Peanut (Arachis hypogaea L.) is an important and widely-consumed food legume in the world. However, peanut contamination by aflatoxins is a critical food safety issue in most countries in sub-Saharan Africa including Zambia (Njoroge et al., 2016) mostly because of lack of detection, monitoring and enforcement of existing regulatory measures to safeguard the food supply chain. Aflatoxins are naturally-occurring toxic metabolic substances produced by certain fungi predominantly, Aspergillus flavus and A. parasiticus. These fungal species are ubiquitous and thrive well in soil environments that are prohibitive to other soil-borne microorganisms (Richard and Payne, 2003). They are adapted to moisture deficits and elevated soil temperatures often associated with end-of-season drought stress (Jordan et al., 2018).
The ability to predict pre-harvest aflatoxin risk offers an opportunity for timely and appropriate interventions when the risk is high. The most critical period in peanuts is the pod-development stage which occurs in the last six weeks of pod-development. Using relationships between environmental conditions during the pod-development stage of peanut growth, researchers have shown that aflatoxin content at harvest can be predicted successfully (Henderson et al., 2000; Craufurd et al., 2005; Chauhan et al., 2010; Bowen and Hagan, 2015). At field level, late season plant exposure to prolonged moisture deficits and high soil temperatures is strongly associated with elevated aflatoxin content in harvested kernels (Hill et al., 1983; Rachaputi et al., 2002; Torres et al., 2014; Kachapulula et al., 2017; Jordan et al., 2018).
Using relationships between environmental conditions during the pod development stage of peanuts growth and aflatoxin content in harvested kernels, research has revealed that pre-harvest aflatoxin risk can be predicted successfully through regression analysis (Craufurd et al., 2005; Chauhan et al., 2010; Bowen and Hagan, 2015, Kachapulula et al., 2017). Some of the existing models are relatively complex and heavily dependent on computer simulations to calculate model parameters. There is, therefore, a need for simpler models such as the work of Bowen and Hagan (2015) that are easy to use and based on easily measurable soil and whether data parameters. This study aimed to formulate statistical models that predict pre-harvest aflatoxin contamination in peanuts using selected soil and weather parameters that affect the growth of A. flavus in the geocarposphere (immediate soil environment surrounding peanut pods). Further we determined total aflatoxin content in harvested kernels and quantified relationships between crop growth conditions during pod development and total aflatoxin contents.
Site description
Field experiments were conducted at the University of Zambia, Field Research Station (15.4665 °S, 28.3328° E) for two successive rain-fed cropping seasons. The site is located in Lusaka district (Figure 1) and falls within the agro-ecological region ІІa of Zambia characterized by mean annual rainfall of 800-1000 mm (Soil Survey Branch, 2002). The rain-fed cropping season at the site runs from mid-December to late April (Table 1). The soil type at the site had a sandy loam texture and was classified as typic isohyperthemic paleustalf using the USDA Soil Taxonomy (Banda and Chabala, unpublished report).
Field experimental set up and treatments
Three field experiments (plant population density, phased planting and cultivar susceptibility experiments) were conducted for two years to evaluate the impact of soil moisture content and soil temperature conditions on pre-harvest aflatoxin incidence in peanuts grown under rain-fed conditions. The first set of experiments was conducted in the 2016/2017 and repeated in the 2017/2018 season. In all the experiments, experimental plots measured 4 m x 4 m with a 1 m border. Land was prepared by ploughing to a depth of 20 cm using a hand-hoe. Seed beds were prepared by levelling and breaking clods in the ploughed area. Peanut seed was sown on the flat seed beds. In the plant population density and phased planting experiments, a virginia market type peanut cultivar, MGV 4, was grown to full maturity. As for the cultivar susceptibility experiment, four aflatoxin susceptible virginia market type medium kernelled cultivars with a bunch growth habit were evaluated. These cultivars were MGV4, MGV6, MGV7 and Chishango. Crop management practices included regular weeding and application of pesticides to control pests, mostly black aphids. Soil moisture content and day-time soil temperature were measured daily during the last 30 days of pod development.
In the plant population density experiments, the recommended inter-row spacing of 75 cm was maintained and used three intra-row plant spacing of 5, 10 (recommended) and 15 cm to achieve three plant densities of 266 667, 133 333 and 66 667 plants per hectare, respectively. This experiment aimed to vary the soil moisture and soil temperature conditions under the crop canopy by varying the planting density. Thus, the experiment evaluated the effects of the three plant population densities on soil moisture content and soil temperature under the canopy and related them to pre-harvest aflatoxin content in peanut kernels.
