Full Length Research Paper
ABSTRACT
We set up six light conditions to investigate the changes in the development of Arabidopsis thaliana hypocotyls and roots. Seedlings grown for 96 h under darkness were scored with shorter roots and longer hypocotyls. In shoot-shaded conditions, seedlings were unable to carry out photosynthesis, resulting in insufficient stored nutrients for root development. In the three groups of different light intensities applied to the roots, total light caused stress in the entire seedlings and the length of roots and hypocotyls were shorter than in conditions when roots were growing within light-dark gradients. Importantly, root lengths were higher within light-dark gradients than in total light. Different light treatments did significantly affect root growth and hypocotyl growth. The addition of ATP-competitive mTOR kinase inhibitor (AZD), drastically reduced root, however, this did not occur with hypocotyl length.
Key words: Total light, total dark, gradient light, shoot dark with light blocker, light blocker, shoot dark.
INTRODUCTION
To adapt to a changing environment, all living organisms have to respond appropriately to circumstances. Unlike animals, plants are unable to move away from extremes in their surrounding environment or move towards a nutrition source. However, plants have a flexible pattern of development that allows them to adjust their organ number and size (architecture) to the changing environment. The fundamental body plan of the mature plant is generated during the early stages of embryogenesis (Ju?rgens et al., 1991). This process involves the production of shoot and root meristem, cotyledons, radicle and hypocotyls.
In animals, most organs are already present by the time the embryo is fully formed. On the contrary, most organs in plants are formed after embryogenesis is finished. Once dormancy is broken, the seeds begin to germinate with the formation of primary plant organs: roots, shoots, and leaves (Kadereit et al., 2014). Roots emerge from root meristems located at the tip of the root, while the aboveground shoot system generates from shoot meristems (Brand et al., 2001). Roots are the underground part of the plant body that is required for anchorage in the substrate, uptake of water and ion, and synthesis of phytohormones (Kadereit et al., 2014). The root apex also has an oscillatory zone (Baluška and Mancuso, 2013). The root apex is subdivided into four zones: Meristematic (Kadereit et al., 2014), transition (Verbelen et al., 2006, Baluška et al., 2010), elongation, and differentiation zones. The root cap is the structure that detects the pull of gravity and thus controls the downward growth of roots (Petricka et al., 2012). Statocytes are specialized root-cap cells that contain amyloplasts, which will precipitate if the root is reoriented (Kadereit et al., 2014). Cells in the elongation zone elongate and allow root growth (Crang et al., 2018). Root growth is regulated and fine-tuned by several phytohormones. The first phytohormone to be discovered was auxin, which is crucial for cell elongation and lateral root growth (Petricka et al., 2012).
Light is one of the most important environmental factors for plant development. Light cues of varied intensity and quality cause plants to change their morphological traits (Yadav et al., 2020). Besides that, light and temperature also modulate phytochrome growth via phytochrome (Ibrahim and František, 2022). Light perceived by photosensory systems in above-ground tissues can affect the roots via long-distance signal transduction pathways (Qu et al., 2017). Sunlight, on the other hand, can reach root tissues that are several centimeters underground (Qu et al., 2017). Plants use dedicated photoreceptors to receive light signals of various wavelengths. Activated photoreceptors trigger a signal transduction cascade, which results in a wide range of gene expression modifications that affect physiological and developmental responses (Su et al., 2017). Most known plant photoreceptors, including phytochromes, cryptochromes, phototropins, and ultraviolet receptors (UVR), are expressed in the root tissues (Qu et al., 2017). Phytochromes are a class of red (R)/far-red (FR) light photoreceptors in plants that mediate the expression of various genes and are involved in root development and structure (Briggs et al., 2001).
When seedlings receive light, the elongation of the hypocotyls is carefully regulated to match the intensity and quality of light around them, and the phenomenon of de-etiolation occur, in which the development of leaves and chloroplasts inhibits stem elongation and promotes root growth and lateral root development, a process known as photomorphogenesis. The initial response of plants to light is photomorphogenesis, in which the shoot and root meristems are activated, and a series of changes, such as cell division and expansion, lead to changes in the differentiation structure and function of plant cells, and eventually the formation of tissues and organs (McNellis and Deng, 1995).
