ABSTRACT
Tropical forest soils have potential to mitigate climate change and support biodiversity. Human activities in these forests threaten biodiversity and alter the ability of the soil to sequester carbon. Many tropical countries experience rampant anthropogenic activities in the forests, yet the extent to which these activities affect biodiversity and soil organic carbon and the relationship between the two is not well studied. In this study, the correlation of soil organic carbon (SOC) and ground beetles was assessed in both control and disturbed sites in Uzungwa Scarp Nature Reserve (USNR). Disturbance activities included logging for timber, tool handles and building poles; fire, hunting, footpaths, collection of fuel wood, and clearing for agriculture. Pitfall trapping, active searching during the day, and active night searching were methods used to collect the ground beetles. Soil samples were collected at three depths 0-15, 15-30 and 30-45 cm in twelve plots: six in disturbed and six in control sites. A total of 890 ground beetles comprising 30 species were collected. The species richness of carabid beetles was high in the control sites (26 species) and low in disturbed site (16 species), with the respective Shannon-Wiener being Hꞌ= 2.103 and Hꞌ = 1.327. The difference in species diversity was statistically significant. Abundance of carabid beetles was also significantly higher in control sites compared to disturbed sites. Mean SOC was low in disturbed sites and high in control sites at all three depths. In disturbed sites, the correlation between SOC and species richness was weakly negative but not significant, and positively correlated with abundance, though it was not statistically significant. In control sites, there was a significant positive correlation between SOC and carabid abundance, but not with species richness of carabid beetles. To conclude, protection of natural forests is prerequisite for biodiversity and ecosystem services. We recommend that management improvement is urgently required, because ongoing human activities seem to contribute to diminished SOC stock.
Key words: Soil organic carbon, disturbance, ground beetles, correlation.
Soil is an important carbon pool in tropical areas, storing about 30% of the carbon in the world (Batjes, 1996; Scharlemann et al., 2014). Comparatively, the amount of carbon stored in soil is greater than the total amount of carbon stored in the atmosphere and the living biomass when combined (Ciais et al., 2013). Soil organic carbon (SOC) is among the five carbon pools recognised by the Intergovernmental Panel on Climate Change (IPCC), other pools include above ground, below ground, dead wood and litter (IPCC, 2006).
Healthier and functioning tropical forests and their diversity are known to enhance productivity, and soil carbon storage, among other ecosystem services (Sheil et al., 2016; Chen et al., 2018). Thus, on-going widespread destructive anthropogenic activities in the tropical forests as a result of human activities such as encroachment for farming, logging, hunting mining, and fire, among other factors, greatly causes loss of biodiversity and affects ability of soil to sequester carbon (Houghton, 2007; Sheil et al., 2016)
In recent years, studies are emerging to assess the correlation between ecosystem carbon stock and biological diversity of different groups of taxa (Strassburg et al., 2010; Kessler et al., 2012; Gilroy et al., 2014; Basham et al., 2016; De Beenhouwer et al., 2016). Some report that carbon stock can have a positive influence on the biodiversity of tropical forests (Strassburg et al., 2010; Venter, 2014). Most of current studies, however, deal with either above-ground carbon stock (Gilroy et al., 2014; Basham et al., 2016)and/or total carbon stock, including SOC (Kessler et al., 2012; De Beenhouwer et al., 2016). Few studies have specifically reported how soil carbon relates with biological diversity of soil dwelling invertebrates, especially ground beetles in agroforestry systems (Kessler et al., 2012; De Beenhouwer et al., 2016).
Existing studies on ground beetles and carbon are based in agroforestry systems and are contradictory, with some reporting that there is a relationship (Kessler et al., 2012), while others report no relationship (De Beenhouwer et al., 2016). However for other taxa, according to (Venter, 2014), a higher carbon stock correlates with higher biodiversity. Likewise, Gilroy et al. (2014)reported that in a secondary forest, both birds and dung beetles were favoured by an increase in non-soil carbon stock. A similar trend was observed in a regenerating secondary forest in tropical Andes (Basham et al., 2016)with regard to amphibian species richness and abundance; also Strassburg et al. (2010) reported correlation between above-ground carbon and selected vertebrates. However, the opposite trend has also been observed (Beaudrot et al., 2016).
More research, therefore, is still needed to understand the relationship between SOC and ground beetles in natural forest settings. Similar to other tropical forests in the world, USNR in Tanzania has been facing disturbances from several anthropogenic activities such as unsustainable farming activities, fire, honey harvesting, collection of fuel wood, building materials, timber, tool handles, forest encroachment for agriculture, illegal hunting and trespassing (Zilihona et al., 1998; Topp-Jørgensen et al., 2009; Rovero et al., 2012).
