ABSTRACT
This article reports the floristic and phytosociology of a solid ground forest, located in the Environmental Park Antonio Danubio Lourenço da Silva, municipality of Ananindeua, Pará, Brazil. The inventory of 1 ha considered individuals with diameter at breast height (DBH) ≥ 10 cm, allocated twenty plots in each field, all of size 20 m x 25 m (500 m2) arranged randomly. A total of 239 plants was registered, distributed in 61 species, 51 genera and 27 families, referring to biocenose area. The families with most species, in a decreasing order, were: Leguminosae, Lecythidaceae, Sapotaceae, Urticaceae, Anacardiaceae, Annonaceae, Caryocaraceae, Vochysiaceae, Lauraceae, Arecaceae and Malvaceae. In relation to the importance value index (IVIi), the highest values ​​were presented by the species: Oenocarpus bacaba, Nectandra cuspidata, Theobroma grandiflorum, Diplotropis purpurea, Piptadenia suavelons, Inga lauriana, Cecropia obtusifolia, Cecropia sciadophylla, Tapirira guianensis and Jacaranda copaia. These species represent 56.06% of the individuals sampled per hectare and 58.63% of the IVIi. The diversity index of Shannon-Weaver (H '= 3.53) indicates that the park has a floristic diversity less than other native forest fragments in State of Para, Brazil. This is probably due to the park being part of an anthropic area near the federal highway BR-316, where a large number of vehicles circulate daily.
Key words: Phytosociology, biodiversity, conservation, identificacion.
Vegetation plays essential roles in urban centers, because from a physiological point of view, it improves the urban environment through the ability to produce shade, filter noise, lessen noise pollution, improve air quality, increase oxygen content and moisture, and absorb carbon dioxide, as well as moderate the temperature, among other contributions (Graziano, 1994). The trees exert a vital role for the welfare of urban communities, because they have the unique ability to control many of the adverse effects of the environment, and thus should contribute to a significant improvement in quality of life (Volpe-Filiket et al., 2007). Consequently, there is a growing need to manage urban green areas for the benefit of the whole society.
The Danube Environmental Park Antonio Lourenço da Silva, located in Ananindeua municipality in the State of Pará, is an Area of ​​Relevant Ecological Interest (ARIE) (Brazil, 2000), created by the Municipal Law no. 2472, on January 5, 2011, with the following objectives: (i) maintain the natural ecosystem of regional and local importance; (ii) to ensure the preservation and protection of the existing fauna and flora; (iii) promote the use of natural components in environmental education, in order to make the community a partner in the conservation of the natural heritage of the municipality; and (iv) provide the population able to exercise cultural, educational, recreational and leisure activities in a balanced natural environment. Studies on the floristic composition and phytosociological structure of forest formations are of fundamental importance, because they offer information for understanding the structure and dynamics of these formations - essential parameters for the management, regeneration and conservation of different plant communities (Silva et al. 2002; Chaves and Del, 2013).
According to Oliveira et al., (2003), among the most important phytosociological parameters are: Frequency, indicating the occurrence of the taxon in the sample units; Density, which basically refers to the number of individuals of a given species per unit area (Mueller-Dombois and Ellenberg, 1974); Dominance, which expresses the influence or taxon contribution in the community, usually calculated in indirect values ​​of biomass; Importance Value Index, which allows the sorting of species hierarchically according to their importance in the community (Durigan, 2003); Coverage Value Index, which is defined as the vertical projection of the crown or root área of a species on the ground (Mueller-Dombois and Ellenberg, 1974); and Shannon-Weaver Diversity Index (H'), which accounts for both abundance and evenness of the species present in the area surveyed.
Considering the socioenvironmental importance of the Antônio Danúbio Environmental Park for the municipality of Ananindeua in the State of Pará, the floristic surveys and the phytosociological studies of the area can provide useful information in the elaboration and planning of actions that aim at the conservation or even the forest recovery of the area studied (Watzlawick et al., 2005). Accordingly, this study aims to characterize the floristic composition, structure and functioning of the dynamic distribution of this vegetation in the Environmental Park Antonio Danubio, located in the city of Ananindeua, Pará, Brazil, thus seeking a theoretical basis for the elaboration of conservation projects in these kinds of urban forest fragments.
