ABSTRACT
The artificial lighting conditions which promoted growth of the gametophytes and sporophytes of brown alga Undaria pinnatifida were examined. The seaweed was subjected to continuous or intermittent white, blue, or red light. There were notable, but not significant, differences in gametophyte and sporophyte growth between continuous and intermittent (104 Hz) white light conditions. Gametophyte growth was promoted most notably by white, followed by blue light. Sporophyte growth length was promoted most notably under intermittent white light, while body length and blade area were promoted notably under continuous white light. Sporophytes under blue or red light withered considerably. The results showed that white light is more beneficial for growth of both U. pinnatifida gametophytes and sporophytes compared with blue or red light. Male and female gametophyte grew more robustly under white light regardless of whether the pattern was intermittent or continuous light. However, the results further indicated that overall continuous white light promoted growth to a greater degree than did intermittent white light. Finally, white light promoted U. pinnatifida sporophyte growth to a greater degree than blue or red light.
Key words: Light wavelength, intermittent light, Undaria pinnatifida, seaweed culture, sporophyte growth.
The development of light emitting diode (LED) devices in recent years has improved control of photo environments and resulted in great improvements to technologies for culturing plants and algae in artificial environments (Mori et al., 2002). For commercial plant culture, photo environments that promote the growth of target plant species while saving energy are needed (Takatsuji, 2010).
The brown alga Undaria pinnatifida is useful as a feed additive for fish farming and food for human, and is cultivated widely in coastal areas around East Asia (FAO, 2016). Techniques for culturing U. pinnatifida in the sea are well established (Saito, 1956a; Akiyama, 1965). However, field culture production is unstable when weather conditions are poor. To ensure stable production under an artificial environment, it is necessary to identify the most effective photo environment characteristics for cultivating U. pinnatifida, such as the optimal light intensity, irradiance rhythms, and wavelength.
There have been some reports on the relationship between the growth of large marine plants such as U. pinnatifida and light intensity. It was reported that U. pinnatifida grows well under light intensities of 50–100 μmol m−2s−1 for gametophytes and 50 μmol m-2s-1 for sporophytes. (Akiyama, 1965; Saito, 1958; Baba, 2008; Zou et al., 2003; Morelissen et al., 2013).
Further, irradiance rhythms promote gametophyte growth and maturing sporophyte growth. Notoya et al. (1995) examined the relationship between the growth of young U. undarioides sporophytes and light intensity, and found that a light intensity of 80 μmol m-2s-1 and irradiance rhythm (14 h light: 10 h dark) promoted sporophyte growth. There have also been studies concerning the influence of light color on U. pinnatifida growth. Saito (1956b), Matsui et al. (1992), and Xu et al. (2005) examined the effects of light color on U. pinnatifida growth, and demonstrated that blue light is suitable for promoting the growth and maturation of gametophytes and sporophytes. However, these reports did not discuss the influence of wavelength distribution on growth in detail.
Recently, it was reported that intermittent light promotes the growth of phytoplankton (Yago et al., 2012) and lettuce (Watanabe, 1997; Yanagi et al., 1996) better than continuous light. However, no studies have examined the influence of intermittent light on the growth of macroalgae such as U. pinnatifida. Thus, the appropriate photo environment for U. pinnatifida gametophyte and sporophyte growth is unknown.
To determine a suitable photo environment for stable productive culture of the brown alga U. pinnatifida, the influences of intermittent light and various colors of light on gametophytes and sporophytes growing under laboratory conditions were investigated.