These activities are among some of the immediate drivers of habitat degradation in developing countries, including Tanzania (URT, 2010; Kissinger et al., 2012); however, to what extent they affect SOC and invertebrates specifically ground beetles in USNR is not very well studied. Some of the known research in the USNR include studies on vertebrates (Fjeldså, 1999; Menegon and Salvidio, 2005; Stanley and Hutterer, 2007; Rovero et al., 2012)and those on invertebrates only confined at the Kihansi waterfall (Zilihona and Nummelin, 2000; Zilihona et al., 2004). Other studies on invertebrates include Scharf (1992) and Sorensen et al. (2004) but did not address the impact of ongoing disturbances on ground beetles. Thus, little is known about the impact of the ongoing human activities on soil carbon stock and ground beetle diversity in the USFR.
Studies of carabid beetles in Tanzania have been recorded by surveys carried out in the Uluguru Mountains by Basilewsky (1962, 1976), these were museum collections, and do not provide ecological information. Other available research include Zilihona and Nummelin (2000) and Zilihona et al. (2004) that address the impact of Kihansi gorge construction in USNR, and Nyundo and Yarro (2007) on designing inventory methods, as well as Belousov and Nyundo (2013) on taxonomy of some new species in Udzungwa Mountain National Park. Therefore, there is limited information on the impact of ongoing activities on ground beetles in USNR.
Ground beetles have been chosen for this study for several reasons: (a) they can be sampled using simple methods (McGeoch, 1998; Rainio and Niemela, 2003), b) they are abundant in most ecosystems and they are good indicators of habitat disturbance, c) they occur with species that possess strong habitat preferences, d) most of the ground beetle species show association with specific microclimate conditions, e) they show a rapid response to changes in vegetation and overall landscape ecology, and f) they have a high functional importance (Rosenberg et al., 1986; McGeoch, 1998; Rainio and Niemela, 2003).
The present study aimed to enhance understanding of the impact of anthropogenic disturbances on biodiversity and ecosystem services in USFR, which is an area with high endemism (Myers et al., 2000; Rovero and Menegon, 2005), and has a possible high rate of species extinction and rampant anthropogenic activities (Fjeldså, 1999; Menegon and Salvidio, 2005; Stanley and Hutterer, 2007; Rovero et al., 2012).
Specifically, the study aimed at: a) evaluating the effect of anthropogenic disturbances on ground beetle diversity, b) assessing the impact of anthropogenic disturbances on SOC, and c) examining the relationship between ground beetle diversity and SOC. Since management and conservation of forest embraces both ecosystem services and biodiversity, this study may provide insight on the relationship that exists between SOC and ground beetles. Detailed information about carbon and biodiversity patterns can help in the formulation of policy objectives such as reducing emissions from deforestation and forest degradation (REDD+). Moreover, the impact of forest disturbance leading to degradation is less well known in Tanzania (Burgess et al., 2010); hence, this study will provide an understanding on how forest disturbance impacts SOC. This may help in developing mitigation measures at both local and international level.
Study site
The study was carried out in the USNR, an area of about 207 km2 located in the Udzungwa Mountains within the Eastern Arc Mountains (EAM) of Tanzania and Kenya (Myer et al., 2007). The USNR lies between latitudes 35° 50' and 36° 05' E and longitude 8°10' and 8° 37' S in southern central Tanzania within Morogoro and Iringa Regions (Figure 1). Its altitude ranges from 300 to 2,068 m a.s.l. (MNRT, 2010). The area has an estimated average temperature between 20°C maximum in December and 15°C minimum in July; while in lowland areas, temperatures reach a maximum of 27°C in December and a minimum of in 19°C in July. Annual rainfall varies from 1,350 to 2,000 mm and sometimes exceeds 3000 mm in wetter areas (MNRT, 2010).
Study sites were selected based on presence/absence of signs of human activities such as log stumps, snares for trapping animals, footpaths, presence of abandoned human habitats, collected fuel wood and tool handles, and pit sawing sites. A site was considered as disturbed if five or more recent (<2-year) activities mentioned earlier were encountered within the plot or within 50 m outside the plot perimeter. Study sites were located between 466 and 740 m. a. s. l. (Table 1).
Data collection
A total of twelve plots, each 1 ha in size (100 m × 100 m), were established at each site. Each site was characterized as either disturbed or control, based on the intensity of human activities. Within a 1 ha plot, sampling for both carabid beetles and soil was done. Sampling took place in November and December 2016 and July and August 2018; these periods mark the end of dry and end of wet season, respectively.
Data on Carabid beetles
Carabid beetles were sampled using three methods: pitfall traps, active searching (day), and active searching (night) (Greenslade, 1964; Nyundo and Yarro, 2009). Geographical position and altitude of each site were recorded using Garmin GPS 60 (Table 1).
Forty pitfall traps made of plastic containers (12 cm top width, 15 cm depth, 1 L capacity) sunken in the ground and half filled with a preservative (propylene glycol), were set at a distance of 10 m apart around the perimeter of the 1-ha plot. Traps were checked after one week. Each trap constituted a “sample”. Ground beetles from pitfall traps were sieved and collected using forceps.
