ABSTRACT
Malaria is a leading cause of morbidity and mortality in the world. In general, malaria is easily treated but in a subset of cases it develops to severe disease. Severe malaria has a high rate of mortality even with the best available care. This creates the need for intensive research to fully characterise the pathogenesis of the disease in order to create future novel therapies. One of the hallmarks of malaria infections is its ability to adhere to specific sites in the body leading to severe disease syndromes such as cerebral malaria and pregnancy associated malaria. In this review, the platelet-mediated clumping adhesion phenotypes of malaria-infected erythrocytes were discussed in the context of infected erythrocyte adhesion phenotypes such as cytoadhesion and rosetting. Platelet-mediated clumping refers to a phenomenon of P. falciparum-infected erythrocytes whereby they agglutinate to form large aggregates held together by activated platelets. This unique phenotype is important because it has been associated with severe malaria in both children and adults in diverse geographical and transmission settings. The precise mechanisms by which platelet-mediated clumping occurs are yet to be precisely described. The platelet receptors implicated in this phenotype include CD36, P-Selectin and gC1qR. The parasite derived ligands that mediate this phenotype are yet to be described.
Key words: Malaria, platelets, platelet-mediated clumping, infections.
According to the WHO, 212 million people contracted malaria in 2015 whereby 90% of them happened in Africa (WHO, 2016). It is estimated that there were 429,000 malaria related deaths in 2015 a majority of whom were children under 5 years old. In general the scale up of malaria control programmes over the past decade has led to a decrease in malaria-related mortality and morbidity.
Malaria is caused by the protozoan parasite known as Plasmodium falciparum that is transmitted by female Anopheles mosquitoes during blood meals. The injected sporozoites travel from the site of infection on the skin to the liver where they infect hepatocytes. After appro-ximately 10 days of development and division in the hepatocytes merozoites are released into the blood stream where they infect erythrocytes. Merozoitesdevelop through a 48 h cycle in the erythrocytes that ends in the release of more merozoites that re-infect fresh erythrocytes. This intra-erythrocytic parasite stage coincides with the symptomatic phase of malaria infections. The mature stage of the intra-erythrocytic parasite known as the trophozoite exports and inserts parasite-derived antigens into the erythrocyte plasma membrane modifying its flexibility and adhesive properties. This enables infected erythrocytes (IEs) to avoid splenic clearance by sequestering to tissues and organs through endothelial cell receptors in the microvasculature.
Malaria is in general an easily treated disease with antimalarial drugs. However, in the absence of effective treatment, the infection develops to severe disease that is characterised by three syndromes namely: Impaired consciousness, respiratory distress, cerebral malaria and severe malaria anaemia (Marsh et al., 1995). The adhesive properties of mature IEs are thought to contribute to the pathogenesis of severe malaria by causing disturbed blood flow, leading to localized host inflammatory responses and deprived oxygen concentrations. This adhesion is thought to further lead to disruption of endothelial cell function and apoptosis
ADHESION PHENOTYPES OF P. FALCIPARUM INFECTED ERYTHROCYTES
The adhesion of infected erythrocytes to various human host cell receptors is referred occurs in three different forms namely cytoadherence, rosetting and platelet-mediated clumping. Cytoadherence happens when infected erythrocytes bind human host endothelial cells though receptors. Rosetting occurs when infected erythrocytes bind to uninfected erythrocytes.
Adhesion phenotypes of infected erythrocytes are important to study because they have been associated with specific disease syndromes and therefore could be targeted in development of a new class of antimalarial drugs. Adhesion of IEs to brain endothelial cells through the receptor intercellular adhesion molecule-1 (ICAM-1) has been associated with cerebral malaria. Furthermore, binding to chondroitin sulfate A (CSA) in synctiotrophoblasts has been associated with pregnancy associated malaria whereas resetting has been associated with severe malaria anaemia. These examples illustrate why there is a compelling need to precisely study and describe the mechanisms that lead to the development of these adhesion phenotypes. In this review the literature on platelet-mediated clumping of infected erythrocytes was discussed (Rowe et al., 2009).