Experimental apparatus
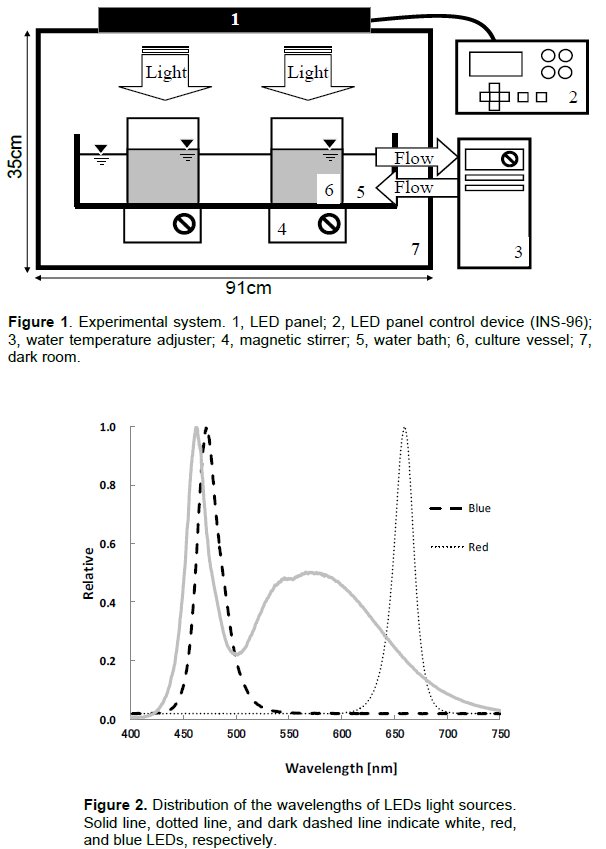
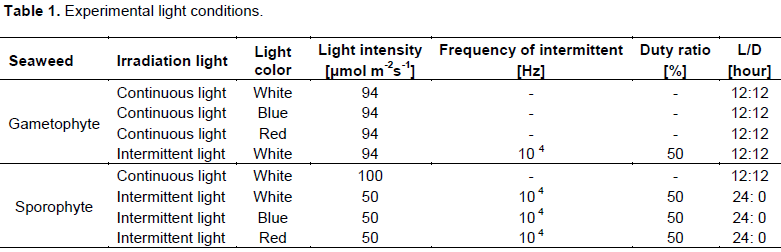
An experimental apparatus controlled the light conditions and temperature in a chamber. LED bulb panels (CCS Inc., Kyoto, Japan) were used as the light source (Figure 1). The panel was set in the constant-temperature chamber, and the lighting conditions were controlled by an external control device (INS-96; CCS Inc.). The control panel enabled adjustments to the times that lights were turned on and off, the light and dark period (below LD cycle), the frequency of intermittent lighting (10-2 to 105 Hz) and the duty ratio (0 to 100%) of the LED panel. The LED panel included white, blue, and red lights. The spectral distributions of the LED panel lights are shown in Figure 2. The white LED had peaks at 460 and 570 nm wavelengths, while the blue and red LEDs had peaks at 470 and 660 nm wavelengths, respectively. The experimental apparatus was set in a dark room to prevent light from outside the device from entering.
Gametophyte and sporophyte
The experiments were divided two parts, according to the life stages of U. pinnatifida, as follows: (1) the gametophyte stage, which lasts for 14 days after substrate adhesion of the zoospore and (2) the sporophyte stage. The effects of the lighting conditions on growth were investigated during both stages.
Gametophyte stage
U. pinnatifida sporophytes cultivated off of Katsuura, Chiba, Japan, were carried to the laboratory immediately. The sporophytes were dried in the shade for 1 h, and the blades of the sporangium were cut. Then, to release the zoospores, the sporangium was dipped into seawater sterilized by autoclave. The released zoospores were then poured into a 15-cm diameter, 9-cm tall petri dish that contained 1 L of sterilized seawater. Then, a glass slide was placed into the petri dish. The dish was set aside for 30 min, and the zoospores adhered to the slide glass. The adhesion density of the zoospores was ca. 400 ind./cm2. The slide glass with the zoospores was moved to a culture vessel (Petri dish, 15 cm diameter, 9 cm height), which contained PESI culture medium (Tatewaki, 1966). The culture vessel was set in a constant-temperature room (20°C) and the zoospores were cultivated under various light conditions. The slide glass was taken out on the 7 and 14th days, and photographs of 30 individual gametophytes on the slide glass were taken using an optical microscope (100× magnification; BM-2, Olympus Co., Tokyo). Motic Image Plus 2.0S image analysis software (Motic China Group Co., Hong Kong) was used to measure the body length of gametophytes. The experiments were conducted twice for each light condition.