Within the same plots, active searching for 1 h constituted a sample. Active searching was done both day (for three hours) and night (for three hours) at each site. The activity involved searching for ground beetles under logs, rotting logs and in leaf litter. Leaf litter was scooped onto a 1-m2 white cloth; and carabid beetles were collected by hand or using a pooter collection device. Specimens were kept in labelled plastic bags containing 75% alcohol and transported to the University of Dar es Salaam (UDSM) for sorting and identification.
Data on soil samples
Soil samples were collected in 1-m2 quadrats established at three points within each plot boundaries using a soil auger. Soil samples were collected at three depths 0-15, 15-30 and 30-45 cm for determination of SOC. The soil was mixed according to their specific layers to form composite. A sub-sample mixture from each layer was kept in sealed polythene bags with labels. Using a cylinder steel core, soil was sampled for determination of bulk density; the known volume of core cylinder was used. While being careful, without disturbing the top layer, the soil samples were collected and kept in polythene plastic bags with labels for further laboratory analysis at the UDSM. For bulk density analysis, samples were oven dried at 105°C for 24 h then weighed for sample dry weight. Volume of the core cylinder and weight of the dry sample were used to calculate the soil bulk density. Soil organic carbon content was determined using Walkley and Black’s potassium dichromate method as described in Nelson and Sommers (1982). Soil organic carbon in tonnes per hectare (t/ha) was calculated using Equation 1 (Bross and Baldock, 2008).
Soil organic carbon (t/ha) = depth (cm) × bulk density (g/cm3) × % organic carbon (1)
Carabid beetle identification was carried out by use of identification keys (Basilewsky, 1953) and specimens available in the collections of Zoology and Wildlife Department of the UDSM. Identification considered all external visible features excluding genitalia. Identification was made to species level whenever possible; whenever there were difficulties to identify species level, we identified genus or subfamily.
Data analysis
Diversity of ground beetles was computed using the Shannon-Wiener Index and compared using a special diversity t-test. The Shannon-Wiener diversity index takes into consideration the number of individuals (abundance) and the number of taxa (species richness) (Magurran, 1988). The difference in abundance between disturbed and control sites was assessed using a Mann-Whitney test.
SOC (t/ha) estimated from the three sampling points for each depth was averaged to get the estimate of SOC per site. Not all data were normally distributed; therefore data were logarithmic-transformed. Carbon stock between disturbed and control sites, and at three depths, was analysed using parametric tests; namely, two-sample t-test and ANOVA, respectively.
The relationship between carbon and carabid beetle diversity was assessed using a Pearson linear correlation (r). Simple linear regression was performed using Reduced Major Axis (RMA) for coefficient of determination (R2). Total SOC for each site was regressed against ground beetles. Finally, PAST software Version 3.20 (Hammer, 2018) was used for all statistical tests and graphs generation.
Abundance and diversity of carabid beetles in control and disturbed sites
Overall, carabid beetle abundance was high in control sites with 596 individuals and low in disturbed sites with 294 individuals. In control sites, the minimum number of carabid beetles per sample was 0 while the maximum was 18, while in disturbed sites, the minimum and maximum number of carabid beetles per sample was 0 and 9, respectively. The mean number of carabid beetles was 0.725 ± 0.065 in control sites and 0.356 ± 0.038 in disturbed sites. A Mann-Whitney test showed that the difference in abundance between control and disturbed sites was not statistically significant (p > 0.05).
Overall, a total of 890 carabid beetles represented by 30 species of carabid beetles were collected. The species richness of carabid beetles was high in control sites (26 species) and low in disturbed site (16 species). Some species appeared only in disturbed sites, while others were strictly in control sites; and others were found in both disturbed and control sites in a varying composition. Shannon-Wiener diversity index (Hꞌ) revealed that species diversity was high in control sites (Hꞌ = 2.082) and low in disturbed sites (Hꞌ = 1.260). Carabid beetle species diversity differed significantly between disturbed and control sites (p = 1.807 E-14).
Comparison of the amount of soil organic carbon between disturbed and control sites
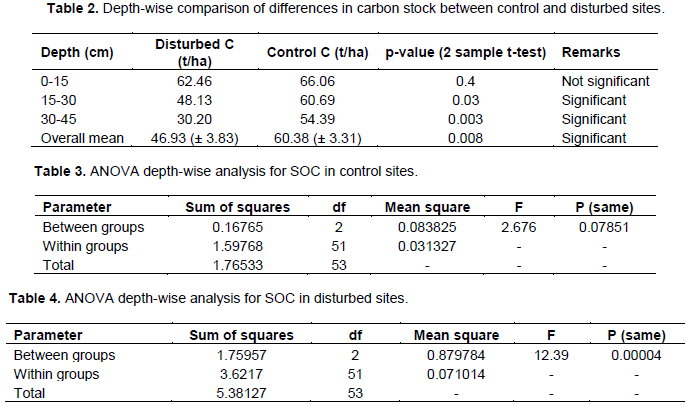
Overall results show that, at all three depths, disturbed sites contained a lower amount of soil organic carbon when compared with control sites (Figure 2). Also, there was a significant difference in SOC between disturbed and control sites for 15-30 and 30-45 cm depth, but not for 0-15 cm. The respective mean SOC was 46.93 and 60.38 t C/Ha (Table 1). The two sample T-test revealed that the difference was statistically significant (p = 0.008). Testing the level of significance by depth, within control sites, there was no significant difference in SOC stock (Table 3), while in disturbed sites, carbon differed significantly with depth (Table 4).
