing regions, ascertain the effect of management practices and plantation age on FW incidence, as well as investigate farmers’ knowledge regarding the symptoms and spread of FW in Uganda. Individual interviews were conducted in 119 farms using a pre-tested questionnaire and field observations during a survey in major banana growing regions. Results indicate that FW is widely distributed across the banana growing areas with more occurrences (70%) in Kapchorwa district and majority of respondents (63.4%) reported increasing disease prevalence. A chi-square test performed revealed significant association between FW incidence and plantation age with more incidences (51.6%) recorded in older plantations (>20 years of establishment) than newly (1-5 years) established ones (11.1%). FW incidence was significantly associated with plantation management with higher incidences (86.9%) recorded in well managed plantations. Half of interviewed farmers could explain and distinguish symptoms associated with FW from other diseases, but only 38.4% of these could tell how the disease spreads; thus, a need for more concerted efforts in building the capacity of farmers to identify the symptoms and spread of FW for effective management program. We identified preliminary evidence that field abandonment is sometimes used as a last option for coping up with FW. Understanding the mechanism behind this requires more detailed research as well as establishing how farmers are managing FW culturally.
Key words: Fusarium wilt, farmers’ knowledge, spatial distribution, Uganda.
INTRODUCTION
Banana and plantain are one of the major staple foods for over 50% of the population in Uganda (Karamura et al., 2012). In addition, banana is considered a key food security crop and a source of income for resource poor farmers. Uganda has the highest global per capita consumption levels of banana, estimated at 0.4-0.7 kg per day (FEWS NET, 2017). Seventy percent of the bananas grown in Uganda are consumed locally and the rest is sold on the domestic market to generate household income (UBOS, 2019). Depending on genome,banana serves several purposes, for instance; AAA (Cavendish, Gros Michel and East African Highland Bananas-EAHBs) are majorly used as deserts and cooking respectively, AAB (Pisang Awak, Silk and Pome) are grown for preparing juice and desert, AB (Sukali Ndizi, Kisubi) for dessert, ABB (Bluggoes) and AAAA (FHIAs mainly, 03, 17, 21, 23 and 25) for juice, desert and cooking. Therefore, the production of banana is demand-driven with the EAHBs also locally known as “matooke/cooking type”, the most cultivated ones (Karamura et al., 2012). However, in the recent years, investing in dessert varieties especially Gros Michel is becoming lucrative and has become major export commodity not only in Uganda but in East Africa and the world. According to Fruitrop (2016), the global production of Gros Michel is at 14910.16 tonnes; of this, 4.1% come from East Africa mainly: Uganda (352.8 tonnes), Tanzania (173.1 tonnes), Burundi (86.496 tonnes), Kenya (72.1 tonnes) and Rwanda (70 tonnes).
Despite its importance, banana productivity has remained low (6.3 t ha-1 yr-1) compared to the potential yield of 70 tonnes/ha/year obtained on station under proper management practices (Nalunga et al., 2015). Pests and diseases, declining soil fertility and drought stress are among the major factors attributing to this yield gap in Uganda (Nyombi, 2013; Tinzaara et al., 2014). Among the most devastating diseases is Fusarium wilt (FW), also known as Panama disease. It has been reported among diseases posing substantial threat to banana production especially to the dessert varieties (Karangwa et al., 2018; Kangire et al., 2000; Tushemereirwe et al., 2004).
FW has caused almost more than 60% in the field losses (Buregyeya et al., 2020). FW is a soil borne fungus caused by Fusarium oxysporum f. sp cubense (Foc). At present, four races of Foc have been documented (Mostert et al., 2017). These include race 1 that causes manifestation of the disease in Gros Michel (AAA), Silk (AB), Pome (AAB) and Pisang Awak (ABB), race 2 which attacks Bluggoe (ABB), race 3 that attacks Heliconia species and tropical race 4 (TR4) which attacks mainly Cavendish (AAA) varieties and all varieties susceptible to race 1 and 2 (Mostert et al., 2017; Dita et al., 2018), Race 1 has remained problematic to non-Cavendish varieties as recent surveys in Mozambique still confirm the status quo (Viljoen et al., 2020). Although this classification still holds, there is a growing concern yet to be confirmed that TR4 does not necessarily affect all species susceptible to Race 1 (Molina personal communication). The race system of Foc classification has been proven to be insufficient in distinguishing different Foc isolates from different parts of the world and therefore the Vegetative Compatibility Group (VCG) system has been used (Mostert et al., 2017). Currently, only race 1 has been reported in Uganda mainly on Gros Michel, Sukali Ndizi and Pisang awak under the VCGs 0124, 0125, 0128, 01212,01220 and 01222 (Karangwa et al., 2018; Tushemereirwe et al., 2004).