Sporophyte stage
Sporophytes cultivated under natural light conditions were used. The average body length of a sporophyte was 30.7±2.8 cm (n=12) at the start of the experiment. To determine the growth of the blade, a circular hole was made in the center of the blade, 3 cm from where the stipe meets the blade. Then, 2,000 mL of Provasoli’s enrichment (PESI) culture medium was poured into a beaker (13 cm diameter, 20 cm height), and a sporophyte was placed in the beaker. The beakers were set in the same experimental apparatus system as the gametophytes (Figure 1). The magnetic stirrer in Figure 1 was replaced with an aeration apparatus to circulate the medium in the beaker. The temperature of the medium was 15 ± 0.5°C. The experiments were conducted three times under each lighting condition. Every 2 days, 1,000 mL of fresh PESI medium was replaced in the beaker. Sporophytes were cultured for 28 days. The body length, blade growth length, and blade area of the sporophytes were measured every 7 days. Blade growth was determined by the distance from the circular hole to 3 cm from the spot where the stipe meets the blade. A digital camera was used to photograph the sporophyte blades. Then, the blade area (including side leaves) was calculated using Adobe Photoshop CS image editing software and Lia32 image analysis software (http://www.agr.nagoyau.ac.jp/~shinkan/LIA32/LIAMan.htm).
Lighting conditions
The lighting conditions for each experiment are shown in Table 1. In the gametophyte experiments, continuous white, blue, and red light and intermittent white light were used. In the sporophyte experiments, intermittent white, blue, and red light and continuous white light were used. The intensity of the continuous light was 94 μmol m-2s-1 in the gametophyte experiment and 100 μmol m-2s-1 in the sporophyte experiment. The intensity of the intermittent light was 94 μmol m-2s-1 in the gametophyte experiment and 50 μmol m-2s-1 in the sporophyte experiment.
LD cycles were 12 h: 12 h continuous/intermittent light in the gametophyte experiments and 24 h: 0 h intermittent light (104 Hz) in the sporophyte experiment. Thus, the total daily quantum irradiation amounts in the gametophyte experiment and in the sporophyte experiment were equal.
Effects of lighting conditions on gametophyte growth
The effects of different light conditions on U. pinnatifida gametophyte growth are shown in Table 2. The size of the U. pinnatifida zoospores that adhered to the substrate slide glass was ca. 5 μm before experimental culture. Under continuous white light, the body length of the gametophytes was 46.8 μm on the 7th day, and those of male and female gametophytes on the 14th day were 231 μm and 179.6 μm, respectively. Under, intermittent white light (104 Hz), the body length of the gametophytes was 56.5 μm on the 7th day, and those of male and female gametophytes were 220.9 and 179.7 μm, respectively, on the 14th day.
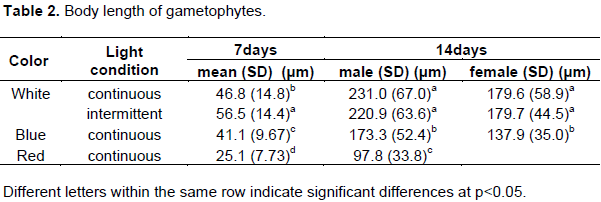
The body length of gametophytes under blue light was 41.1 μm on the 7th day, and those of male and female gametophytes were 173.3 and 137.9 μm, respectively, on the 14th day. The body lengths of gametophytes under red light on the 7th and 14th days were 25.1 and 97.8 μm, respectively. Under red light, the gametophytes grew slowly; thus, we were not able to judge the sex of the gametophytes on the 14th day.
There was no significant difference in the body length of female gametophytes on the 14th day under continuous and intermittent white light (p > 0.05) according to a Tukey-Kramer test for multiple comparisons. However, the gametophyte length under intermittent and continuous white light was significantly greater than the corresponding lengths under blue and red light (p < 0.01). Thus, we found that white light effectively promotes U. pinnatifida gametophyte growth.