Correlation between SOC and carabid beetle diversity
In examining the relationship between ecosystem service (soil organic carbon) and biodiversity of the carabid beetles at the sampling sites, a mixed pattern of associations was found, some aspects showing positive while others showing a negative relationship.
Species richness was positively correlated to SOC for control sites, while in disturbed sites it was negatively correlated, however not significant (r = 0.318, p = 0.538 and r = - 0.256, p = 0.625, respectively) (Figure 2a and b). The coefficient of determination was R2 = 0.102 for control sites. Abundance was positively correlated to SOC in both disturbed and control sites (r = 0.322, p = 0.534, and r = 0.829, p = 0.041, respectively) (Figure 2c and d). The coefficients of determination were R2 = 0.072 and 0.687, respectively.
The mean soil organic carbon estimated in the present study for 0-15 and 15-30 cm is lower than the values reported by Munishi and Shear (2004)from the Uluguru and Usambara Mountains. Historical records of threats in the reserve date back to the 1990s (Hunter, 1992; Shangali et al., 1998). Also studies by Rovero et al. (2005) and Rovero (2012) show that human activities are wide spread in the reserve. Therefore, both historical and ongoing anthropogenic activities in the USNR might have contributed to depletion of the stock.
The present results show that soil organic carbon was higher in control sites and lower in disturbed sites at all three soil depth (Table 2), providing evidence that on-going human activities in the reserve reduce the capacity of soil to sequester carbon. Similar observations were reported by Chiti et al. (2018)when comparing natural and degraded forest in Kenya and also Kessler et al. (2012) when comparing natural forest and agroforest systems. Despite the fact that little is known on the effect of forest degradation on SOC (Berenguer et al., 2014), the present study establishes evidence that upper layer soils 0-45 cm are very sensitive to forest degradation.
The results reveal that soil organic carbon stock decreases from the upper depth (0-15 cm) to the lower depth (30-45 cm). This trend of higher SOC in the top layer might be attributed by higher rate of litter decomposition and might be suggesting that the upper layer is associated with other biological activities (Alamgir and Al-Amin, 2008; Dinakaran and Krishnayya, 2008; Sheikh et al., 2009). A similar decreasing trend was also noted in Uluguru and Usambara Mountains (Munishi and Shear, 2004)for 0-15 and 15-30 cm depth. When depth-wise comparison was considered, it was revealed that for the upper depth the difference in soil organic carbon stock was not significantly different between control and disturbed sites; this was contrary to the middle and lower depth which showed significant differences in soil organic carbon stock (Table 1). Also, the amount of SOC stored among different depths differed significantly in disturbed sites (Table 4) when compared with control sites (Table 3). This might be suggesting that in disturbed sites, there is less input in the upper depth (0-15cm), thus lower SOC is moving down. The present findings are in agreement with Dinakaran and Krishnayya (2008).
Disturbed sites hold a lower amount of SOC when compared with control sites. There may be several possible explanations. First, the altered tree species composition in disturbed sites as a result of disturbance might have altered the quality of litter input. Moreover, the different tree species may increase the quality of litter production and increase rate of decomposition processes, which adds carbon to the soil. Review studies on litter decomposition (Hättenschwiler, 2005; Hättenschwiler et al., 2005), have revealed that decomposition rate of litter from species-rich plant communities are higher than the rate of decomposition of a single species litter. Furthermore, the presence of plant communities with high species richness usually supports and enhances abundance of primary herbivores and numerous microbial activities associated with them. This in turn, will act on litter and hasten decomposition rates adding SOC to the soil. This was also reported by several authors (Fornara and Tilman, 2008; De Deyn et al., 2011; Lange et al., 2015). The second reason for lower SOC in disturbed sites might be associated with the number of stems; disturbed sites had few stems when compared with control sites. This situation leaves the soil bare and prone to mineralization, erosion and decomposition. This may lead to carbon losses (De Beenhouwer et al., 2016). Several studies concur with the present study (Omoro et al., 2013; De Beenhouwer et al., 2016).
Contrary to the present study, Kessler et al. (2012) reported no variation in soil organic carbon stock when comparing an agroforestry and natural forest system. These results might have been attributed by the fact that agroforestry systems do not involve total removal of trees and for this reason the soils are neither left bare nor exposed to severe erosion. The remaining trees prevent soil erosion and mineralization processes and enhance retention of SOC (Sepúlveda and Carrillo, 2015). Also, Dawoe et al. (2013) reported high soil organic carbon in areas with increased management intensification, which involved slashing and burning.