PLATELET-MEDIATED CLUMPING
Platelet-mediated clumping refers to the aggregation of mature P. falciparum IEs in close contact with platelets (Pain et al., 2001). Apparent autoagglutination of IEs was initially reported in a laboratory parasite line IT/C10 and later in patient Kenyan patient isolates (Roberts et al., 2000; Roberts et al., 1992). Subsequent work on parasite line IT/C10 showed that this autoagglutination was mediated by platelets and that it is a common phenotype in patient isolates (Pain et al., 2001; Chotivanich et al., 2004).
Platelet-mediated clumping assay
The platelet-mediated clumping phenotype of a given parasite isolate is determined in vitro by counting the number of IEs in clumps as a proportion of the total number of IEs. A clump is defined as an aggregation of three or more IEs. The level of platelet-mediated clumping of isolates varies among parasites.
Platelet-mediated clumping is a specific adhesion phenotype that is characteristic of a subset of malaria parasites. Previous studies show that the clumping phenotype is sensitive to assay conditions (Arman and Rowe 2008; Arman et al., 2013). The platelet-mediated clumping assay attempts to measure a property of parasites that has not been showed to occur in vivo in clinical malaria, although platelet accumulation in malaria has been reported in the brain of children that died from cerebral malaria (Grau et al., 2003). It has been shown that CD36-binding IEs may bind to activated endothelia through vWf-decorated platelets as a bridge on surfaces that may otherwise lack CD36 (Bridges et al., 2010). If the clumps of IEs observed in vitro in platelet-mediated clumping assays could form in vivo, it is plausible that they could obstruct of blood flow in post-capillary venules.
P. falciparum interaction with platelets might also lead to platelet activation and release of inflammatory mediators (Srivastava et al., 2008; McMorran et al., 2009; McMorran et al., 2012)however, the precise role of platelets in malaria pathology remains unclear. The assessment of P. falciparum clumping is affected by the precise conditions used to set up the clumping assay in vitro, with parasitaemia and haematocrit having a profound effect on the outcome of the assay. For field isolate studies, it is crucial that the effect of these parameters on clumping is taken into account during experimental design; otherwise the higher parasitaemia usually seen in parasite isolates from severe malaria patients compared to uncomplicated malaria controls could bias results. Possible solutions to the confounding effect of parasitaemia on studies of clumping and malaria severity include adjustment of all isolates to a standardized parasitaemia, or matching of cases and controls. For laboratory experiments on clumping, it is important that the limitations of the assay (e. g. the difficulty in assessing the true maximum clumping frequency of a parasite because of the formation of giant clumps that cannot be counted accurately) are taken into account. However, the assay conditions alone cannot explain the variable association of clumping with severe malaria in different studies, which requires further investigation using carefully designed experiments and standardized techniques.
CLINICAL STUDIES ON PLATELET-MEDIATED CLUMPING
Thus far there is no study that has formally shown that platelet-mediated clumping occurs in vivo in patients with malaria. However circumstantial evidence points to a possibility of clumping occurring in vivo. Firstly, platelet deposits were reported in post-mortem studies of children that died from malaria in Malawi along with infected erythrocytes, hemozoin and leucocytes (Grau et al., 2003). Secondly, the likelihood of the clumping phenotype occurring in vivo is shown by the size of clumps observed in vitro. If the size of clumps observed in vitro would occur in vivo it is plausible that they would obstruct post-capillary venules. Thirdly, some parasite isolates have been shown to have multiple adhesion phenotypes therefore it is possible that the adhesion phenotypes would occur concurrently including adhesion to endothelial cells on vascular endothelia, rosetting uninfected erythrocytes and platelet-mediated clumping.