Effects of lighting conditions on sporophyte growth
Variations in body length, blade growth, and blade area of sporophytes under different light conditions over time are shown in Table 3. The measurements were conducted on the 7th, 14th, and 21st days. However, we could not take measurements on the 28th day because the tip of the blades had been destroyed, meaning that the circular holes made in the center of the blades were lost.
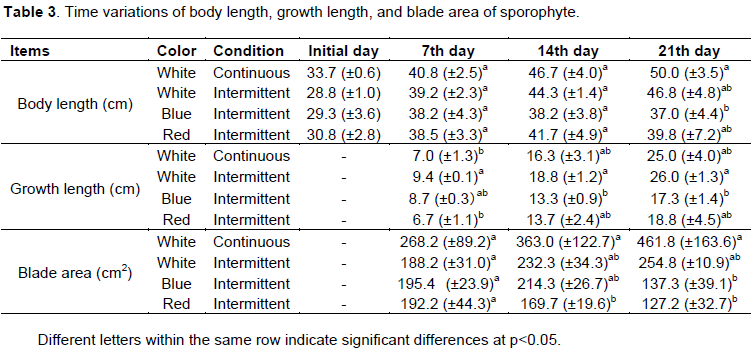
The body length and growth of sporophytes under continuous white light were 40.8 and 7.0 cm on the 7th day, 46.7 and 16.3 cm on the 14th day, and 50.0 and 25.0 cm on the 21st day, respectively. In contrast, body length and growth under intermittent white light were 39.2 and 9.4 cm on the 7th day, 44.3 and 18.8 cm on the 14th day, and 46.8 and 26.0 cm on the 21st day, respectively. During the experiment, the tip of the sporophyte blade deteriorated remarkably. The growth of sporophyte under intermittent light on the 7th and 14th days was 1.3 and 1.2, times greater than under continuous light. Growth on the 21st day was almost the same under continuous and intermittent light. There was a significant difference in sporophyte growth on the 7th day between continuous light and intermittent light (Scheffe’s F test, p < 0.05). However there was no significant difference after the 14th day (p > 0.05). There were no significant differences in blade area under continuous and intermittent light (p > 0.05). Nevertheless, the blade area was larger under continuous white light than under intermittent white light.
The growth of sporophytes under blue and red light increased over time. Growth after the first week under
white light was 9.4 cm, which was the greatest among the three colors of light. In contrast, growth under the red light was the least at 6.7 cm. Growth on the 21st day was 26.0, 18.8, and 17.3 cm under white, red, and blue light, respectively. Growth under white light was 1.4 to 1.5 times greater than under the other colors.
Differences in body length, blade growth, and blade area under different color lighting were examined by multiple comparison tests. There was a significant difference between body length under continuous white light and that under intermittent blue light (p < 0.05). There was a significant difference between blade growth under intermittent white light and that under intermittent blue light, and between blade area under continuous white light and those under intermittent red and blue light (p < 0.01). Body length increased most under continuous white light, while blade area increased most under continuous white light. Sporophyte blade area decreased over time under red light.
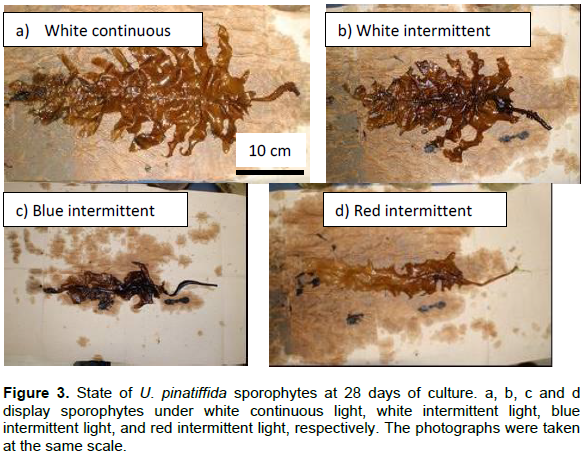
Features of sporophytes on the 28th day under each light condition are shown in Figure 3. Side leaves of the sporophytes under continuous white light grew remarkably faster than those under intermittent white light. Development of side leaves under intermittent white light was better than that under intermittent blue and red light. Blades under blue and red light did not develop and withered.