The Environmental Park Danube Antonio Lourenço da Silva has a total area of ​​3,544 ha and is located along the BR 316 highway, 5 km, in the urban area of ​​the city of Ananindeua in the State of Pará, Brazil, between the coordinates 01° 40' 62.80'’ S and 48° 44’ 78.76'’ W (Figure 1). The vegetation data were obtained employing the methods of fixed area or multiple portions, as recommended by Mueller and Dombois (1974). The surveyed sites were allocated twenty (20) plots in each field, all of size 20 m x 25 m (500 m²) arranged randomly, totaling 10,000 m² (1 ha). The vegetation data collections were conducted in the period from January to May 2016.
In each of the 20 sample units, the circumferences of all trees were obtained using a tape measure based on the criterion of diameter at breast height (DBH), measured at 130 cm in height on the trunk, for trees with diamteters that were greater than or equal to 10 cm. Specimen identification was conducted at Pará State University Herbarium using classical taxonomy used by the standards, based on vegetative and floral morphological characters. The names of species and plant families have been updated using the database Species List Flora of Brazil (Flora do Brasil, 2020) and Missouri Botanical Garden (Tropicos, 2014). From the database file of all plots, a phytosociological analysis for the total sample was obtained, in order to characterize the tree community studied. For each sampled species, the following were calculated to characterize the Phytosociology (Mueller-Dombois and Ellenberg, 1974; Lamprecht, 1964):
Absolute Density (ADi) = ni / A
Relative Density (DRi) = ni / N
Absolute Frequency (AFi) = ji / k
Relative Rrequency (RFi) = AFi / Σ AF
Relative Dominance (RDOi) = BAi / Σ TBA
Coverage Value Index (CVIi) = DRi + RDOi
Importance Value Index (IVIi) = FRi + DRi + RDOi
where:
ni = Number of individuals of the species i
N = Total number of sampled individuals
ji = Total number of times the species i occurs in parcels
k = Total number of parcels
BAi = Basal Area of ​​species i
TBA = Total Basal Area
The diversity of species was analyzed by Shannon diversity index (H '). The Shannon index is calculated using the following equation (Magurran, 1988):
H ' = - Σ pi ln pi
where pi = ni / N; ni = abundance of the ith species; and N = total abundance of all species.
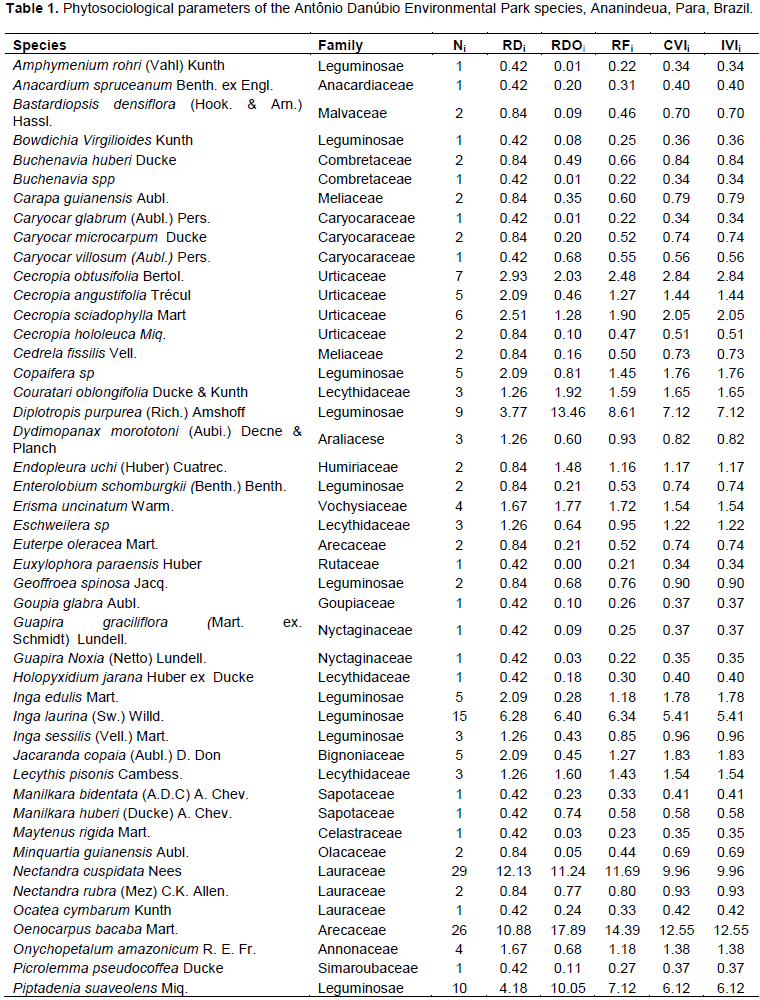
In the Antônio Danúbio Environmental Park area, 239 individuals were sampled, distributed into 61 species, 51 genera and 27 families (Table 1). The families with most species were Leguminosae 13 (thirteen), Lecythidaceae, Sapotaceae and Urticaceae with 4 (four) each, Anacardiaceae, Annonaceae, Caryocaraceae, Vochysiaceae and Lauraceae with 3 (three) each, Arecaceae and Malvaceae with 2 (two) each (Figure 2).