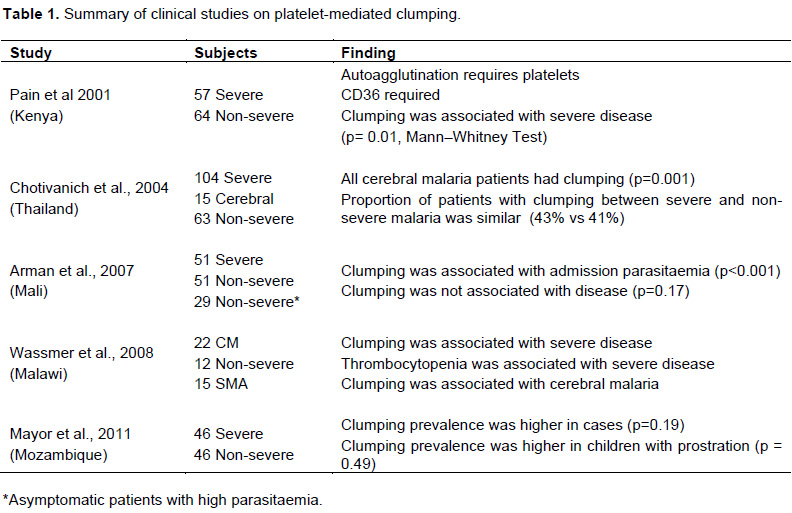
Platelet-mediated clumping has been associated with severe disease in four clinical studies thus far (Pain et al., 2001; Chotivanich et al., 2004; Wassmer et al., 2008; Mayor et al., 2011) but one study associated it with high parasitaemia and not disease severity (Arman et al., 2007). Interestingly, platelet-mediated clumping was associated with severe disease in Thailand, a study that, unlike the others, was conducted in an area of unstable malaria transmission and in adults (Chotivanich et al., 2004). The rest of the studies were conducted with isolates from children from sub-Saharan Africa where malaria is endemic. In multivariate analysis it was found that both parasitaemia and clumping were independently associated with severe disease, so to investigate this further, Arman et al. (2007) enrolled children with non-severe hyperparasitaemia as controls to investigate the association of platelet-mediated clumping and disease severity. This led to the recommendation of using standardized parasitaemia in clinical studies investigating the platelet-mediated clumping phenotype (Arman and Rowe, 2008). In the Mozambican case-control study, platelet-mediated clumping was associated with severe disease both with standardised and admission parasitaemia. This study further associated platelet-mediated clumping with prostration (Mayor et al., 2011). Table 1 summarizes the results from the studies on platelet-mediated clumping.
Platelet mediated clumping has been studied in both laboratory parasite lines and in field isolates as discussed previously and is thought to be important in the pathogenesis of severe disease in diverse clinical and geographical settings. Although not all studies thus far that investigated the platelet-mediated clumping phenotype agrees on its importance and relevance in malaria pathogenesis. The dissenting results have been explained by the sensitivity of the clumping assay to experimental conditions and it has been noted that earlier studies were conducted under different experimental conditions. A further challenge was that the studies were conducted in a relatively small number of samples.
MECHANISMS FOR PLATELET-MEDIATED CLUMPING
The requirement of CD36 for the platelet-mediated clumping phenotype has been reaffirmed in more studies both in field isolates and in laboratory parasite lines (Chotivanich et al., 2004; Wassmer et al., 2008; Mayor et al., 2011; Arman et al., 2013). Platelets with the platelet Naka phenotype that do not express CD36 do not support clumping while anti-CD36 monoclonal antibodies inhibit the clumping phenotype (Pain et al., 2001; Arman et al., 2013) Two more receptors have been reported to mediate platelet-mediated clumping, namely, P-Selectin and gC1qR (Biswas et al., 2007; Wassmer et al., 2008; Mayor et al., 2011).