The plant population density has an effect on the crop canopy structure and subsequently the amount of shading under the canopy. Also, plant population density has an effect on water uptake from the root-zone. Both shading and water uptake by plants have impacts on soil moisture and soil temperature dynamics in the root and pod zones. Treatments were laid out in a randomized complete block design (RCBD) and replicated four times.
In the phased plating experiment, the aim was to expose the peanut crop to different soil moisture and soil temperature conditions during the pod development stage. Therefore, the experiment evaluated the effects of selected planting dates on pre-harvest aflatoxin incidence under rain-fed conditions. A virginia market type peanut cultivar with a bunch growth habit, MGV4 was planted successively at irregular intervals from the on-set of the planting season for each of the two years. Peanut seed was sown on dates when the soil was visibly moist for optimal germination. In the 2016/2017 season, peanut seed was sown in five phases. In the 2017/2018 season, crops were planted in seven phases. Some of the planting dates exposed the crop to the end-of-season drought in the final weeks prior to physiological maturity. Treatments were laid out in a randomized complete block design (RCBD) and replicated four times.
In the cultivar susceptibility experiment, the aim was to evaluate the effect of selected cultivars on soil moisture content and soil temperature under the canopy and subsequently the pre-harvest aflatoxin contamination risk in kernels. Treatments were four aflatoxin-susceptible virginia market type medium-kernelled cultivars with a bunch growth habit. The selected cultivars were MGV4, MGV6, MGV7 and Chishango. The named cultivars have the same maturity period of 120-130 days after planting. These treatments were laid out in a Latin square experimental design and replicated four times.
Determination of soil moisture and temperature
In-situ soil moisture content and soil temperature in the pod zone were measured daily in the final 30 days of pod development, which is a critical period for pre-harvest aflatoxin development in the peanut growth cycle according to Sanders et al. (1985).
Considering that most peanut pods are formed within 5 cm depth from the soil surface, all measurements were taken within this depth under the plant canopy. Six evenly spread focal points within the net plot (excluding the outer plant rows) were chosen as measurement points. Soil temperature and moisture readings from the focal points were averaged to represent an entire experimental unit. These focal points were located under the crop canopy in the intra-row spaces. Soil temperature was measured using a soil temperature probe (HI98331, Hanna Instruments, Kungsbacka, Sweden). Soil moisture content under the canopy was measured using the SM150 Soil Moisture Kit (SM150T, Delta-T Devices, Cambridge, England).
Sample preparation and determination of total aflatoxin content
Physiologically mature pods were harvested by digging using a hand hoe. The pods were then dried to 10% gravimetric moisture content in an electric vacuum drier (D-6450 Hanau, Heraeus Instruments, Germany). Samples for laboratory analysis were obtained from shelled kernels. The samples were ground into powder using an ordinary kitchen grinder and temporarily stored at room temperature in airtight plastic jars.
Total aflatoxin content in samples was determined within seven days after grinding using Neogen Afla Reveal® Q+ aflatoxin kit (Neogen Corporation, USA). For each treatment, three sub-samples each weighing 10.0 g were taken and analyzed for total aflatoxin content. Thirty milliliters of 65% ethanol obtained by diluting 95% ethanol was added to the peanut powder. The mixture was shaken on a rotary shaker (ISO-9001-2000, Navyug, India) at 120 rpm for 3 min and then filtered the suspension through Whitman 42 filter paper. Five hundred microliters of the diluent buffer solution were added into a sample dilution cup using a standard 500 µl micro pipette and then thoroughly mixed with 100 µl of each sample extract using a clean, sterile micro pipette. One hundred microliters of the sample/diluent solution mixture was transferred into a measuring cup and then one Afla Reveal® Q+ test strip was placed inside the cup and allowed to react for 6 min. This was done for each sample and after 6 min; the aflatoxin content was determined by placing the strip into a strip holder and read by an aflatoxin reader application installed on a computer tablet (K011, ASUS Corporation, USA). The lower and upper limits of detection of aflatoxin were 1 and 50 µg/kg, respectively. Aflatoxin standard solutions for peanuts were used to ensure that the aflatoxin reader was functional during the analysis.