The composition of the families presented in the park under consideration confirms the representativeness of fitocenose in other studies of green areas and urban conservation areas in the metropolitan region of Belém (Laus and Jardim, 2013; Trinity et al., 2007; Santos and Garden, 2006)
Particularly, Environmental Park Antonio Danubio comprised a few families that have obtained a large number of species. This can happen possibly because families are restricted in terms of species in the study area and are therefore more difficult to be located. In the study by Carim et al. (2013), on the floristic composition of a fragment of Atlantic Forest in the extreme north of Brazil, a good representation at the family level has been identified; in the community only 17% had a single species, whereas at the Park this value was 37.5%. The families with the highest number of individuals are Leguminosae (59), Lauraceae (32), Arecaceae (28), Malvaceae (24), Urticaceae (20), Lecythidaceae (10), Vochysiaceae, Anarcadiaceae, Annonaceae and Sapotaceae with 7 (seven) each and Bignoniaceae with 5 (five) each (Figure 2). Among the ten (10) sampled, Leguminosae was the family that most stands out in both species richness and number of individuals.
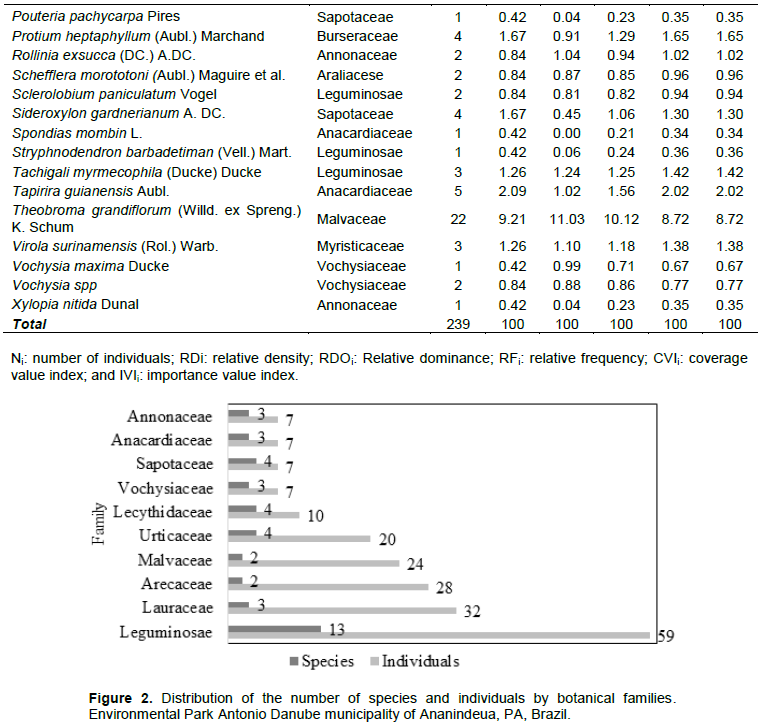
In Brazil, the family Leguminosae or Fabaceae appears among the main families that comprised the flora of the diverse ecosystems (Souza and Lorenzi, 2005). In floristic studies related to the family, Silva et al. (2013) cited Leguminosae with values expressive in numbers of species for the state of Pará, Brazil. A significant number of individuals (67.21%) have diameters ranging from 10.00 to 135.40 cm, whereas the latter class of diameter from 511.60 cm trees has two maxima Ducke Vochysia and Endopleura Uchi (Huber) Cuatrec (Figure 3).