To our knowledge, there is no published study that has attempted to identify the parasite ligands used by IEs to interact with platelet receptors in platelet-mediated clumping. This is an important research question because platelet-mediated clumping has been associated with severe disease, making the clumping ligand a potential marker for severe disease or a candidate for development of adjunctive therapies to supplement existing malaria drug regimens. The cellular and molecular interactions that lead to the development of platelet-mediated clumping are not entirely understood. Although the parasite ligand that mediates platelet-mediated clumping has not been identified, the asexual stage of the parasite that exhibits the platelet-mediated clumping phenotype also adheres to endothelial cell receptors (Arman and Rowe, 2008). It is plausible that the parasite ligand for platelet-mediated clumping is the same or is similar to parasite ligands for other endothelial receptors.
Some characteristics of the platelet-mediated clumping ligand are similar to PfEMP-1, the adhesion for other IE adhesion phenotypes including rosetting (Rowe et al., 1994), CD36 (Robinson et al., 2003), P-Selectin (Senczuk, 2001), PECAM-1(Fernandez et al., 1998). Furthermore, two of the known clumping ligand receptors, CD36 and P-Selectin are known receptors of PfEMP-1 (Senczuk, 2001; Robinson et al., 2003). Platelets also express PECAM-1, another receptor for PfEMP-1. Taken together, this evidence could lead to a hypothesis that places PfEMP-1 as an important ligand for platelet-mediated clumping.
IEs have protrusions on the surface that are sites of expression of PfEMP-1 known as knobs. Truncation of the kahrp gene, that encodes KAHRP, leads to development of knobless IEs. Knobless IEs are known to have reduced ability to bind to purified receptors compared to knobbed IEs (Horrocks et al., 2005). Recent published data has shown that clumping parasites can be selected from knobless parasite line DD2 (Arman et al., 2013). It is possible however that these parasites are not completely knobless but have a lower expression of the knobby phenotype. It is not clear if the knobless parasite line DD2 is entirely knobless or that it has a lower expression of knobs compared to other parasite liens. It is possible that the positive selection method selected for parasites with knobs and therefore a higher surface expression of CD36 binding ligands compared to the rest of the population.
There are several perceived contradictions on what is known about platelet-mediated clumping. An interesting paradox exists in the relationship of the platelet-mediated clumping phenotype and severe disease. On one hand, platelet mediated clumping is associated with severe disease while on the other hand platelet-mediated clumping is dependent on CD36, which on the endothelium is associated with non-severe malaria causing IEs (Pain et al., 2001; Robinson et al., 2003; Ghumra et al., 2012; Pleass, 2009). Furthermore, virtually all field isolates bind to CD36 whereas not all of them exhibit the platelet-mediated clumping phenotype. This could be explained by one of two scenarios. In the first scenario, the clumping ligand and the adhesion ligand for CD36 exploit different distinct epitopes on CD36, while in the second scenario, the clumping ligand and the adhesion ligand may bind to different platelet receptors (Pain et al., 2001).
The parasite ligand for platelet-mediated clumping is hypothesized to be a PfEMP-1. However, this presents another paradox since CD36-binding PfEMP-1 variants are associated with non-severe disease whereas platelet-mediated clumping which has also been associated with severe disease relies on CD36.
Another compelling question to malaria researchers is the contribution of the clumping phenotype to development of cerebral malaria. It is plausible that platelet-mediated clumping contributes to development of cerebral malaria although current evidence cannot entirely explain the mechanisms behind it. Two clinical studies so far found that all parasite isolates from cerebral malaria patients were positive of the clumping phenotype (Chotivanich et al., 2004; Mayor et al., 2011). If cerebral malaria can be partly explained by obstruction of post-capillary venules in the brain it can be conjectured that clumping could contribute to cerebral malaria. Furthermore platelets are CD36-rich and have been found to bridge CD36-binding parasites to brain endothelia that lacks CD36 therefore since platelet-mediated clumping brings parasites together it is plausible that if clumping occur in brain endothelia it would contribute to cerebral malaria.