From the above results, continuous white light promoted growth to a greater degree than did intermittent white light. Furthermore, white light promoted U. pinnatifida sporophyte growth more than blue or red light.
Effects of intermittent light on seaweed growth
There are many reports that the cell numbers of unicellular phytoplankton under intermittent light of various frequencies (10 Hz to 50 kHz) are greater than those under continuous light (Grobbelaar et al., 1996; Park and Lee, 2000; Janssen et al., 2001; Yoshioka et al., 2012; Yago et al., 2012). Furthermore, the same phenomenon has been reported in land plants. Watanabe (1997) reported that lettuce grew well under intermittent light conditions at a high frequency (100 Hz or more). These studies have suggested that the reason intermittent light promotes plant growth is that no light exposure is needed for the dark reaction period of photosynthesis (Park and Lee, 2000).
However, in this study, U. pinnatifida gametophyte and sporophyte growth were not promoted by intermittent light at a frequency of 104 Hz. The reason for this is not clear. In previous studies (Park and Lee, 2000), diurnal LD cycles were not used in intermittent light experiments. In the current experiment, diurnal LD cycles were used for the gametophyte experiment because a previous study showed that LD cycles affect U. pinnatifida growth (Notoya et al., 1995).
In this study, the light intensity of the light period in the intermittent light treatment for the gametophyte experiment was set at 188 μmol m-2s-1. Arakawa and Matsuike (1992) reported that the growth of U. pinnatifida gametophytes was not promoted by light intensity greater than 10.000 Lx (166 μmol m-2s-1). Thus, the light intensity we used for the intermittent lighting may have been too strong.
There were no significant differences between the growth of U. pinnatifida sporophytes under continuous and intermittent light. However, the development of side leaves under the intermittent light was poor (Figure 3). For sporophyte growth, the effect of having no LD cycle might be greater than the promotion of growth under intermittent light.
Effects of light color on macrophyte growth
There are many reports concerning the suitable light color environment for macrophytes (Xu et al, 2005; Matsui et al., 1992; Murase et al., 2014; Dring and Luning, 1975; Takada et al., 2011; Yago et al., 2014). The suitable light color for E. bicyclis (Murase et al., 2014), Scytosiphon lomentaria (Dring and Luning, 1975) and Ulva prolifera (Takada et al., 2011) growth has been examined using LEDs. These studies suggested that E. bicyclis and S. lomentaria gametophytes grew well under blue LED light and U. prolifera grew well under blue and red LED light. It has been suggested that the differing effects on growth by different light colors originate from the varying spectral absorptions of different species’ blades.
The appropriate wavelengths for promoting U. pinnatifida gametophyte and sporophyte growth were investigated. It was found that both gametophytes and sporophytes grew well under white light and poorly under blue and red light.
Matsui et al. (1992) examined the effects of light color on gametophyte and sporophyte growth in several species of Laminariales. They reported that sporophytes cultured under green light and red light had an abnormal shape, while those grown under blue light grew normally. These results disagree with our current findings; however, a fluorescent lamp was used in Matsui’s studies. The wavelength range of blue fluorescent light is wider than that of blue LED light (dominant wavelength; 470 nm), which may have influenced sporophyte growth. Furthermore, in contrast to blue light, the spectrum of white LED light has peaks near 500 nm and 550–650 nm. We propose that white LED light promoted U. pinnatifida growth because the spectral distribution of white LED light is near the photosynthetically active spectrum for U. pinnatifida.
The quantity of light absorbed in the blade can be determined by multiplying the photosynthetically active spectrum of U. pinnatifida sporophyte blades (Calogero et al., 2014) and the spectrum of each color of LEDs used in our experiment. According to these calculations, the light absorption of U. pinnatifida with white LED light should equal 1, and the light absorption ratios with blue and red LEDs should equal 1.3 and 0.25, respectively. Thus, the light of the blue LEDs is advantageous for photosynthesis in terms of the light absorption of the blade. However, white light promoted growth to a greater degree than blue light in our study, and the shape of sporophyte blades under white light was closer to the natural appearance of U. pinnatifida. Exposure to blue and red light resulted in much smaller sporophyte blades in the current study. White LED light includes a very strong blue band, a strong green band, and a weak red band. In contrast, the photosynthetically active spectrum of U. pinnatifida blades (Calogero et al., 2014) has two peaks at the blue and red bands. U. pinnatifida blades do not absorb the green light band.