The behavior shown in definition tends to distribute inverted "J" in diameter distribution of the classes, as the concentration of individuals is considerably on greater numbers in the first tracks. According to Andrade et al. (2017), the distribution of "j" inverted observed in upland forest of Rondônia (Curia Ecological Station), suggests its own natural dynamics arising from mortality and recruitment conducted in the first classes, which ultimately reflect the regeneration intensity in component tree.
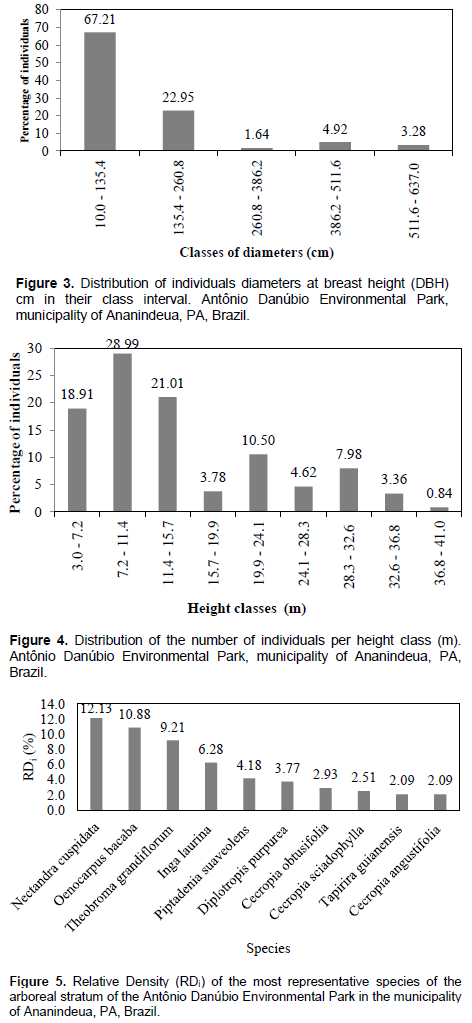
In Figure 4, it is possible to observe that, among the individuals sampled, those in the corresponding class from 7.20 m to 11.40 m in height are where the highest percentage of individuals occur, about 28.99%, followed by the class in which the height is between 11.40 to 15.70 m, representing 21.01% of the inhabited individuals. Within the class range that presents the smallest number of individuals (only 0.84%), it comprises the highest individuals with a height of 36.80 to 41.00 m. In a study conducted by Laus and Jardim (2013) at APA Combu Island, the height distribution obtained the highest concentration in the first two classes which corresponds to 60% of the total of individuals, and the lowest in the last classes follows along the curve one distribution. The species that obtained the highest values of relative density (Figure 5) were: Nectandra cuspidata, Oenocarpus bacaba, Theobroma grandiflorum, Inga laurina, Piptadenia suaveolens, Cecropia obtusifolia, Cecropia sciadophylla, Tapirira guianensis and Cecropia angustifólia. These species represent 17.54% of the total sampled species; however, it holds 61.08% of the total number of individuals per hectare.
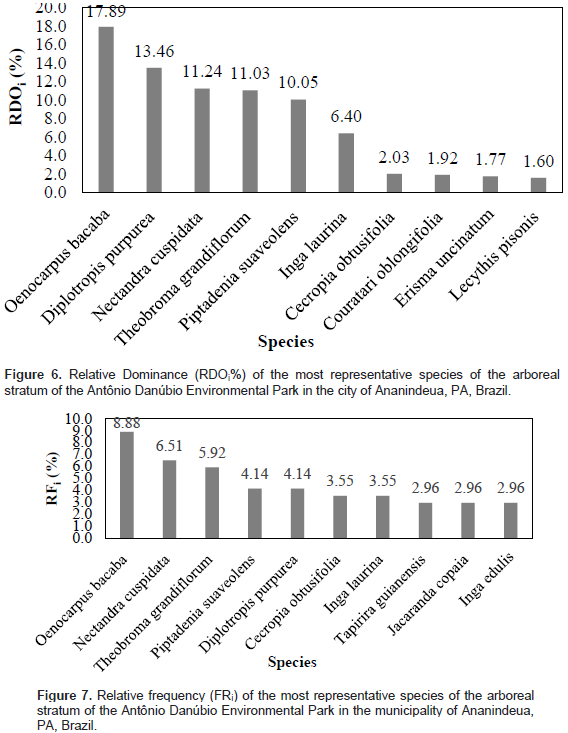
In relation to the species with greater relative dominance (Figure 6), the species Oenocarpus bacaba presented the highest basal area per hectare, followed by the species, Diplotropis purpurea, Nectandra cuspidata, Theobroma grandiflorum, Piptadenia suaveolens, Inga laurina, Cecropia obtusifolia, Couratari oblongifolia, Erisma uncinatum and Lecythis pisonis. These species together hold 77.38% of the basal area per hectare. The species that obtained the highest values ​​of the relative frequency (Figure 7) are: Oenocarpus bacaba (arecaceae), Nectandra cuspidata (Lauraceae), Theobroma grandiflorum (Malvaceae), Piptadenia suaveolens (Leguminosae), Cecropia obtusifolia (Urticaceae), Inga laurina (Leguminosae), Tapirira guianensis (Anacardiaceae), Jacaranda copaia (Bignoniaceae) and Inga edulis (Leguminosae).