Carabid beetle species diversity, abundance and species richness was high in control sites and lower in disturbed sites. The difference in the diversity of ground beetles between disturbed and control sites is an indication that on-going human activities affect ground beetles’ diversity in USFR. Disturbance might have created habitats suitable for only a few generalist species, because it contained few and less tree stands when compared with control sites (pers. observ.), which could create a more homogeneous microclimate and alter soil moisture content by increasing temperature and lowering the moisture content of the soil; this condition might have had an effect on carabid beetles species richness and diversity as suggested by Ings and Hartley (1999). Also, increase in soil temperature and low moisture content have negative effects on SOC (Chen et al., 2018). On the other hand, increase in tree species in control sites may influence creation of diverse habitats and food resources, because increase in vegetation diversity supports an increase in primary productivity (Hooper et al., 2005); this would support herbivore arthropods, and as a consequence the biomass of consumers will increase (Borer et al., 2012). This situation may affect the diversity of ground beetles positively. This observation was also supported by several prior studies for insects (Winter and Möller, 2008; Axmacher et al., 2009; Schuldt et al., 2010).
High carabid species diversity in control sites is supported by the “Enemies hypothesis” (Root, 1973), which postulates that plant communities with diverse tree species will support more predators than simple plant communities with few species; thus, high plant diversity increases the ability of predator to catch prey (Russell, 1989). The present findings are also in agreement with Andow (1991). Likewise, high species diversity in sites with high plant species diversity is in agreement with species-energy ecological theory by Wright (1983). Moreover, the control sites had an undisturbed layer of leaf litter, which would provide habitat for cryptic carabid beetles. The situation may support diverse species to co-occur when compared with disturbed sites where habitats are unsuitable and too few food resources are available to support diverse species.
Contrary to the present study, other investigators (De Beenhouwer et al., 2016; Latty et al., 2006) report no variation in carabid beetle abundances in forests with different management types. In the current study, abundance of carabid beetles and SOC for control sites showed highly positive significant correlation with high values of coefficient of determination. In disturbed sites, the correlation was positive but not significant and coefficient of determination was low. These results suggest that, in absence of human disturbance, SOC is a better predictor of the abundance of ground beetles compared to a disturbed one. It is likely that disturbance is altering SOC, and this changes other conditions, such as soil temperature and moisture content, which directly affect carabid beetles. A recent study by Chen et al. (2018) reported that SOC is negatively correlated with high temperature and low moisture.
Species richness showed a positive correlation with SOC, but not significant for control sites; while disturbed sites showed only a marginal negative correlation, however not significant. It seems that disturbance may be affecting SOC and other factors that are important for different species of ground beetles. Several other factors are important for existence of ground beetles, factors such as specific microclimate condition, food resources that are crucial; and these factors may have been changed as a result of disturbance and changes in carbon. The findings suggest that in disturbed sites, apart from carbon, there might be other environmental parameters that are affected in a similar way to carbon, and likely have influenced the relationship between SOC and carabid beetles.
Similar to the present study, Kessler et al. (2012)reported a positive correlation between carbon and carabids species richness, when only forest species and total (below and above ground) carbon in natural forest was considered; and a negative correlation in agroforestry systems. Other studies examining other taxa are in agreement with the present study; however they used non-soil carbon (Basham et al., 2016)for amphibians, and Gilroy et al. (2014)for dung beetles and birds, and at a global scale (Strassburg et al., 2010)for selected vertebrates. Conversely to the present study, De Beenhouwer et al. (2016)reported no relationship between total carbon and carabid beetles.
CONCLUSION AND RECOMMENDATION
Overall, the present study showed that ongoing human activities in the USNR affect both biodiversity (carabid beetles) and ecosystem serviced (soil organic carbon). These activities should not be overlooked when updating management plans. Therefore in USFR, forest degradation should be kept minimal or halted completely. The positive correlation noted in control sites provides information that maintaining a natural forest can embrace biodiversity and climate mitigation, thus initiatives such as REDD+ activities may serve both biodiversity and climate mitigation. Further research should also include carbon pools such as leaf litter and dead wood in relation to carabid beetles.
The authors have not declared any conflict of interests.
The study was financed by Norwegian Programme for Capacity Building in Higher Education and Research for Development (NORHED) project under the Centre for Climate Change Studies (CCCS) of the University of Dar es Salaam. The authors also thank Tanzania Forest Agency (TFS) for permissions, Rispael Mmanyi, Michael Mpwage, Deo Wayinga, and Bahati Luvinga for logistics and assistance during the field work.
REFERENCES
|
Alamgir M, Al-Amin M (2008). Allometric models to estimate biomass organic carbon stock in forest vegetation. Journal of Forestry Research 19(2):101.
Crossref
|
|
|
|
Andow DA (1991). Vegetational diversity and arthropod population response. Annual Review of Entomology 36(1):561-586.
Crossref
|
|
|
|
|
Axmacher JC, Brehm G, Hemp A, Tünte H, Lyaruu HVM, Müller-Hohenstein K, Fiedler K (2009). Determinants of diversity in afrotropical herbivorous insects (Lepidoptera: Geometridae): plant diversity, vegetation structure or abiotic factors?. Journal of Biogeography 36(2):337-349.