In this study, an experiment using blue and red light at the same time was not conducted. The energy ratio of the blue band (400-500 nm) and red band (600-700 nm) in white light was calculated according to their respective strengths and found that the ratio was 35:29. Thus, it was suggested that blue light and red light of the almost same strength are necessary for promoting healthy U. pinnatifida sporophyte growth.
Watanabe (1997) reported that lettuce exposed to 8% blue light and red light grew well and had the same shape and color as that cultivated under natural light. Mizuta et al. (2007) also investigated the effects of light color on the growth and shape of Laminaria japonica. They concluded that blue light assists with the formation of the sporangium, while red light restrains sporangium formation and promotes formation of the rhizoid and leaf stalk. They suggested that the use of both red and blue wavelengths is important for cultivation of large seaweed species.
In the current study, it was found that continuous white light was the most suitable artificial photo environment for U. pinnatifida culture. That is, the light of both blue and red wavelength bands is necessary to grow healthy U. pinnatifida. However, the white light also had a lower absorption by the blade than the other two colors according to the active photosynthetic spectrum of U. pinnatifida. To clarify the ideal photo environment for efficient U. pinnatifida growth, future studies should examine the appropriate ratios of blue and red light intensities for optimal growth.
The authors have not declared any conflict of interests.
REFERENCES
|
Akiyama K (1965). Studies of ecology and culture of Undaria pinnatifida â…¡. Environmental factors affecting the growth and maturation of gametophyte. Bull. Tohoku Nat. Fish. Res. Inst. 25:143-170.
|
|
|
|
Arakawa H, Matsuike K (1992). Influence on insertion of zoospores, germination, survival, and maturation of gametophytes of brown algae exerted by sediments.Nippon Suisan Gakkaishi. 58:619-625.
|
|
|
|
|
Baba M (2008). Effects of temperature, irradiance, and salinity on the growth of Undaria pinnatifida from Niigata Prefecture, Central Japan. Rep. Mar. Ecol. Res. Inst. 11:7-15.
|
|
|
|
|
Calogero G, Citro I, Marco GD, Minicante SA, Morabito M, Genovese G (2014). Brown seaweed pigment as a dye source for photoelectrochemical solar cells. Spectrochim. Acta A Mol. Biomol. Spectrosc. 117:702-706.
|
|
|
|
|
Dring MJ, Luning K (1975).Induction of two-dimensional growth and hair formation by blue light in the brown alga Scytosiphon lomentaria. Z. Pflanzenphysiol. 75:107-117.
|
|
|
|
|
FAO (2016). The state of world fisheries and aquaculture 2016, Contributing to food security and nutrition for all. Rome. 200 p.
|
|
|
|
|
Grobbelaar JU, Nedbal L, Tichý V (1996). Influence of high frequency light/dark fluctuations on photosynthetic characteristics of microalgae photoacclimated to different light intensities and implications for mass algal cultivation. J. Appl. Phycol. 8:335-343.
|
|
|
|
|
Janssen M, Slenders P, Tramper J, Mur LR, Wijffels RH (2001). Photosynthetic efficiency of Dunaliella tertiolecta under short light/dark cycles. Enzyme Microb. Technol. 29:298-305.
|
|
|
|
|
Matsui T, Ohgai M, Ohshima Y, Kohara K (1992). The effects of light quality and quantity on gametophyte growth and fertility, and young sporophyte growth, in several species of Laminariales. Nippon Suisan Gakkaishi 58:1257-1265.
|
|
|
|
|
Mizuta H, Kai T, Tabuchi K, Yasui H (2007). Effects of light quality on the reproduction and morphology of sporophytes of Laminaria japonica (Phaeophyceae). Aquact. Res. 38:1323-1329.