The families of the species found reveal the characteristic of an area that is in the secondary forest phase, both by the behavioral analysis of some that appear after anthropic disturbance (Urticaceae, Malvaceae and Anarcardiceae) and by the successional analysis of families that are commonly present in the early stages (Leguminosae) of areas.
In particular, the species Oenocarpus bacaba and Cecropia obtusifolia are typical of the Amazon region, where they can grow in the shade, but prefer more open areas. The first one is fire resistant, being found in capoeiras and pastures (Cymerys, 2005). The second is found either in open forest areas (recent clearings or more advanced clearings), near the slopes of water courses, less crowded places, or in depleted soils (Berg, 1978). The species with the highest coverage value index were: Oenocarpus bacaba, Nectandra cuspidata, Theobroma grandiflorum, Diplotropis purpurea, Piptadenia suaveolens, Inga laurina, Cecropia obtusifolia, Cecropia sciadophylla, Erisma uncinatum and Couratari oblongifolia as shown in Figure 8. These species represent 56.06% of the total individuals per ha and 65.94% of the cover value index (CVI%).
Oenocarpus bacaba, Nectandra cuspidata, Theobroma grandiflorum, Diplotropis purpurea, Piptadenia suavelons, Inga Lauriana, Cecropia obtusifolia, Cecropia sciadophylla, Tapirira guianensis and Jacaranda copaia are the species with the highest value index of importance (Figure 9). These species represent 56.06% of the individuals sampled per ha and 58.63% of the importance value index (IVIi). The species with the highest values of importance consequently are those that obtained the highest values of frequency, density and absolute dominance, since the value of importance is the sum of these results, and attributes to these species are greater ecological importance within this plant community (Table 1).
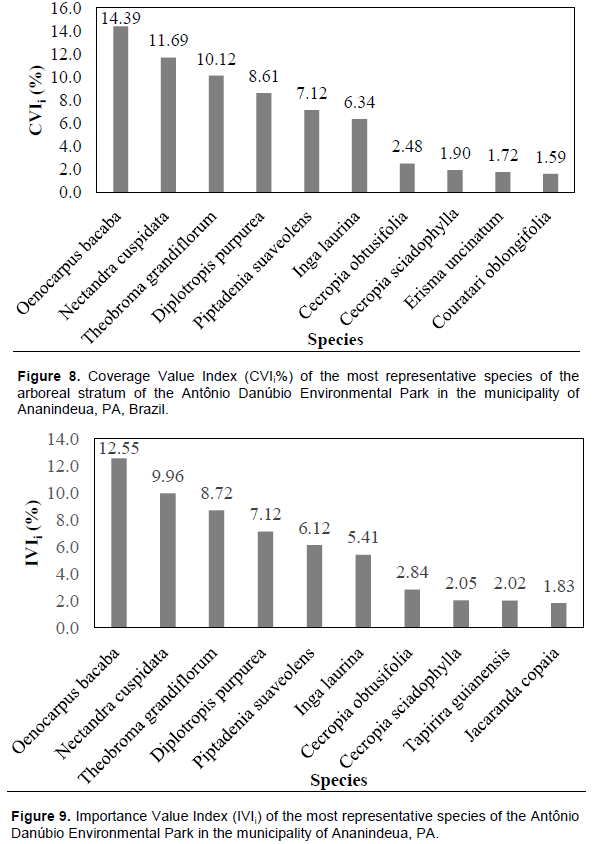
Carim et al. (2013) assumed that by the characteristic rate associated with the three parameters, phytosociological confers actual weight in each species’ ecological importance that is present within the community. In ascending order, among the species with the highest levels of importance, Oenocarpus bacaba value stood out by presenting IVI of 23.21; thus, leaving the front Nectandra species cuspidata which has the greatest number of individuals in the community (29) to obtain a lower level (20.82).