Data management and analysis
The data were checked for outliers and observations with a magnitude 3 times the inter-quartile range classified as extreme outliers and were removed from the data set. The Levene’s test of homogeneity of variance was then applied on data sets from individual experiments. The analysis of variance (ANOVA) was done to determine the effects of plant population density, phased planting and cultivar susceptibility on total aflatoxin content in kernels. Total aflatoxin content in kernels was predicted using the selected parameters by pooling data from individual experiments before performing linear regression analysis. Data pooling was done in order to strengthen the resultant regression models. This was because the number of observations from individual experiments was sometimes as low as only 12 measurements. The selection of predictor variables involved conducting Pearson’s correlation analysis to identify variables that significantly correlated with the output variable. Significantly correlated variables were then subjected to stepwise multiple linear regression analysis in order to formulate regression models to predict aflatoxin contamination risk in harvested kernels.
Statistical significance of all the tests was judged at 5% level of significance. Statistical analysis was conducted using IBM SPSS software package version 20.0 (SPSS Inc, Chicago, USA).
Effect of plant population density on pre-harvest aflatoxin content in peanut kernels
A higher plant population density was associated with lower aflatoxin content in kernels in both seasons. During the 2016 season, the total aflatoxin content in peanut kernels for the treatment with 66,667 plants per hectare was 23 and 38% higher than the total aflatoxin content in kernels for treatments with 133, 333 and 266, 667 plants per hectare, respectively (Table 2). Although, the total aflatoxin content tended to be lower in kernels from the treatment with 266, 667 compared with 133, 333 plants per hectare, the difference was not significant (Table 2). In the 2017 season, the mean total aflatoxin content in the kernels from treatments 66,667 and 133,333 plants per hectare was respectively 1.54 and 1.56 times higher than aflatoxin content in kernels from 266,667 plants per hectare treatment (Table 2). Additionally, the mean total aflatoxin content in kernels was positively correlated with soil temperature and negatively correlated with soil moisture content in both season. This result is consistent with the findings of Chalwe et al. (2016) who reported observed lower aflatoxin content at higher soil moisture content during pod development. Pitt et al. (2013) showed that a favorable soil moisture status is linked to greater soil microbial activity, which tends to increase competition among soil fauna. This minimizes the dominant tendencies of A. flavus and A. parasiticus.
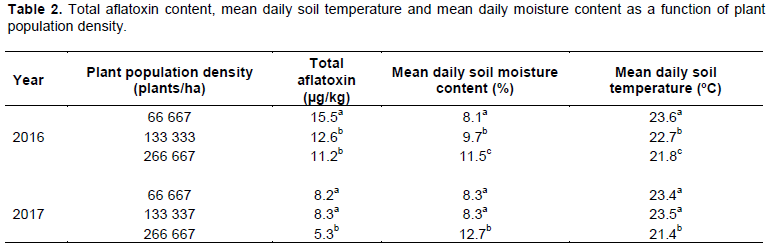
Treatments followed by the same letter in the same column did not show significant differences in total aflatoxin content per season.
Effect of phased planting on total aflatoxin content in peanut kernels
Planting peanut seed in phases did not show a consistent relationship with pre-harvest aflatoxin content in peanut kernels (Table 3). During the 2016 season, there were no significant differences in aflatoxin content attributable to the variation in planting dates. During the 2017 season, the total aflatoxin content decreased by 62% between the fifth and seventh planting dates. Generally, the mean total aflatoxin content in harvested kernels was 59% lower in the first season than in the second season. The lower aflatoxin level during 2016 season was associated with higher mean daily volumetric soil moisture content in the last 30 days of pod development which was 2.3 times more compared with 2017 season. Nevertheless, there was a strong negative correlation (r = -0.642) between total aflatoxin content in kernels and the mean daily soil moisture content in the pod zone. On the contrary, soil temperature did not significantly correlate with the total aflatoxin content in kernels (Table 3).
The use of phased planting to estimate aflatoxin contamination risk is based on the effect of crop exposure to end-of-season drought often associated with high temperatures and low soil moisture. As documented by other authors (Hill et al., 1983; Craufurd et al., 2005), soil temperature and soil moisture content are among the most important soil parameters affecting the activity of Aspergillus fungi and their capacity to produce aflatoxins in the field. In this study, it was observed that the effect of drought during pod development on aflatoxin development depended on mean soil moisture content and soil temperature as independent factors each having a significant effect on aflatoxin development.
One of the challenges of using the duration of exposure to end-of-season drought stress to predict aflatoxin contamination risk in peanut kernels is that drought stress is not always associated with high soil and ambient temperature. In Zambia, both ambient and soil temperature have a decreasing trend during the growing season (Table 1), signifying the need to determine actual temperatures during pod development.