The authors have not declared any conflict of interests.
REFERENCES
|
Arman M, Adams Y, Lindergard G, Rowe JA (2013). A method for positive and negative selection of Plasmodium falciparum platelet-mediated clumping parasites and investigation of the role of CD36. PloS one. 8(2):e55453.
Crossref
|
|
|
|
Arman M, Raza A, Tempest LJ, Lyke KE, Thera MA, Koné A, Plowe CV, Doumbo OK, Rowe JA (2007). Platelet-Mediated Clumping of Plasmodium falciparum–Infected Erythrocytes Is Associated with High Parasitemia but Not Severe Clinical Manifestations of Malaria in African Children. Am. J. Trop. Med. Hyg. 77(5):943-946.
|
|
|
|
|
Arman M, Rowe JA (2008). Experimental conditions affect the outcome of Plasmodium falciparum platelet-mediated clumping assays. Malar J. 7(1):243.
Crossref
|
|
|
|
|
Biswas AK, Hafiz A, Banerjee B, Kim KS, Datta K, Chitnis CE (2007). Plasmodium falciparum uses gC1qR/HABP1/p32 as a receptor to bind to vascular endothelium and for platelet-mediated clumping. PLoS Pathog. 3(9):1271-1280.
Crossref
|
|
|
|
|
Bridges DJ, Bunn J, van Mourik JA, Grau G, Preston RJ, Molyneux M, Combes V, O'Donnell JS, de Laat B, Craig A (2010). Brief report Rapid activation of endothelial cells enables Plasmodium falciparum adhesion to platelet-decorated von Willebrand factor strings. Blood 115(7):1472-1474.
Crossref
|
|
|
|
|
Chotivanich K, Sritabal J, Udomsangpetch R, Newton P, Stepniewska KA, Ruangveerayuth R, Looareesuwan S, Roberts DJ, White NJ (2004). Platelet-induced autoagglutination of Plasmodium falciparum-infected red blood cells and disease severity in Thailand. J. Infect. Dis. 189(6):1052 1055.
Crossref
|
|
|
|
|
Fernandez V, Treutiger CJ, Nash GB, Wahlgren M (1998). Multiple adhesive phenotypes linked to rosetting binding of erythrocytes in Plasmodium falciparum malaria. Infect. Immun. 66(6):2969-2975.
|
|
|
|
|
Ghumra A, Semblat JP, Ataide R, Kifude C, Adams Y, Claessens A, Anong DN, Bull PC, Fennell C, Arman M, Amambua-Ngwa A (2012). Induction of strain-transcending antibodies against Group A PfEMP1 surface antigens from virulent malaria parasites. PLoS pathog. 8(4):e1002665.
Crossref
|
|
|
|
|
Grau GE, Mackenzie CD, Carr RA, Redard M, Pizzolato G, Allasia C, Cataldo C, Taylor TE, Molyneux ME (2003). Platelet accumulation in brain microvessels in fatal pediatric cerebral malaria. J. Infect. Dis. 187(3):461-466.
Crossref
|
|
|
|
|
Horrocks P, Pinches RA, Chakravorty SJ, Papakrivos J, Christodoulou Z, Kyes SA, Urban BC, Ferguson DJ, Newbold CI (2005). PfEMP1 expression is reduced on the surface of knobless Plasmodium falciparum infected erythrocytes. J. Cell Sci. 118(11):2507-2518.
Crossref
|
|
|
|
|
Marsh K, Forster D, Waruiru C, Mwangi I, Winstanley M, Marsh V, Newton C, Winstanley P, Warn P, Peshu N, Pasvol G (1995). Indicators of life-threatening malaria in African children. New Engl. J. Med. 332(21):1399-1404.