The result cited is related to the weight that the dominance and relative frequency of individuals earned in relation to species Arecaceae family. The species that showed higher importance local conditions are theoretically the most suitable (Estigarribia et al., 2017). The diversity of trees found in the study area was 3.53 nats/ind. by Shannon-Wiener index (Table 2). Compared to other arboreal strata of the metropolitan area, the Environmental Park Antonio Danube has a flora diversity below the others. Only the front of Combu Island, one of the factors that lead to this reduced number has low number of species found.
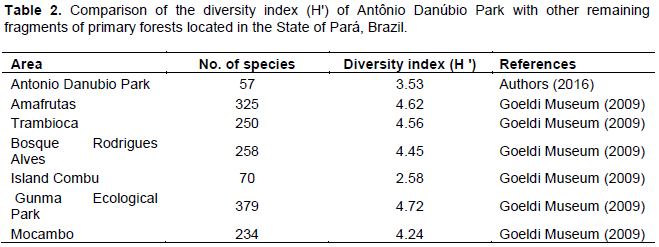
Silva et al. (2014) obtained a diversity index of 4.27 for primary upland forest. Already, Andrade et al. (2017) in Cuniã Ecological Station (RO) found a range of 3.76. In the latter, the authors considered that the low biodiversity in relation to other regions of the Amazon did not void the importance of the area, since the recognition becomes a contribution to the protection of the floristic composition. One explanation for the diversity found in the park may be linked mainly to the condition of being located in an urban area, where the characteristics of size, lack of connectivity with other green areas, isolation time and its disturbing history are variables that interfere with abiotic components of the medium and thus perpetuating the species.
The results show the existence of common floristic and structural variations of vegetation located in urban areas. The Shannon diversity index was inferior compared to other forest fragments located in the state of Pará, probably due to the Antônio Danúbio environmental park being part of an anthropic area near the federal highway BR-316, where a large number of vehicles circulate daily. In this sense, although the number of individuals was small, the study is of great relevance, since it represents the physiognomy of this fragment of forest that still remains in the municipality of Ananindeua, located in the state of Pará, Brazil.
Considering the low number of species observed, it is suggested that the municipal public authority, manager of the Antônio Danúbio Park, develop a plan to recover the area, introducing species endemic to the Amazon. In addition to implementing other actions related to the protection and management of forest resources in the area, both the awareness of the local population and the importance of maintenance, as well as the permanent studies of the forest fragment is recommended. It is observed from the results that continuous efforts are needed to achieve a more balanced environment of fauna, flora, water, air and other elements.
The authors have not declared any conflict of interests.
REFERENCES
|
Andrade RTG, Pansini S, Sampaio AF, Ribeiro MS, Cabral GS, Manzatto AG (2017). Fitossociologia de uma floresta de terra firme na Amazônia Sul-Ocidental, Rondônia, Brasil. Biota Amazônia 7(2):36-43.
|
|
|
|
Brazil (2000). Law no. 9985 of 18 July. Establishes the National System of Conservation Units of Nature and other measures. Available at: View
|
|
|
|
|
Berg DC (1978). Cecropia species of the Brazilian Amazon. Amazon Acta 8(2):49-182.
Crossref
|
|
|
|
|
Carim MJV, Guillaumet JB, Guimaraes JRS, Tostes LCL (2013). Composition and forest structure Rain extreme Dense North State of Amapa, Brazil. Biota Amazon 3(2):1-10.