Since being identified in Uganda in 1952 (Leaky, 1970) entire fields of dessert banana varieties of Bogoya (Gross Michel), Kayinja (Pisang Awak), Sukali Ndizi and Kisubi (Ney Poovan) have almost been wiped out (Tushemereirwe et al., 2004). Fusarium wilt symptoms include wilting of the old leaves, splitting at the corm base, the xylem becomes reddish brown, plugged, thus hindering water and nutrient transport as the plant eventually dies (Ploetz, 2015a; Viljoen et al., 2017). Initially, yellowing of the leaves begins with the margin advancing towards the midrib and the petioles becomes brown and buckles, pseudostem splits above, discoloration of the corm and dead leaves hang around the pseudostem appearing like a skirt (Viljoen et al., 2017; Thangavelu et al., 2020). Expression of such symptoms has been widely used to identify Banana Fusarium wilt and collect samples for further laboratory characterisation and hence (Karangwa et al., 2018; Viljoen et al., 2017).
Foc can survive in the soils for more than 20 years as hard-cased chlamydospores, making it very difficult to eradicate (Ploetz, 2015b; Dita et al., 2018). Management of Fusarium wilt in other parts of the world where the Cavendish industry is grossly affected by the virulent TR4 has been through attempts at use of fungicides and chemicals, soil fumigation and complete destruction of Foc-infected plants (Viljoen et al., 2019). Most of these methods have proven expensive and unsustainable (Veena et al., 2014). Fusarium wilt being a soil borne disease needs to be sustainably managed (Dita et al., 2018).
For sustainable management of the disease, especially with the imminent threat of the presence of TR4 in neighbouring Mozambique in 2013, there is need to monitor diseases progress, understand how farmers are managing the disease as well documenting current knowledge about the same basing on community perception for an informed and participatory breeding program. Such information in Uganda is limited. The last study with closely related information was by Kangire et al. (2000); it is more than 20 years now and certainly a lot has changed. For instance, there are growing unsubstantiated claims that Fusarium wilt is more prevalent in well managed than abandoned plantations, others argue that Fusarium wilt is common in older plantations than recently established ones. In addition, knowledge about the spatial distribution of FW in Uganda is limited yet such information in vital in understanding disease pressure to map out hot spot experimental areas (Madden and Hughes, 1995), sampling program for disease losses (Liu et al., 2015) and determining areas where concerted management efforts should be put (Ristaino and Gumpertz, 2000). Information on spatial patterns of FW has been widely studied (Meldrum et al., 2013; Gudero et al., 2018; Liu et al., 2015; Heck et al., 2021) in other countries not Uganda.
Therefore, this study aimed at: (1) understanding the distribution (spatial and within Musa spp.) and status of FW based on symptomatology within major banana growing areas, (2) investigate what is known by farmers in regard to symptom identification and spread and (3) ascertain the effect of plantation age and management practices on Fusarium wilt.
MATERIALS AND METHODS
The study used two different tools, namely: (1) individual interviews, (2) field observation based on symptomatology.
Individual interviews
Farmers from major banana producing areas of eastern, central and western Uganda were selected based on having at least 5 mats (a mat refers to collection of banana plants interconnected to original plant) of FW susceptible varieties. The sample was stratified according to altitude differences: (1) districts at high altitude (1450-1950), (2) districts at medium altitude (1200-1450 masl), and (3) districts at low altitude (1000-1200 masl). A total of 119 farmers were selected from four districts with contrasting altitudes namely, Kapchorwa, Isingiro, Kabarole and Sironko for high altitude; Ntungamo, Rubirizi, Rakai and Mukono for medium altitude; Mbale, Masaka, Luweero; and Kayunga for lower altitude. For each district, three sub counties were randomly selected and ten respondents with at least 5 mats of FW susceptible varieties chosen per sub county. Banana growing households distant from each other by at least five km with acreage between 0.5-20 were selected. The farmers were interviewed using an open-ended questionnaire aimed at generating data on demographic characteristics, plantation history, and knowledge about Fusarium wilt (FW), as well as information on resistance on grown banana varieties/clones.
Field observation
Following a Fusarium wilt disease symptom and management manual by Viljoen et al. (2017), a transect walk was conducted in plantation for each individual respondents to identify the presence or absence of FW symptoms on desert bananas (diseases incidence). For each plantation, symptomatic plants as explained by Viljoen et al. (2017) were split and checked for discoloration of the corm to confirm the presence of the disease on desert bananas, and upon this confirmation, the number of mats affected were counted and recorded.
Data analysis
Data were subjected to three packages for analysis: (1) Quantum GIS to develop maps on spatial distribution of the disease in study areas (2) Stata Version 15 (Stata Corporation. 2003) and Microsoft excel for inferential and descriptive statistics mainly means and frequencies as well as testing for associations between variables. Qualitative data was consolidated and disaggregated using queries to generate required tables for analysis. The filter function of Microsoft Excel was employed to eliminate outliers and check for wrong entries. The cleaned data was subjected to Pivot Table function of Microsoft and Stata version 15 for descriptive and inferential statistics. The chi-square test was performed to test the association between Fusarium wilt incidence and management status of banana plantations. All tests on inferential statistics were conducted at 95% confidence level.
RESULTS
Demographic factors within selected banana growing regions of Uganda
According to the individual interviews, male (63%) still dominates banana production as compared to their female counterpart (37%). On appropriateness of information provided, most farmers (77.3%) had completed primary school and above. However, 22.7% of the respondents engaged in banana farming had never received any formal education. It was interesting to note that university graduates owned a notable number of plantations 8.4%. Nevertheless, farmers who never went to school were reported (Table 1).