Crossref
|
|
|
|
|
Basham EW, González del Pliego P, Acosta-Galvis AR, Woodcock P, Medina Uribe CA, Haugaasen T, Edwards DP (2016). Quantifying carbon and amphibian co-benefits from secondary forest regeneration in the Tropical Andes. Animal Conservation 19(6):548-560.
Crossref
|
|
|
|
|
Basilewsky P (1953). Carabidae (Coleoptera, Adephaga): Exploration du Parc National de," Mission G. F. Witte 10:1-152.
|
|
|
|
|
Basilewsky P (1962). Mission Zoologique de l'I.R.S.A.C. en Afrique orientale (P. Basilewsky et N. Leleup, 1957). LX. Coleoptera Carabidae. Annales Musée Royal de l'Afrique Centrale, Tervuren, Série in 8°, Sciences Zoologiques 107:48-337.
|
|
|
|
|
Basilewsky P (1976). Mission entomologique du Musee royal de I'Afrique Centrale auxm Monts Uluguru, Tanzanie. 19. Coleoptera Carabidae," Revue Zoologique Africaine 90:671-722.
|
|
|
|
|
Batjes NH (1996). Total carbon and nitrogen in the soils of the world. European Journal of Soil Science 47(2):151-163.
Crossref
|
|
|
|
|
Beaudrot L, Kroetz K, Alvarez-Loayza P, Amaral I, Breuer T, Fletcher C, Andelman S (2016). Limited carbon and biodiversity co-benefits for tropical forest mammals and birds. Ecological Applications 26(4):1098-1111.
Crossref
|
|
|
|
|
Belousov IA, Nyundo BA (2013). A new species of Pachytrechodes Jeannel, 1960 (Coleoptera: Carabidae :.Trechinae) from Tanzania, with a key to species. Zootaxa 3637(1):065-073.
Crossref
|
|
|
|
|
Berenguer E, Ferreira J, Gardner TA, Aragão LEOC, De Camargo PB, Cerri CE, Barlow J (2014). A large-scale field assessment of carbon stocks in human-modified tropical forests. Global Change Biology 20(12):3713-3726.
Crossref
|
|
|
|
|
Borer ET, Seabloom EW, Tilman D (2012). Plant diversity controls arthropod biomass and temporal stability. Ecology Letters 15(12):1457-1464.
Crossref
|
|
|
|
|
Burgess ND, Bahane B, Clairs T, Danielsen F, Dalsgaard S, Funder M, Zahabu E (2010). Getting ready for REDD+ in Tanzania: a case study of progress and challenges. Oryx 44(03):339-351.
Crossref
|
|
|
|
|
Ciais P, Sabine C, Bala G, Bopp L, Brovkin V, Canadell J, Chabra A, DeFries R, Galloway J, Heimann M, Jones C, Le Quéré C, Myneni RB, Piao S, Thornton B (2013). Carbon and other biogeochemical cycles, in Climate Change: The Physical Science Basis, Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate. Cambridge University Press, Cambridge, UK, and New York, NY, USA pp. 465-570.
|
|
|
|
|
Chen S, Wang W, Xu W, Wang Y, Wan H, Chen D, Bai Y (2018). Plant diversity enhances productivity and soil carbon storage. Proceedings of the National Academy of Sciences 115(16):4027-4032.
Crossref
|
|
|
|
|
Dawoe EK, Quashie-Sam JS, Oppong SK (2013). Effect of land-use conversion from forest to cocoa agroforest on soil characteristics and quality of a Ferric Lixisol in lowland humid Ghana. Agroforest System 88:87-99.
Crossref
|
|
|
|
|
De Beenhouwer M, Geeraert L, Mertens J, Van Geel M, Aerts R, Vanderhaegen K, Honnay O (2016). Biodiversity and carbon storage co-benefits of coffee agroforestry across a gradient of increasing management intensity in the SW Ethiopian highlands. Agriculture, Ecosystems & Environment 222:193-199.
Crossref
|
|
|
|
|
Dinakaran J, Krishnayya NSR (2008). Variations in type of vegetal cover and heterogeneity of soil organic carbon in affecting sink capacity of tropical soils. Current Science pp. 1144-1150.
|
|
|
|
|
Fjeldså J (1999). The impact of human forest disturbance on the endemic avifauna of the Udzungwa Mountains, Tanzania. Bird Conservation International 9(01):47-62.
Crossref
|
|
|
|
|
Fornara DA, Tilman D (2008). Plant functional composition influences rates of soil carbon and nitrogen accumulation. Journal of Ecology 96(2):314-322.
Crossref
|
|
|
|
|
Gilroy JJ, Woodcock P, Edwards FA, Wheeler C, Baptiste BLG, Medina Uribe CA, Edwards DP (2014). Cheap carbon and biodiversity co-benefits from forest regeneration in a hotspot of endemism. Nature Climate Change 4(6):503-507.