Crossref
|
|
|
|
|
Mayor A, Hafiz A, Bassat Q, Rovira-Vallbona E, Sanz S, Machevo S, Aguilar R, Cisteró P, Sigaúque B, Menéndez C, Alonso PL (2011). Association of severe malaria outcomes with platelet-mediated clumping and adhesion to a novel host receptor. PLoS One 6(4):e19422.
Crossref
|
|
|
|
|
McMorran BJ, Marshall VM, de Graaf C, Drysdale KE, Shabbar M, Smyth GK, Corbin JE, Alexander WS, Foote SJ (2009). Platelets kill intraerythrocytic malarial parasites and mediate survival to infection. Science 323(5915):797-800.
Crossref
|
|
|
|
|
McMorran BJ, Wieczorski L, Drysdale KE, Chan JA, Huang HM, Smith C, Mitiku C, Beeson JG, Burgio G, Foote SJ (2012). Platelet factor 4 and Duffy antigen required for platelet killing of Plasmodium falciparum. Science 338(6112):1348-1351.
Crossref
|
|
|
|
|
Pain A, Ferguson DJ, Kai O, Urban BC, Lowe B, Marsh K, Roberts DJ (2001). Platelet-mediated clumping of Plasmodium falciparum-infected erythrocytes is a common adhesive phenotype and is associated with severe malaria. Proceed. Natl. Acad. Sci. 98(4):1805-1810.
Crossref
|
|
|
|
|
Pleass RJ (2009). Platelet power: sticky problems for sticky parasites? Trends Parasitol. 25(7):296-299.
Crossref
|
|
|
|
|
Roberts DJ, Craig AG, Berendt AR, Pinches R, Nash G, Marsh K, Newbold CI (1992). Rapid swtching to multiple antigenic and adhesive phenotypes in malaria. Nature 357(25):689.
Crossref
|
|
|
|
|
Roberts DJ, Pain A, Kai O, Kortok M, Marsh K (2000). Autoagglutination of malaria-infected red blood cells and malaria severity Effect of local radiotherapy for bone pain on urinary markers of osteoclast activity. 355:1427-1428.
|
|
|
|
|
Robinson BA, Welch TL, Smith JD (2003). Widespread functional specialization of Plasmodium falciparum erythrocyte membrane protein 1 family members to bind CD36 analysed across a parasite genome. Mol. Microbiol. 47(5):1265-1278.
Crossref
|
|
|
|
|
Rowe A, Berendt AR, Marsh K, Newbold CI (1994). Plasmodium falciparum: A family of Sulphated Glycoconjugates Disrupts Erythrocytes Rosettes. Exp. Parasitol. 79:506-516.
Crossref
|
|
|
|
|
Rowe JA, Claessens A, Corrigan RA, Arman M (2009). Adhesion of Plasmodium falciparum-infected erythrocytes to human cells: molecular mechanisms and therapeutic implications. Expert Rev. Mol. Med. 11:e16.
Crossref
|
|
|
|
|
Senczuk AM, Reeder JC, Kosmala MM, Ho M (2001). Plasmodium falciparum erythrocyte membrane protein 1 functions as a ligand for P-selectin. Blood 98(10):3132-3135.
Crossref
|
|
|
|
|
Srivastava K, Cockburn IA, Swaim A, Thompson LE, Tripathi A, Fletcher CA, Shirk EM, Sun H, Kowalska MA, Fox-Talbot K, Sullivan D (2008). Platelet factor 4 mediates inflammation in experimental cerebral malaria. Cell Host Microbe 4(2):179-187.
Crossref
|
|
|
|
|
Wassmer SC, Taylor T, MacLennan CA, Kanjala M, Mukaka M, Molyneux ME, Grau GE (2008). Platelet-induced clumping of Plasmodium falciparum-infected erythrocytes from Malawian patients with cerebral malaria—possible modulation in vivo by thrombocytopenia. J. Infect. Dis. 197(1):72-78.
Crossref
|
|
|
|
|
World Health Organization (WHO) (2016). World Malaria Report 2016. Available at:
View
|
|