Effect of cultivar susceptibility to aflatoxin contamination on total aflatoxin content in peanut kernels
There was no significant difference in total aflatoxin content in kernels attributable to the type of cultivar (Table 4). This could be because all the cultivars evaluated in this study were susceptible to aflatoxin contamination. In addition, there were no significant differences in mean daily soil temperature and the daily soil moisture content during pod development between cultivars. Nevertheless, total aflatoxin content in kernels significantly correlated with soil temperature and soil moisture content (Table 4). Therefore, soil temperature and soil moisture content were key determinants of aflatoxin contamination risk in the different cultivars.
Predicting total aflatoxin content in peanut kernels using soil temperature
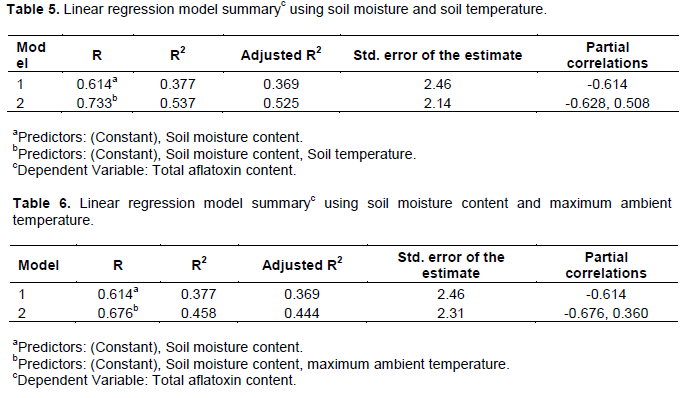
There was a significant positive linear relationship between total aflatoxin content in kernels and soil temperature at 5 cm soil depth under the canopy during the last 30 days to physiological maturity (Figure 2). Total aflatoxin content was greater at higher mean daily soil temperature in the pod zone during pod development. Based on soil temperature, total aflatoxin content in harvested kernels could be predicted according to the equation:

Where, = Total aflatoxin content (µg/kg) and = Mean soil temperature (°C) at 5 cm depth under the crop canopy.
According to Blankenship et al. (1984), the mean threshold geocarposphere temperature of 25.7 to 27ºC in the last 60 days of the growing season was necessary for aflatoxin development and there was 95.7% colonization of undamaged mature kernels by A. flavus due to heat treatment raising the mean soil temperature to 30.5ºC. This was proportionate to aflatoxin content in harvested kernels, which was higher with greater colonization of pods. Although Blankenship et al. (1984) suggested non-existence of aflatoxin contamination in treatments with geocarposphere temperatures of 23.6 ºC or less, a study by Chauhan et al. (2010) reported that the formation of aflatoxins occurred at soil temperatures ranging from 22 to 35ºC under water deficit conditions. According to Chauhan et al. (2010), soil temperatures below 22ºC were assigned a zero to the calculated soil temperature function of the model.
In the current study, there was a considerably high total aflatoxin content in kernels (4.7 µg/kg) even at low mean daily soil temperature of 21.4ºC. This result could be attributed to other confounding crop growth factors such as soil moisture content. Another possible explanation is that there could have been days of higher than the recorded average soil temperature. Such periods of high temperature could have allowed for aflatoxin development in the current study. As observed in the current study, ignoring soil temperatures less than 22ºC can lead to an underestimation of the aflatoxin content in peanut kernels.
Predicting total aflatoxin content in peanut kernels using soil moisture content
The total aflatoxin content in peanut kernels was higher at lower mean daily soil moisture content during the final 30 days of pod development until harvesting (Figure 3). According to the fitted regression line (unstandardized R2 = 0.38), moisture alone accounted for 38 % of the observed variations in aflatoxin content. The incidence of pre-harvest total aflatoxin content in kernels followed the equation:
Where, = Total aflatoxin content (µg/kg), and = Mean volumetric soil moisture content (%) in the top 5 cm soil
layer under the canopy.