Crossref
|
|
|
|
|
Chaves A, Del CG (2013). A importância dos levantamentos florísticos e fitossociológicos para a conservação e preservação das florestas. ACSA - Agropecuária Científica no Semiárido, Campina Grande 9(2):43-48.
|
|
|
|
|
Cymerys M (2005). Bacaba. Oenocarpus bacaba. in:Mart. Fruit trees and useful plants in Amazonian life. Available at:
View
|
|
|
|
|
Durigan G (2003). Methods for woody vegetation analysis. In Cullen Junior L , Rudran R, Valladares-Padua C (Eds). Research Methods in Conservation Biology and Wildlife Management. Curitiba: UFPR, Boticário Foundation for Nature Protection.
|
|
|
|
|
Estigarribia F, Aparicio WCS, Galvão, FG, Gama LCB (2017). Vegetation structure of forest fragments in the Federal University of Amapá - Brazil. Biota Amazon 7(3):17-22.
|
|
|
|
|
Flora do Brasil (2020). Em construção (2019). Jardim Botânico do Rio de Janeiro. Available at:
View
|
|
|
|
|
Graziano TT (1994). Municipal nurseries. Department of Horticulture - FCAVJ - UNESP: Lecture Notes.
|
|
|
|
|
Laus AV, Jardim MAG (2013). Floristic and structure of the tree community in a lowland forest in the Environmental Protection Area, Combu Island, Belém, Pará. Magazine Biota Amazon. Open Journal System, Macapa 3(2):88-93.
Crossref
|
|
|
|
|
Lamprecht H (1964). Ensayo sobre la structure de la floristic part suroriental del Bosque University "El Caimital" - Barinas state. For Rev.. Venez., Mérida 7(1):10-11.
|
|
|
|
|
Mueller-Dombois D, Ellemberg H (1974). Aims and methods of vegetation ecology J. Wiley & Sons, New York.
|
|
|
|
|
Magurran AE (1988). Ecological diversity and its measurement (pp 179). New Jersey: Princeton University Press.
Crossref
|
|
|
|
|
Oliveira NA, Amaral IL, Nobre AD, Couto LD, Sado RM (2003). Composition and floristic diversity in one hectare of a upland forest dense in Central Amazonia, Amazonas, Brazil. Biodiversity and Conservation (In press).
|
|
|
|
|
Santos GC, Garden MAG (2006). Floristic and structure of the tree layer of a floodplain forest in the municipality of Santa Bárbara do Pará, Para State, Brazil. Acta Amazonica 36(4):437-446.
Crossref
|
|
|
|
|
Silva WLS, Gurgel ESC, Santos JUM, Silva MF (2013). Inventory and geographical distribution of Leguminosae in the archipelago of Marajó, PA-Brazil. Hoehnea 40(4):627-647.
Crossref
|
|
|
|
|
Silva WAS, Carim MJV, Tostes, LCL (2014). Composition and plant diversity in a flooded forest in southwestern stretch of the Amapá State, Eastern Amazonia, Brazil. Biota Amazon 4(3):31-36.
Crossref
|
|
|
|
|
Silva L O, Costa DA, Filho KES, Ferreira HD, Brandão D (2002). Levantamento Florístico e Fitossociológico em duas áreas de cerrado sensu strictono parque estadual da serra de Caldas Novas, Goiás. Acta Botânica Brasilica 16(1):43-53.
Crossref
|
|
|
|
|
Souza VC, Lorenzi H (2005). Botânica sistemática: guia ilustrado para identificação das famílias de angiospermas da flora brasileira, baseado em APG II (640p). Nova Odessa: Instituto Plantarum.
|
|
|
|
|
Trinity SYM, Andrade CR, Sousa LAS (2007). Floristic and phytosociology of Utinga Reserve, Belém, Pará, Brazil. Journal of Biosciences, Porto Alegre 5(2):234-236.
|
|
|
|
|
Tropicos (2014). Missouri Botanical Garden. Available at:
View
|
|
|
|
|
Volpe-Filik A, Silva LF, Lima AMLP (2007). Evaluation of neighborhood streets afforestation San Dimas in the city of Piracicaba / SP through qualitative parameters. Journal of the Brazilian Society for Urban Forestation 2(1):1-10.
|
|
|
|
|
Watzlawick LF, Sanquetta CR, Valerio AF, Silvestre R (2005). Characterization of the floristic composition and structure of a Mixed Rain Forest, in the municipality of General Carneiro / PR. Ambience1(2):229-238.
|
|