Crossref
|
|
|
|
|
Greenslade PJM (1964). Pitfall trapping as a method for studying populations of Carabidae (Coleoptera). The Journal of Animal Ecology pp. 301-310.
Crossref
|
|
|
|
|
Hammer Ø (2018). PAleontological STatistics (PAST Version 3.20), Reference manual, Natural History Museum University of Oslo. pp. 264.
|
|
|
|
|
Houghton RA (2007). Balancing the global carbon budget. Annual Review of Earth and Planetary Sciences 35:313-347.
Crossref
|
|
|
|
|
Hättenschwiler S (2005). Effects of tree species diversity on litter quality and decomposition. In Forest Diversity and Function. Springer. pp. 149-164.
Crossref
|
|
|
|
|
Hättenschwiler S, Tiunov AV, Scheu S (2005). Biodiversity and litter decomposition in terrestrial ecosystems. Annual Review of Earth and Planetary Sciences 36:191-218.
Crossref
|
|
|
|
|
Hooper DU, Chapin FS, Ewel JJ, Hector A, Inchausti P, Lavorel S, Naeem S (2005). Effects of biodiversity on ecosystem functioning: a consensus of current knowledge. Ecological Monographs 75(1):3-35.
Crossref
|
|
|
|
|
Ings TC, Hartley SE (1999). The effect of habitat structure on carabid communities during the regeneration of a native Scottish forest. Forest Ecology and Management 119(1-3):123-136.
Crossref
|
|
|
|
|
Intergovernmental Panel on Climate Change (IPCC) (2006). 'Guidelines for National Greenhouse Gas Inventories' In: (Eggleston HS, Buendia L, Miwa K, Ngara T, Tanabe K (Eds.), National Greenhouse Gas Inventories Programme. Institute for Global Environmental Strategies, Japan.
|
|
|
|
|
Kessler M, Hertel D, Jungkunst HF, Kluge J, Abrahamczyk S, Bos M, Tscharntke T (2012). Can Joint Carbon and Biodiversity Management in Tropical Agroforestry Landscapes Be Optimized? PLoS ONE 7(10).
Crossref
|
|
|
|
|
Kissinger GM, Herold M, De Sy V (2012). Drivers of deforestation and forest degradation: a synthesis report for REDD+ policymakers. Lexeme Consulting. Vancouver Canada.
|
|
|
|
|
Lange M, Eisenhauer N, Sierra CA, Bessler H, Engels C, Griffiths RI, Scheu S (2015). Plant diversity increases soil microbial activity and soil carbon storage. Nature Communications 6:6707.
Crossref
|
|
|
|
|
Latty EF, Werner SM, Mladenoff DJ, Raffa KF, Sickley TA (2006). Response of ground beetle (Carabidae) assemblages to logging history in northern hardwood-hemlock forests. Forest Ecology and Management 222(1-3):335-347.
Crossref
|
|
|
|
|
Magurran A (1988). Ecological diversity and its measurement Princeton NJ: Princeton University Press.
Crossref
|
|
|
|
|
Mcgeoch M (1998). The selection, testing and application of terrestrial insects as bioindicators. Biological Reviews 73(2):181-201.
Crossref
|
|
|
|
|
Menegon M, Salvidio S (2005). Amphibian and Reptile Diversity in the Southern Udzungwa Scarp Forest Reserve, South-Eastern Tanzania. In African Biodiversity. Boston, MA: Springer US. pp. 205-212
Crossref
|
|
|
|
|
Munishi PKT, Shear TH (2004). Carbon storage in Afromontane rain forests of the Eastern Arc mountains of Tanzania: their net contribution to atmospheric carbon. Journal of Tropical Forest Science 16(1):78-93.
|
|
|
|
|
Nelson DW, Sommers LE (1982).Total carbon, organic carbon and organic matter. In: Page AL (Eds), Methods of soil analysis. Part 2. Agronomy Monographs 9. ASA and SSSA, Madison. WI. pp. 539-579.
|
|
|
|
|
Nyundo B, Yarro J (2009). An assessment of methods for sampling carabid beetles (Coleptera: Carabidae) in a montane rain forest. Tanzania Journal of Science 33(1):41-49.
Crossref
|
|
|
|
|
Omoro LMA, Starr M, Pellikka PKE (2013). Tree biomass and soil carbon stocks in indigenous forests in comparison to plantations of exotic species in the Taita Hills of Kenya. Silva Fennica 47(2):935-953.
Crossref
|
|
|
|
|
Rainio J, Niemela J (2003). Ground beetles (Coleoptera: Carabidae) as bioindicators. Biodiversity and Conservation 12:487-506.
Crossref
|
|
|
|
|
Rosenberg DM, Danks HV, Lehmkuhl DM (1986). Importance of insects in environmental impact assessment. Environmental Management 10:773-783.
Crossref
|
|
|
|
|
Root RB (1973). Organization of a plant-arthropod association in simple and diverse habitats: the fauna of collards (Brassica oleracea). Ecological Monographs 43(1):95-124.