Light, as we have seen, has a rapid and dramatic impact on root development and physiology. Light is shown to have a great impact on adventitious roots and hypocotyles (Zeng et al., 2022). That aspect should be taken into account while doing experiments with plants, particularly those focusing on roots. Seeds are usually planted in transparent agar medium in hyaline Petri dish plates in laboratory settings, exposing roots and shoots to light in a similar way. Sucrose is also added to the growth medium of the classic agar plate culture technique (TPG, traditional plant-growing). However, because this is not a natural environment for roots, certain artifacts may occur (Xu et al., 2013). Improved approaches are available to make root experiments more efficient. An improved agar-plate method (IPG, improved plant-growing) is one example, in which shoots are lighted while roots are grown in a media without sucrose under dark circumstances. When comparing IPG to TPG, the root and lateral root lengths are both shorter, and the root hair density is lower. The primary root, on the other hand, was much longer. As a result, IPG provides a better and more natural environment for investigating A. thaliana root development and responses (Xu et al., 2013).
The target of rapamycin (TOR) is a large and highly conserved protein belonging to the family of phosphatidylinositol 3-kinase-related kinases (PIKKs). In response to environmental changes such as nutrients, energy status, and growth factors, TOR serves as a key sensor of cell growth and metabolism. Recent research has discovered that the conserved TOR pathway is vital in coordinating plant development at the whole plant level (Barrada et al., 2015). Furthermore, the Arabidopsis genome seems to have a single critical TOR gene, which down-regulation results in reduced plant growth, stress resistance (Menand et al., 2002), and increased life span (Ren et al., 2013). Moreover, Arabidopsis plants silenced for TOR expression display significantly reduced polysome abundance (Deprost et al., 2007), indicating that TOR plays a function in plant translational regulation. TOR inhibitors restrict the meristematic cell proliferation capability by reducing the number of cells in the MZ, mostly via encouraging differentiation (Montané and Menand, 2013). One of the most effective TOR inhibitors is AZD. As a second-generation mTOR inhibitor (known as ATP-competitive mTOR kinase inhibitor), it is developed not only to suppress mammalian TOR for cancer therapy but also to inhibit TOR in plants. AZD can bind to the TOR kinase domain within the ATP?binding pocket and inactivates the TOR complex (Montané and Menand, 2013). The aim of this study was to show the effect of AZD on Arabidopsis seedlings grown for 96 h under different light conditions. We could show that when Arabidopsis is treated with AZD and grown for 96 h under different light conditions, the root and hypocotyl are significantly modulated.
MATERIALS AND METHODS
Arabidopsis thaliana seedlings grown under different light conditions
Growth media preparation
The growth medium was prepared by mixing the MS medium salt (with vitamins), saccharose and dH2O. After adding each to a 1 L container, the pH was adjusted to 5.8 using KOH or HCl. After that, 4 g of phytagel was added to 1 L. The medium was mixed and autoclaved at 120ºC. The medium was placed in Petri dishes of different sizes and prepared under a sterile bench, for further usage. For the stupor experiment with A. thaliana seedlings, medium was added on round Petri dishes with AZD at 5 μM concentration.
Seeds preparation
A. thaliana seeds were sterilized in a plastic tube for 3 min with 1 mL of 70% ethanol. This was followed by a 5 min treatment with 1 mL sodium hypochlorite solution. The plastic tube was inverted many times in each phase. The seeds were washed five times in distilled water. Sterilized seeds were sown on square Petri dishes with ½ MS medium under the sterile bench. The square Petri dishes with sterilized seeds were stored in the fridge for stratification for 48 h at 4°C and transferred to the growth chamber for 96 h for seed germination.
Experimental preparation with AZD
To investigate the influence of AZD on A. thaliana root and hypocotyl growth, A. thaliana seedlings were transferred to round Petri dishes with phyto agar and AZD (5 μM). The 48 h stratified seedlings were placed side by side with straightened roots in a horizontal position. Control plates were treated in the same way but, only containing phyto agar. After placing the seedlings, all dishes were sealed with parafilm and then transferred to different light conditions (Plate 1). Each treatment was repeated in triplicate. The six light conditions used are as follows: (1) Total light (TL): Round Petri dishes with A. thaliana seedlings were placed under the light of the growth chamber with the intensity of 100 μmol s-1 m-2. (2) Total dark (TD): Plants were kept in total darkness (covered with aluminum foil) for 96 h. Moreover, shaded seedling roots create two forms of light; gradient light and light blocker. Shoot dark and shoot dark with a light blocker were two types of light conditions created by shading seedling shoots. (3) Gradient light (GL): Plants in the Petri dish were introduced in a black box, where the roots were inside the box and the hypocotyl outside, resulting in a slight gradient with a value of 39.74 μmol s-1 m-2. (4) Light blocker (LB): A light blocker strip was placed inside the medium, perpendicular to the Petri dish, preventing light from reaching below the blocker. Subsequently, they were introduced into a black box, resulting in light intensity of 7.27 μmol s-1 m-2. (5) Shoot dark (SD): The hypocotyls of A. thaliana were covered resulting in light intensity of 7.91 μmol s-1 m-2. (6) Shoot dark with light blocker (SDB): The light blocker strip (same as LB) was placed on medium in a round Petri dish and then the hypocotyls of the seedlings were covered, resulting in light intensity of 2.03 μmol s-1 m-2.