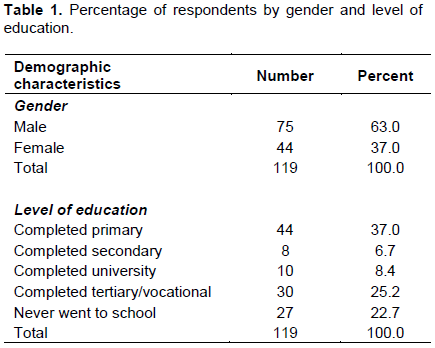
Farmers’ experience on growing banana and land size allocated to banana
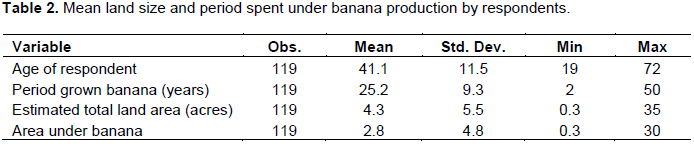
Interviewed farmers had an average land size of 4.3 acres with some farmers owning up to 35 acres of land. Land ownership may not necessarily mean banana production; so to understand this, an interview was held to discern amount of land allocated to banana, and it was revealed that majority (66.3%) allocate their land to banana production. The interviewed farmers were majorly small-scale banana farmers with average banana acreage of 2.8 although very small pieces of 0.3 acres were recorded. Nevertheless, some farmers had scaled up banana production to 30 acres. Among these, the youngest was 19 years and the oldest 72, with an average age for all at 41. Additionally, all farmers had at least two years’ experience in growing banana with an average experience at 25 years. The results also indicated that some farmers had grown banana for 50 years (Table 2).
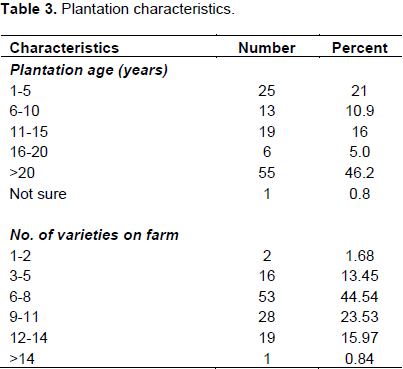
Plantation characteristics in interviewed banana production areas
An assessment to understand plantation age revealed that majority (46.2%) were old plantations established 20 years and above. Albeit a notable number of new plantations were reported (21%), of these 5 were established after abandoning those severely hit by FW. It was also reported that majority of farmers (44.5%) prefer growing a mixture of varieties as opposed to pure stands of a single variety (1.7%) (Table 3).
Distribution and status of FW in selected banana growing regions
Spatial distribution
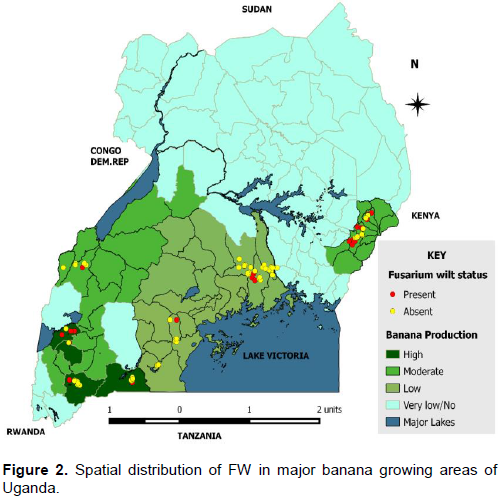
Symptoms associated with FW were widespread within selected banana growing areas with more prevalence in the eastern region of the country (Figure 2). There was a significant difference (p < 0.05) between FW incidence and districts. Generally, FW was more prevalent in eastern Uganda with Kapchorwa district showing highest incidence (70%), followed by Mbale (60%), and Sironko (60%) (Figure 2). Results further indicated that altitude did not have a significant contribution on FW incidence as this is evidenced by occurrence of high disease incidences in districts at high, medium and low altitudes.
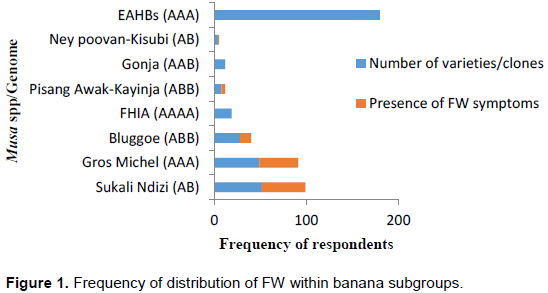
Distribution of FW symptoms within Musa spp. according to farmers’ perspective
EAHBs continue to be the dominant banana subgroup grown in Uganda. Farmers’ perception according to resistance, ranked Sukali Ndizi (AB) as the most susceptible Musa species followed by Gros Michel (AAB) and Bluggoe (ABB). Symptoms associated with FW were more observed in Sukali Ndizi and Gros Michel (Figure 1). Results further indicated that all clones of Pisang Awak (ABB) genome were reported susceptible and neither did any single farmer report symptoms associated with FW on FHIA (AAAA), Gonja (AAB) and KM5 (AAA) nor were such symptoms observed on the same varieties.