Soil moisture content is an important variable influencing the colonization of pods by Aspergillus flavus and the subsequent aflatoxin contamination of kernels (Hill et al., 1983). Craufurd et al. (2005) reported a strong negative linear relationship between kernel infection by aflatoxin and the average simulated fraction of extractable soil water (FESW) from flowering until harvesting. The FESW was calculated using simulated daily soil water content in the root zone in the last 40-25 days of the growing season. According to Wotton and Strange (1987), kernels developing under low soil moisture content have reduced capacity to produce phytoalexin, an antimicrobial substance that inhibits fungal growth and thus offer resistance to the invasion of peanut kernels by A. flavus. This observation implies that higher moisture content promotes resistance of pods to fungal colonization and subsequent aflatoxin contamination of kernels suggesting that soil moisture conservation is an essential factor in minimizing pre-harvest aflatoxin contamination. For instance, the current study showed that higher soil moisture content at higher planting density was associated with lower total aflatoxin content in harvested kernels.
According to Pitt et al. (2013) drought-stressed plants show a reduced natural defence against infection due to reduced metabolism as plants wilt. Additionally, drought stress tends to reduce water activity (aw; the ratio of the vapor pressure of soil to that of pure water) in the soil, resulting in reduced microbial activity while at the same time promoting the growth of the adapted organisms such as, A. flavus and A. parasiticus (Dorner et al., 1989). In a study involving fungal-resistant crop varieties Guo et al. (2008) showed that drought stress negatively affected the expression of genes for resistance against aflatoxin formation.
Predicting total aflatoxin content in peanut kernels using ambient temperature
Total aflatoxin content in harvested peanut kernels was greater at high average maximum ambient temperatures during pod development (Figure 4). The total aflatoxin content in kernels was predicted according to the equation:
Where, = Total aflatoxin content (µg/kg), and =
Mean maximum ambient temperature (°C) during pod development.
This result can be attributed to the strong link between maximum ambient temperature and day-time soil temperature, responsible for aflatoxin development in peanut kernels. Studies by Bowen and Hagan (2015) and Kachapulula et al. (2017) revealed strong positive relationships between aflatoxin content in peanut kernels and ambient temperatures during the cropping season. According to Achar and Sanchez (2006) the maximum growth of A. flavus and the subsequent formation of aflatoxins in freshly harvested mature undamaged groundnut pods occurred at ambient temperatures ranging from 27 to 30°C, while fungal growth was limited at temperatures less than 10°C and above 37°C, with less than detectable concentrations of aflatoxins being measured.
Ambient temperature data are relatively easy to access from most online weather data services. It is also one of the most frequently measured data parameter in almost all modern weather stations. In Zambia, a free weather data service is provided by the Southern African Science Service for Climate Change and Adaptive Land Management (SASSCAL) WeatherNet. This data service is accessible online at http://www.sasscalweathernet.org. Farmers can use these data and determine the aflatoxin contamination risk in their region using the proposed model.
Predicting total aflatoxin content in peanut kernels using soil moisture content, soil temperature and ambient temperature
In the multivariate linear regression models, soil moisture content, soil temperature and maximum ambient temperature were used as input variables to predict total aflatoxin content in peanut kernels. However, maximum ambient temperature was very closely related to soil temperature (tolerance value = 0.14). This relationship is because in the lower atmosphere, the ambient temperature is a function of upward heat transfer from the soil surface to the air above it (Ahrens, 2009). Thus, higher soil temperatures are associated with elevated ambient temperatures. Accordingly, the two variables could not be used simultaneously to predict aflatoxin content in the same regression model. Maximum ambient temperature was excluded through a stepwise regression analysis that initially included all the three parameters as input variables. Therefore, only combinations of soil moisture content with either soil temperature or maximum ambient temperature were considered in the study.
Using soil moisture content and soil temperature to predict total aflatoxin content in peanut kernels yielded the following regression model:
Where, = Total aflatoxin content (µg/kg), = Mean volumetric soil moisture content (%) during pod development, and = Mean soil temperature in the pod zone (°C) during pod development.
From the above model, soil moisture content and soil temperature together could explain 54% (Unstandardized R2 = 0.54) of the variation in aflatoxin content (Table 5).
Although soil temperature is related to soil moisture content, these two variables were independent in the current study. This is because soil temperature is also a function of other factors such as soil surface cover by plants and other soil properties such as color and texture (Brady and Weil, 2010). For example, the soil under shade can be very dry and yet record a low temperature.