Crossref
|
|
|
|
|
Rovero F, Menegon M, Struhsaker TT (2005). Biological importance and urgent need for protection of the Udzungwa Scarp Forest Reserve and other forests of the Udzungwa mountains. Journal of East African Natural History 87:91-100.
|
|
|
|
|
Rovero F, Mtui AS, Kitegile AS, Nielsen MR (2012). Hunting or habitat degradation? Decline of primate populations in Udzungwa Mountains, Tanzania: An analysis of threats. Biological Conservation 146(1):89-96.
Crossref
|
|
|
|
|
Russell EP (1989). Enemies hypothesis: a review of the effect of vegetational diversity on predatory insects and parasitoids. Environmental Entomology 18(4):590-599.
Crossref
|
|
|
|
|
Scharff N (1992). The linyphiid fauna of eastern Africa (Araneae: Linyphiidae): distribution patterns, diversity and endemism. Biological Journal of the Linnean Society 45:117-154.
Crossref
|
|
|
|
|
Scharlemann JP, Tanner EV, Hiederer R, Kapos V (2014). Global soil carbon: understanding and managing the largest terrestrial carbon pool. Carbon Management 5(1):81-91.
Crossref
|
|
|
|
|
Schuldt A, Baruffol M, Böhnke M, Bruelheide, H, Härdtle W, Lang AC, Zhou H (2010). Tree diversity promotes insect herbivory in subtropical forests of south-east China. Journal of Ecology 98(4):917-926.
Crossref
|
|
|
|
|
Sepúlveda RB, Carrillo AA (2015). Soil erosion and erosion thresholds in an agroforestry system of coffee (Coffea arabica) and mixed shade trees (Inga spp and Musa spp) in Northern Nicaragua. Agriculture, Ecosystems and Environment 210:25-35.
Crossref
|
|
|
|
|
Shangali CF, Mabula CK, Mmari C (1998). Biodiversity and human activities in the Udzungwa Mountain forest, Tanzania. Etnhobotanical survey in the Udzungwa Scarp Forest Reserve. Journal of East African Natural History 87:291-318.
Crossref
|
|
|
|
|
Sheikh MA, Kumar M, Bussmann RW (2009). Altitudinal variation in soil organic carbon stock in coniferous subtropical and broadleaf temperate forests in Garhwal Himalaya. Carbon Balance and Management 4(1);6.
Crossref
|
|
|
|
|
Sheil D, Ladd B, Silva LCR, Laffan SW, Heist MV (2016). How are soil carbon and tropical biodiversity related? Environmental Conservation 43(3):231-241.
Crossref
|
|
|
|
|
Sorensen L (2004). Composition and diversity of the spider fauna in the canopy of a montane forest in Tanzania. Biodiversity Conservation 13:437-452.
Crossref
|
|
|
|
|
Stanley WT, Hutterer R (2007). Differences in abundance and species richness between shrews and rodents along an elevational gradient in the Udzungwa Mountains, Tanzania. Acta Theriologica 52(3):261-275.
Crossref
|
|
|
|
|
Strassburg BBN, Kelly A, Balmford A, Davies RG, Gibbs HK, Lovett A, Rodrigues ASL (2010). Global congruence of carbon storage and biodiversity in terrestrial ecosystems. Conservation Letters 3(2):98-105.
Crossref
|
|
|
|
|
Topp-Jørgensen E, Nielsen MR, Pedersen U (2009). Mammalian density in response to different levels of bushmeat hunting in the Udzungwa Mountains, Tanzania. Tropical Conservation Science 2(1):70-87.
Crossref
|
|
|
|
|
United Republic of Tanzania (URT) (2010). Millenium Development Goals. Retrieved December 21, 2018.
|
|
|
|
|
Venter O (2014). REDD+ Policy: Corridors of carbon and biodiversity. Nature Climate Change 4(2):91-92.
Crossref
|
|
|
|
|
Winter S, Möller GC (2008). Microhabitats in lowland beech forests as monitoring tool for nature conservation. Forest Ecology and Management 255(3-4):1251-1261.
Crossref
|
|
|
|
|
Wright DH (1983). Species-energy theory: an extension of species-area theory. Oikos 41(3):496-506.
Crossref
|
|
|
|
|
Zilihona IJE, Niemelä J, Nummelin M (2004). Effects of a hydropower plant on Coleopteran diversity and abundance in the Udzungwa Mountains, Tanzania. Biodiversity and Conservation 13(8):1453-1464.
Crossref
|
|
|
|
|
Zilihona IJE, Nummelin M (2000) Coleopteran diversity and abundance in different habitats near Kihansi waterfall, in the Udzungwa Mountains, Tanzania. Biodiversity and Conservation10:769-777.
|
|
|
|
|
Zilihona I, Shangali C, Mabula CK, Hamisy C (1998). Human Activities Threatening the Biodiversity of the Uzungwa Scarp Forest Reserve, Tanzania. Journal of East African Natural History 87(1):319-326.
Crossref
|
|