Status of FW in sampled banana growing areas
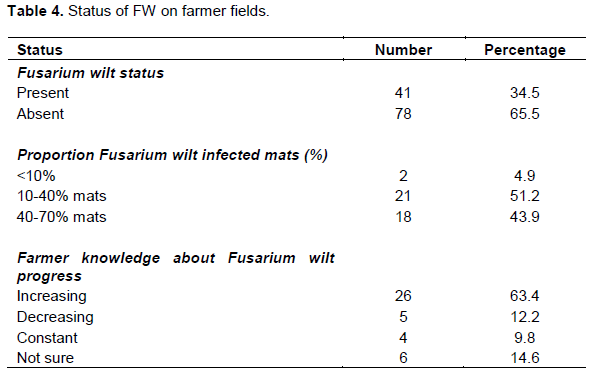
Symptoms associated with FW symptoms were observed in only 34.5% of the surveyed area. Out of these, more than 40% mats were infected, and the disease was reported increasing by majority (63.4%) of respondents (Table 4).
Understanding farmers’ knowledge in regard to FW symptom identification and spread
In phytopathology, the design of an effective and efficient diseases management program depends on clear understanding of symptoms, spread and survival mechanism. We investigated to ascertain if farmers know typical symptoms of FW, how it spreads and how possible to manage it.
Farmers’ knowledge on symptom identification and spread of FW
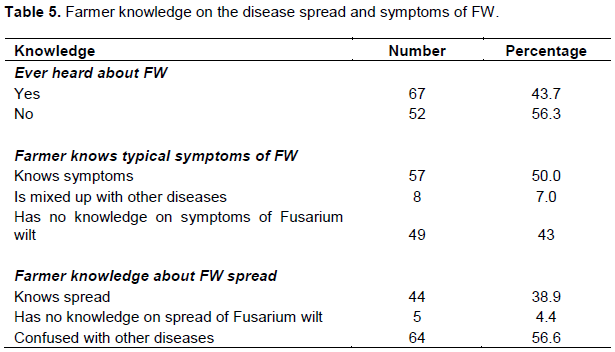
In a detailed interview, farmers were requested to explain the symptoms exhibited in FW infected plants and probed to reveal if such symptoms are not confused with Banana Xanthomonas Wilt (BXW) or other biotic stresses. Half of the respondents (50%) explained typical symptoms of FW (Table 5). Nevertheless, farmers still confuse FW with BXW and other disorders. For instance, farmers in Rubirizi district reported corm discoloration in EAHBs, yet these are known to be resistant to FW (Viljoen et al., 2017; Arinaitwe et al., 2019) (Plate 1). Understanding the spread of FW still remains a paradox to farmers, majority (56.6%) had no knowledge about the spread and only 38.9% could clearly describe the spread by associating it with soil movement from infected site’.

Effect of management practices and plantation age on FW incidence
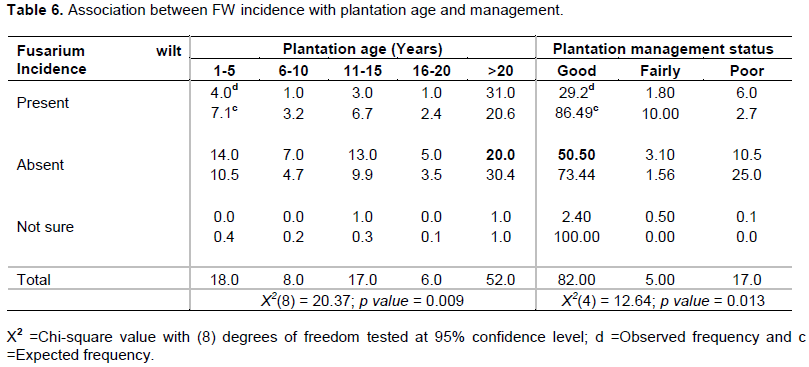
A Chi-squared test performed to test the association between FW incidence and management status of banana plantations was significant (p < 0.05) (Table 6). Remarkably, more incidences (86.5%) of FW were found in well managed farms (weeded, pruned, de-suckered with corms removed) than fairly managed (10%) and poorly/nearly abandoned farms (2.7%). Results further indicated a significant relationship (p < 0.05) between FW incidence and plantation age with highest incidences observed in older plantations established more than 20 years ago (Table 6).
DISCUSSION
Demographic factors within banana growing regions of Uganda
Fusarium wilt remains one of the most devastating diseases in several banana growing regions of the world and thus a global threat to banana production (Ploetz, 2015b; Kema et al., 2020). In this study, we identified the spatial distribution of FW in the banana growing areas and within banana subgroups, ascertained the effect of management practices and plantation age on FW incidence, as well as investigated how much information is known by farmers in regard to the symptoms and spread of FW in Uganda. Results showed that more males (63%) are involved in banana production than female (37%) in the major banana growing areas. The higher involvement of males in banana production suggest that men (males) are the likely more responsible for cash crops management than women as the latter mainly provide labour, although both males and females could be involved in decision making on the farms for increased income (Bjornlund et al., 2019; Rietveld et al., 2018).