|
|
|
|
|
Morelissen B, Dudley BD, Geange SW, Phillips NE (2013). Gametophyte reproduction and development of Undaria pinnatifida under varied nutrient and irradiance conditions. J. Exp. Mar. Biol. Ecol. 448:197-206.
|
|
|
|
|
Mori Y, Takatsuji M, Yasuoka T (2002). Effects of pulsed white LED light on the growth of Lettuce. J. Soc. High Technol. Agric. 14:136-140.
|
|
|
|
|
Murase N, Abe M, Noda M, Suda Y (2014). Growth and maturation of gametophyte in Eisenia bicyclis under different light quality from Light Emitting Diodes (LEDs). J. Nat. Fish. Univ. 62:147-152.
|
|
|
|
|
Notoya M, Kimura H, Ogura H (1995). Life cycle and morphogenesis of Undaria pinnatifida in room culture. Kaiyo Monthly 27:47-52.
|
|
|
|
|
Park KH, Lee CG (2000). Optimization of algal photobioreactors using flashing lights. Biotechnol. Bioprocess Eng. 5: 186-190.
|
|
|
|
|
Saito Y (1956a). An ecological study of Undaria pinnatifida Sur.-1. On the influence of environmental factors upon the development of gametophytes. Bull. Jpn. Soc. Sci. Fish. 22: 229-239.
|
|
|
|
|
Saito Y (1956b). An ecological study of Undaria pinnatifida Sur.-â…¡. On the influence of the environmental factors upon the maturity of gametophytes and early development of sporophytes. Bull. Jpn. Soc. Sci. Fish. 22:235-239.
|
|
|
|
|
Saito Y (1958). An ecological study of Undaria pinnatifida Sur.-3. On the effects of light intensity and water temperature upon the rate of photosynthesis -1. Bull. Jpn. Soc. Sci. Fish. 24:484-486.
|
|
|
|
|
Takada J, Murase N, Abe M, Noda M, Suda Y (2011). Growth and photosynthesis of Ulva prolifera under different light quality from Light Emitting Diodes (LEDs). Aquac. Sci. 59:101-107.
|
|
|
|
|
Takatsuji M (2010). Present status of completely-controlled plant factories. J. Soc. High Technol. Agric. 22:2-7.
|
|
|
|
|
Tatewaki M (1966). Formation of a crustaceous sporophyte with unilocular sporangia in Scytosiphon lomentaria. Phycologia 6:62-66.
|
|
|
|
|
Watanabe H (1997). Light emitting diodes as the irradiation source for plant factories. Rev. Laser Eng. 25:836-840.
|
|
|
|
|
Xu Z, Dapeng L, Hanhua H, Tianwei T (2005). Growth promotion of vegetative gametophytes of Undaria pinnatifida by blue light. Biotechnol. Lett. 27:1467-1475.
|
|
|
|
|
Yago T, Arakawa H, Fukui K, Okubo B, Akima K, Takeichi S, Okumura Y, Morinaga T (2012). Optimum Growth Conditions of Intermittent Light Irradiation for the Production of Microalga Isochrysis galbana. Afr. J. Microbiol. Res. 6:5896-5899.
|
|
|
|
|
Yago Y, Arakawa H, Akima K, Okumura Y, Morinaga T (2014). Effects of flashing light-emitting diode (LED) of several colors on the growth of the microalga Isochrysis galbana. Afr. J. Microbiol. Res. 8:3815-3820.
|
|
|
|
|
Yanagi T, Okamoto K, Takita S (1996). Effects of blue, red, and blue/red lights of two different PPF levels on growth and morphogenesis of lettuce plants. In. International Symposium on Plant Production in Closed Ecosystems. 440:117-122.
|
|
|
|
|
Yoshioka M, Yago T, Yoshie-Stark Y, Arakawa H, Morinaga T (2012). Effect of high frequency of intermittent light on the growth and fatty acid profile of Isochrysis galbana. Aquaculture 338-341:111-117.
|
|
|
|
|
Zou N, Zhou B, Li B, Sun D, Zeng C (2003). Effects of cell density, light intensity and mixing on Undaria pinnatifida gametophyte activity in a photobioreactor. Biomol. Eng. 20: 281-284
|
|