As discussed in the simple regression models, soil moisture and soil temperature play important specific roles in the activity of A. flavus as individual factors. Based on partial correlations of r = -0.628 and r = 0.508 (Table 6) for soil moisture and soil temperature respectively, it was shown that each of the two factors had significant impact on aflatoxin content when you control for either factor. This result implied that neither soil temperature nor soil moisture content alone could sufficiently account for aflatoxin incidence in peanut kernels. Similar results were reported by other researchers for instance, Craufurd et al. (2005) who reported that soil moisture content in the pod zone could be used to predict Aspergillus infection and pre-harvest aflatoxin contamination in peanuts only when soil temperature was high (28-34ºC). Similarly, Hill et al. (1983) reported elevated Aspergillus infection and subsequent pre-harvest aflatoxin contamination in kernels when high pod zone soil temperatures (28.4-29.6ºC) were coupled with very low (soil moisture tension of 18 bars at 5 cm under plant rows) soil moisture content. On the other hand, very low soil moisture content was not associated with higher aflatoxin content in peanut kernels as long as the soil temperature was lower than 23.6ºC (Blankenship et al., 1984).
In the second multivariate model, soil moisture content and the mean maximum ambient temperature were used as input variables to predict total aflatoxin content. According to the model, total aflatoxin content in peanut kernels was predicted as follows:
Where, = Total aflatoxin content (µg/kg), = Mean volumetric soil moisture content (%) during pod development, and = Mean maximum ambient temperature (°C) during pod development.
The above regression model had an R2 value of 0.46, implying that the model could explain 46% of the variation in aflatoxin content (Table 6). Results of the above model (Equation 5) showed that maximum ambient temperature had a significant effect on the model (P = 0.001). This explains why the multivariate model had a higher R2 value than the simple regression models based on either factor suggesting that both factors are important for better predictive ability.
Based on the higher R2 values for the two multivariate models (Equations 4 and 5) proposed in this study, it was shown that multivariate models were better predictors of pre-harvest aflatoxin content in peanut kernels. In particular, combining soil moisture content and soil temperature in one model had better predictive ability (R2 = 0.54) than all the other models.
The current work had notable similarities with published research such as Craufurd et al. (2005) (hereafter referred to as Craufurd’s model), Chauhan et al. (2010) (hereafter referred to as Chauhan’s model); Bowen and Hagan, (2015) (hereafter referred to as Bowen’s model). One of the most important similarities is that all the models were essentially regression models using at least one of the following parameters; soil moisture content, soil temperature and ambient temperature as input data variables. In particular, Equation 5 in the current study can be considered as a modification of Bowen’s model. Notable similarities include the use of stepwise regression analysis as the applied statistical method and the inclusion of maximum ambient temperature as an important input data variable. The cumulative 3-day-dry periods in Bowen’s model can be compared with soil moisture content of the current model.
Nonetheless, there were also significant differences in input data requirements especially with the other models. For instance, Craufurd’s and Chauhan’s models involved the use of computer simulations to generate certain model parameters, which required a wider data base to calculate. In Craufurd’s model, the computer based CROPGRO peanut-growth simulation model was used to simulate the fraction of extractable soil water (FESW) in the last 25 days of pod development as an input variable in the regression model to predict aflatoxin content in peanut kernels. Although the FESW was calculated using simulated daily soil water content and available water content, the overall CROPGRO model had a wider input data requirement as follows: basic crop data (flowering, podding and maturity dates), agronomic data (sowing date, soil water holding capacity and soil type) and weather data (daily rainfall, air and soil temperature, radiation and pan evaporation).
Similarly, the APSIM peanut module applied by Chauhan et al. (2010) heavily relied on computer simulation to calculate the aflatoxin risk index (ARI). The reliance on computer-simulated data may not work well for small-holder farmers in developing countries like Zambia as this category of farmers may not have the required computer skills. The other downside is the non-availability of the input data as some of the data variables are not usually included in most weather monitoring services. Therefore, similar to Bowen’s model, the current study had an advantage of using fewer and easily measurable parameters such as soil moisture content, soil temperature and ambient temperature.
In terms of predictive ability, Equation 4 (R2 = 0.54) of the current study had similar predictive ability with Craufurd’s model (R2 = 0.54) and Bowen’s model (R2 = 0.55). It is noteworthy that all the three other models discussed in this paper applied only at temperatures not very common in the wet and dry tropical conditions of Zambia. The models in current study were not restricted to temperature conditions. The other major concern with application of the existing models is that their application is climate specific. There is therefore need to adapt them to the climatic conditions prevalent in a given region.
Generally, the application of predictive models discussed in this paper requires adherence to sound crop management practices. For instance, results presented in this study were based on peanuts grown on a fertile soil under weed-free conditions and pest management using pesticides as essential crop management practices. Growing conditions other than these may negatively influence the accuracy of the proposed statistical models.