Attaining formal education has been strongly correlated with adoption of management practices (Kikulwe and Asindu, 2020; Ebewore, 2016). In our study, 22.7% of the respondents had never attained any formal education, implying that management practices devised to curtail Fusarium wilt, need to be simplified in a more user-friendly manner and employ practical means like farmer field schools. It was interesting to discover in this study that a notable number (8.4%) of university graduates owned banana farms and were engaged in daily management of plantations. It is envisaged that the future of agriculture lies in the hands of youths, as the need for youths into agriculture has been documented (Irungu et al., 2015; Mukembo and Edwards, 2020). In Uganda, the call for more youths in agriculture has been emphasized in the National Development Plan phase 3 (NDP III) (National Planning Authority, 2020). Finding ten youths already in banana farming in Uganda is evident to show that the country is on the right trend.
Findings further reveal that banana farmers had an average land size of 4.3 acres with about 66.3% allocated to banana production. Banana is a major food security crop accounting for much of farmers’ income and is more profitable than annual food crops such as maize, sweet potatoes and cassava and thus the most important food crop in the country (Kiiza et al., 2004; Nyombi, 2013). The study was represented by farmers with depth in responding to questions related to banana farming as showcased by the average experience in banana production of 25.4 years, with some farmers recorded to have spent 50 years in this business.
Distribution (spatial and within Musa spp.) and status of FW based on symptomatology within major banana growing areas
Data on spatial distribution of Fusarium wilt remains scanty. This makes it difficult to map out hot spot experimental areas, sampling program for disease losses, and conducting cost benefit analysis studies on available management options for Fusarium wilt as highlighted by Staver et al. (2020). This study revealed a wide spatial distribution of symptoms associated with FW disease in all altitudes agrees with Gudero et al. (2018). However, there was a significant difference (p < 0.05) between FW incidence and districts. Kapchorwa, (a district on high altitude, 1950 masl) expressed highest incidence of symptoms associated with FW. The wide distribution of FW agrees with however, the results of altitude and FW contradicts with Karangwa et al. (2016) who reported high symptoms associated with FW at low altitudes. In Kapchorwa, it was observed normal for germplasm exchange between farmers; this traditional exchange of planting materials (sometimes infected) from one field to a healthy one could be the underlying reason for higher incidences of symptoms associated with FW observed in Kapchorwa.
Interestingly, no symptoms of FW were observed in sampled districts within low altitude (Kayunga and Luweero). There is a high likelihood that other factors apart from altitude contributed to this finding. For instance, Kayunga was the district where Banana Xanthomonas Wilt (BXW) was first observed in the early 2000’s, with an impact that was unbearable and that forced farmers to uproot any symptomatic plant and mats, most of these mats were never recovered/ replanted, but rather the land was diversified to other income generating activities (Tushemereirwe et al., 2001). It is envisaged that, continued removal of infected plants could have reduced the inoculum and also reduce population of susceptible varieties, which is in agreement with Tushemereirwe et al. (2004).
In this study, we also found that the EAHBs were the most cultivated Musa species followed by Sukali Ndizi (AB), and Gross Michel (AAA), indicating their economic importance and consumer preferences (Figure 1). For instance, among deserts, Sukali Ndizi (AAB) is the most popular because of its compact bunch, short fingers and very sweet flavour when ripe (Gold et al., 2002). Farmers’ perception according to resistance ranked Sukali Ndizi (AB) as the most susceptible Musa species followed by Gross Michel (AAB) and Bluggoe (ABB). In addition, all clones of Pisang Awak (ABB) genome were reported susceptible and with resistance to FW on FHIA (AAAA), Gonja (AAB) and KM5 (AAA). Our findings are consistent with Kangire et al. (2000) who reported that Sukali Ndizi (AB) variety is very much susceptible to FW. The findings are also consistent with Tushemereirwe et al. (2001) who reported that the FHIA varieties are resistant.
Results also showed that the least grown Musa species were Kayinja (ABB) and Kivuuvu (AB). Despite the low production of Kayinja and Kivuuvu, the contribution by the two in banana production system should not be underestimated. For example, Kayinja produces more juice for beer production as compared to the indigenous beer locally known as “Mbidde” whereas Kivuuvu is preferred in some areas for cooking compared to EAHBs due to low production costs (Bagamba et al., 2006). However, during an epidemic of Banana Xanthomonas wilt in an area, Kayinja and Kivuuvu are the first to be infected (Tushemereirwe et al., 2001). Thus, their susceptibility has tremendously contributed to reduction in mat stands and given that both varieties are susceptible to the two diseases, the decline in mat stands is highly likely to continue unless proper management strategies are reinstated.
Understanding farmers’ knowledge regarding FW symptom identification and spread
Like other diseases, FW can effectively be managed when accurate diagnosis of the diseases through symptomatology and other DNA based methods are correctly done (Dita et al., 2018). Farmers normally rely on symptoms for understanding the disease and devise management strategies. We investigated to ascertain how much is known about symptoms and spread of FW and found out that 50% of the respondents could explicitly explain typical symptoms of FW without confusing it with other Musa spp. diseases such as BXW, of which only 38.9% could understand how the disease spreads. This shows that limited information occurs on identification and spread. FW spread was predominantly confused with BXW as evidenced by farmers practicing removal of male inflorescence and stating that FW was spread through same by the bees, while others were strongly disinfecting tools after cutting a susceptible plant. It is known that being a soil-borne pathogen, dispersal for FW takes place by passive movement of soil particles and spores in soil propagules at short and long distance mainly by water runoff and (or) animals (Dita et al., 2018). The knowledge gap for symptom identification and spread could be one of the underlying factors for continuous spread of FW in sampled areas and more research needs to be directed towards this direction to build the capacity of farmers in understanding symptoms and spread so that they can attach meaning to management practices proposed.
Effect of management practices and plantation age on FW incidence
The study also revealed that plantation age plays a significant role in incidences of FW disease. The highest incidences of FW were observed in older plantations established more than 20 years ago. The effect of plantation age on disease incidence in banana has not received enough attention; nevertheless, scanty studies have been conducted (Mobambo et al., 1996; Karangwa et al., 2016). While conducting a study on distribution and incidence of banana Fusarium wilt in East and Central Africa, Karangwa et al. (2016) found out that plantation age was significantly associated with FW incidence with prevalence more pronounced in plantations of 10-30 years. This is consistent with our findings. Although this interaction has not adequately been investigated, it could be linked with the long duration that the pathogen stays in soil (Stover, 1972). This means that plantations which are already infected will remain as secondary inoculants for a long period. Therefore, as the field becomes old, more and more pathogens could accumulate in the soil if proper soil management practices such as sterilization, application of bio control, soil amendments are not performed over time (Ploetz, 2015b).
The Chi-square conducted revealed an association between Fusarium wilt and management, with higher incidences in well managed plantations, than poorly/nearly abandoned plantations. Well managed plantations involve several agronomy practices for instance mulching and weeding (sometimes with a hoe). These encourage continuous soil disturbance which promotes movement of spores in infected sites (Alabouvette, 1986). For this reason, more incidences are more pronounced in well managed plantations than poorly/nearly abandoned ones which receive less soil disturbance. For the first time, our study reports field abandonment as one of the managements practices used in Uganda (Plate 2). It was reported that instead of cutting down entire plantation, abandoning the field and still harvest a substantial yield is a better option. According to FAO (2019), this practice has been commonly used in Philippines as a worst-case Fusarium wilt management practice. Understanding the mechanism behind this claim will require further research involving field experiments but could also be linked with the concept of suppressive soils being left undisturbed. Alabouvette (1986) reported the role of soil microflora in supressing FW. The author asserts that soils abundant with micro-organisms tend to have a suppressive effect, probably poorly managed or abandoned fields receive minimal disturbance thus encouraging more microbial growth which significantly contribute to soil suppression. In addition, abandoned plantations could be more nutrient-rich due to high microbial activity, and recently, Nowembabazi et al. (2021) revealed the enormous importance of nutrients especially potassium in reducing FW incidence in apple bananas.

CONCLUSION
The study revealed a notable number of growing university graduates in banana production. Fusarium wilt in banana is widely distributed in the major banana growing areas of Uganda with Kapchorwa district showing the highest incidences. According to farmers, Sukali Ndizi (AB), Gross Michel (AAB) and Bluggoe (ABB) were ranked susceptible to FW in that order whereas FHIA (AAAA), Gonja (AAB) and KM5 (AAA) were considered resistant. The disease was reported to be increasing by the majority, and the drivers for this increment were majorly limited knowledge on the spread that encourages management practices similar to BXW as well as traditional exchange of germplasm especially dessert ones. At least, half of the interviewed farmers know how to separate symptoms associated with FW from other banana diseases especially BXW. However, a majority are still challenged with understanding how the disease spreads and how it can be managed. Fusarium wilt symptoms were more pronounced in older plantations with 20 years from date of establishment and such symptoms are common within well managed plantations. Also, higher incidences of FW wilt observed in older plantations as well as well managed plantations need to be further investigated. Interestingly in this study, we found out field abandonment as a strategy for managing already infected field. This study predisposes array of research in near future, for instance the effect of intercrops in supressing FW, a detailed study on how farmers are managing FW in Uganda, understanding the mechanism of field abandonment in managing FW as well as the role of variety mixtures in supressing FW.
CONFLICT OF INTERESTS
The authors have not declared any conflict of interests.
Funding
This study was supported by funds from the CGIAR Research Program on Roots, Tubers and Bananas (RTB).
REFERENCES
|
Alabouvette C (1986). Fusarium-wilt suppressive soils from the Châteaurenard region: review of a 10-year study. Agronomie 6(3):273-284. |
|
|
Arinaitwe IK, Teo CH, Kayat F, Tumuhimbise R, Uwimana B, Kubiriba J, Othman JA HRY (2019). Molecular Markers and Their Application in Fusarium Wilt Studies in Musa spp. SainsMalaysiana 48(9):1841-1853. |
|
|
Bagamba F, Kikulwe E, Tushemereirwe WK, Ngambeki D, Muhangi J, Kagezi GH, Green S (2006). Awareness of banana bacterial wilt control in Uganda: 1. Farmers' perspective. African Crop Science Journal 14(2):157-164. |
|
|
Bjornlund H, Zuo A, Wheeler SA, Parry K, Pittock J, Mdemu M, Moyo M (2019). The dynamics of the relationship between household decision-making and farm household income in small-scale irrigation schemes in southern Africa. Agricultural water Management 213:135-145. |
|
|
Buregyeya H, Tumuhimbise R, Matovu M, Tumwesigye KS, Kubiriba J, Nowankunda K, Tushemereirwe WK, Karamura D, Karamura E, Kityo RM, Rubaihayo R (2020). Journal of Plant Breeding and Crop Science 12(1):16-24. |
|
|
Dita M, Barquero M, Heck D, Mizubuti ES, Staver CP (2018). Fusarium wilt of banana: current knowledge on epidemiology and research needs toward sustainable disease management. Frontiers in Plant Science 9:1468. |
|
|
Ebewore SO (2016). Small scale banana farmers' awareness level and adoption of improved banana varieties in Delta state, Nigeria. Journal of Agriculture and Food Sciences 14(1): 48-59. |
|
|
Food and Agriculture Organization (FAO) (2019). Banana Fusarium Wilt Tropical Race 4: A mounting threat to global banana markets? Food and Agriculture Organization 2019 Food Outlook - Biannual Report on Global Food Markets - November 2019. Rome pp.13-20 |
|
|
FEWS NET (2017). Uganda staple food market fundamentals. Washington, D.C. FEWS NET. |
|
|
FRuiTRoP (2016). World banana statistics 2016: Production, Imports, Exports. CIRAD: Montpellier pp. 86-87. |
|
|
Gold CS, Pinese B, Peña JE (2002). Pests of banana. Tropical fruit pests and pollinators: biology, economic importance, natural enemies and control. pp. 13-56. |
|
|
Gudero MG, Terefe YH, Kesho SA (2018). Spatial distribution and association of banana (Musa spp.) Fusarium wilt (Fusarium oxysporum f. sp. cubense) epidemics with biophysical factors in southwestern Ethiopia. Archives of Phytopathology and Plant Protection 51(11-12):575-601. |
|
|
Heck DW, Dita M, Ponte EMD, Mizubuti E S (2021). Incidence, Spatial Pattern and Temporal Progress of Fusarium Wilt of Bananas. Journal of Fungi 7(8):646. |
|
|
Irungu KRG, Mbugua D, Muia J (2015). Information and Communication Technologies (ICTs) attract youth into profitable agriculture in Kenya. East African Agricultural and Forestry Journal 81(1):24-33. |
|
|
Kangire A, Karamura EB, Gold C, Rutherford MA (2000). Fusarium wilt of banana in Uganda, with special emphasis on wilt-like symptoms observed on East African Highland cooking cultivars (Musa spp., AAA). ActaHorticulturae 540:343-353. |
|
|
Karamura D, Karamura E, Nsabimana A, Ngezahayo F, Bigirimana SC, Mgenzi B, Tendo S (2012). The current classification and naming of the East African highland bananas (Musa AAA) based on morphological characteristics. Banana Cultivar Names, Synonyms and their Usage in Eastern Africa. Biodiversity International Kampala. pp. 6-23. |
|
|
Karangwa P, Blomme G, Beed F, Niyongere C, Viljoen A (2016). The distribution and incidence of banana Fusarium wilt in subsistence farming systems in east and central Africa. Crop Protection 84:132-140. |
|
|
Karangwa P, Mostert D, Ndayihanzamaso P, Dubois T, Niere B, ZumFelde A, Viljoen A (2018). Genetic diversity of Fusarium oxysporum f. sp. cubense in East and Central Africa. Plant Disease 102(3):552-560. |
|
|
Kema GH, Drenth A, Dita M, Jansen K, Vellema S, Stoorvogel JJ (2020). Fusarium Wilt of Banana, a Recurring Threat to Global Banana Production. Frontiers in Plant Science 11 p. |
|
|
Kiiza B, Abele S, Kalyebara R (2004). Market opportunities for Ugandan banana products: National, regional and global perspectives. Uganda Journal of Agricultural Sciences 9(1): 743-749. |
|
|
Kikulwe EM, Asindu M (2020). A contingent valuation analysis for assessing the market for genetically modified planting materials among banana producing households in Uganda. GM Crops and Food 11(2):113-124. |
|
|
Leaky ALC (1970). Diseases of bananas. In: Jameson JD (ed), Agriculture in Uganda. London: Oxford University Press. pp. 143-145. |
|
|
Liu L, Liang CC, Zeng D, Yang LY, Qin HY, Wang GF, Guo LJ, Huang JS (2015). Spatial distribution pattern for the Fusarium wilt disease in banana field and the Fusarium oxysporum f. sp. cubense in different parts of banana plants. Acta Ecologica Sinica 35:4742-4753. |
|
|
Madden LV, Hughes G (1995). Plant disease incidence: distributions, heterogeneity, and temporal analysis. Annual Review of Phytopathology 33(1):529-564. |
|
|
Meldrum RA, Daly AM, Tran-Nyuyen LTT, Aitken EAB (2013). Are banana weevil borers a vector in spreading Fusarium oxysporum f.sp.cubense tropical race 4 in banana plantations? Australasian Plant Pathology 42(5):543-549. |
|
|
Mobambo KN, Gauhl F, Pasberg-Gauhl C, Zuofa K (1996). Season and plant age effect evaluation of plantain for response to black sigatoka disease. Crop Protection 15(7): 609-614. |
|
|
Mostert D, Molina AB, Daniells J, Fourie G, Hermanto C, Chao CP, Viljoen A (2017). The distribution and host range of the banana Fusarium wilt fungus, Fusarium oxysporum f. sp. cubense, in Asia. PLoS One 12(7):e0181630. |
|
|
Mukembo SC, Edwards MC (2020). Improving livelihoods through youth-adult partnerships involving school-based, agripreneurship projects: The experiences of adult partners in Uganda. Journal of International Agricultural and Extension Education 27(2):62-76. |
|
|
Nalunga A, Kikulwe E, Nowakunda K, Ajambo S, Naziri D (2015). Technical report: Structure of the cooking banana value chain in Uganda and opportunities for Value addition and postharvest losses reduction. |
|
|
National Planning Authority (NPA) (2020). The Third National Development Plan (NDPIII) 2020/21 - 2024/25. Kampala, Uganda. |
|
|
Nowembabazi A, Taulya G, Tinzaara W, Karamura E (2021). Effect of integrated potassium nutrition on Fusarium wilt tolerance in apple bananas. African Journal of Plant Science 15(9):257-265. |
|
|
Nyombi K (2013). Towards sustainable highland banana production in Uganda: opportunities and challenges. African Journal of Food, Agriculture, Nutrition and Development 13(2). |
|
|
Ploetz RC (2005a). Panama disease: an old nemesis rears its ugly head: part 1. The beginnings of the banana export trade. Plant Health Progress 6(1):18. |
|
|
Ploetz RC (2015b). Management of Fusarium wilt of banana: A review with special reference to tropical race 4. Crop Protection 73:7-15. |
|
|
Rietveld A, Farnworth CR, Badstue LB (2018). Towards gender-responsive banana research for development in the East-African Highlands: GENNOVATE resources for scientists and research teams. CDMX (Mexico): CIMMYT P 6. |
|
|
Ristaino JB, Gumpertz ML (2000). New frontiers in the study of dispersal and spatial analysis of epidemics caused by species in the genus Phytophthora. Annual Review of Phytopathology 38(1):541-576. |
|
|
Staver C, Pemsl DE, Scheerer L, Perez Vicente L, Dita M (2020). Ex ante assessment of returns on research investments to address the impact of Fusarium wilt tropical race 4 on global banana production. Frontiers in Plant Science 11:844. |
|
|
Stover RH (1972). Banana, plantain and abaca diseases. Banana, plantain and abaca diseases. Commonwealth Mycological Institute, United Kingdom. |
|
|
Thangavelu R, Loganathan M, Arthee R, Prabakaran M, Uma S (2020). Fusarium wilt: a threat to banana cultivation and its management. CAB Reviews 15(004):1-24. |
|
|
Tinzaara W, Karamura EB, Kubiriba J, Ochola D, Ocimati W, Blomme G, Ssekiwoko F (2014). The banana Xanthomonas wilt epidemic in east and central Africa: current research and development efforts. In XXIX International Horticultural Congress on Horticulture: Sustaining Lives, Livelihoods and Landscapes (IHC2014): IX 1114. pp. 267-274. |
|
|
Tushemereirwe W, Kangire A, Ssekiwoko F, Offord LC, Crozier J, Boa E, Smith JJ (2004). First report of Xanthomonascampestrispv. musacearum on banana in Uganda. Plant Pathology 53(6):802-802. |
|
|
Tushemereirwe WK, Kangire A, Smith J, Nakyanzi M, Karyeija R, Kataama D, Musiitwa C (2001). An outbreak of banana bacterial wilt in Mukono and Kayunga districts; a new and devastating disease. NARO/KARI. |
|
|
Uganda Bureau of Statistics Statistical (UBOS) (2019). Uganda Bureau of Statistics Statistical Abstract of 2019. Kampala. |
|
|
Veena DR, Priya HR, Raheesa M, Khatib DJ (2014). Soilborne Diseases in Crop Plants and Their Management. Research & Reviews: Journal of Agriculture and Allied Sciences 3(2). |
|
|
Viljoen A, Ma LJ, Molina AB (2019). Fusarium wilt (Panama disease) and monoculture banana production: resurgence of a century-old disease. Emerging plant diseases and global food security. |
|
|
Viljoen A, Mahuku GS, Massawe C, Tendo Ssali R, Kimunye JN, Mostert G, Coyne D L (2017). Banana diseases and pests: field guide for diagnostics and data collection. International Institute of Tropical Agriculture (IITA), Nairobi, Kenya. |
|
|
Viljoen A, Mostert D, Chiconela T, Beukes I, Fraser C, Dwyer J, Murray H, Amisse J, Matabuana EL, Gladys Tazan G, Amugoli OM, Mondjana A, Vaz A, Pretorius A, BothmaI S, RoseI LJ, Beed F,Dusunceli F; Chao CP, Molina AB (2020). Occurrence and spread of the banana fungus Fusarium oxysporum f. sp. cubense TR4 in Mozambique. South African Journal of Science 116(11-12):1-11. |
|
Copyright © 2024 Author(s) retain the copyright of this article.
This article is published under the terms of the Creative Commons Attribution License 4.0