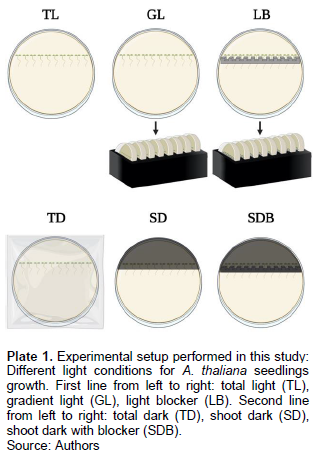
Root and hypocotyl lengths measurements
After 24, 48, 72 and 96 h the round Petri dishes were scanned. Based on the digital images, the root length and hypocotyl length were measured via Fiji software.
Skototropism experimental preparation
To investigate the influence of distance to darkness on A. thaliana roots for the skototropism experiment, A. thaliana seedlings were transferred to round Petri dishes with phyto agar. The seedlings were placed in a vertical position, one below the other with straightened roots. After placing the seedlings, all dishes were sealed with parafilm and then placed in construction that held one-half of the Petri dish in darkness. Dishes were aligned in the construction and set with different distance patterns (0, 10 and 20 mm) from the A. thaliana seedlings to the darkness and then placed under artificial light (100 μmol s-1 m-2) for 96 h in the growth chamber.
Measurements and evaluation
After 96 h, the round Petri dishes were scanned. Based on the digital images, the root bending angle was measured. Values for root bending were sorted into 3 groups: (1) Positive values, showing a bending towards darkness; (2) Negative values, indicating a bending away from darkness; (3) A group zero, exhibiting no visible behavior towards or away from light. Collected data for the experiment were evaluated with Fiji ImageJ software. The length standard for these measurements was set with the help of a ruler which was calibrated with the respective samples. Statistical analysis was performed using Graphpad Prism (version 9.1.1)
RESULTS
Treatment with AZD (5 μM) under shaded light conditions
We found that the growth of the AZD treatment groups was substantially slower than the control groups, but no significant difference was seen in the total dark condition (TD). For control groups, the root length in the total dark condition was always the shortest compared with that in other lighting conditions, and in the gradient light (GL) and light blocker (LB) settings. Importantly, root lengths were longer in the gradient light (GL) and light blocker (LB) conditions than in the total light condition (TL). This shows that root growth speeds up if it grows within light-dark gradients and also there is a clear inhibitory role of AZD in root growth. Figure 1 shows a clear difference after four days of growth.
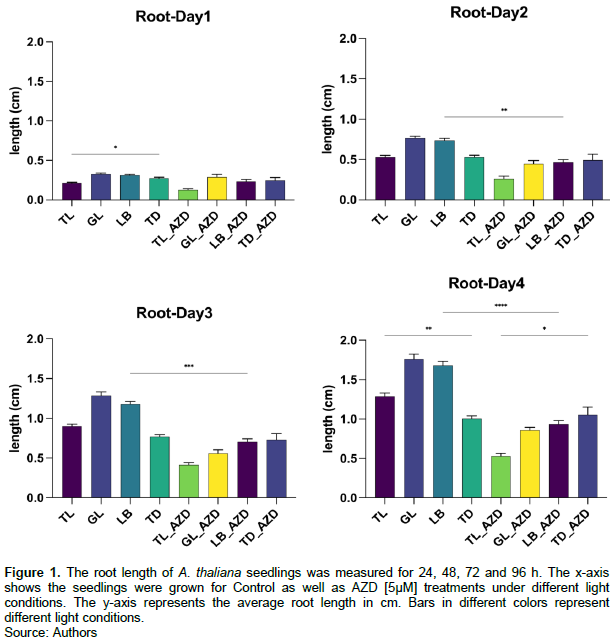
Root growth under different light conditions
When comparing the hypocotyl length in different illumination conditions under the same treatment, it was noted that the length of hypocotyls under total dark conditions (TD) was much larger than that under other illumination conditions and that the length of hypocotyls under complete illumination was always the shortest. Furthermore, there was no significant difference in hypocotyl development between the control and AZD treatment groups (Figure 2). This result shows that AZD has no significant influence on hypocotyl growth under our studied condition.
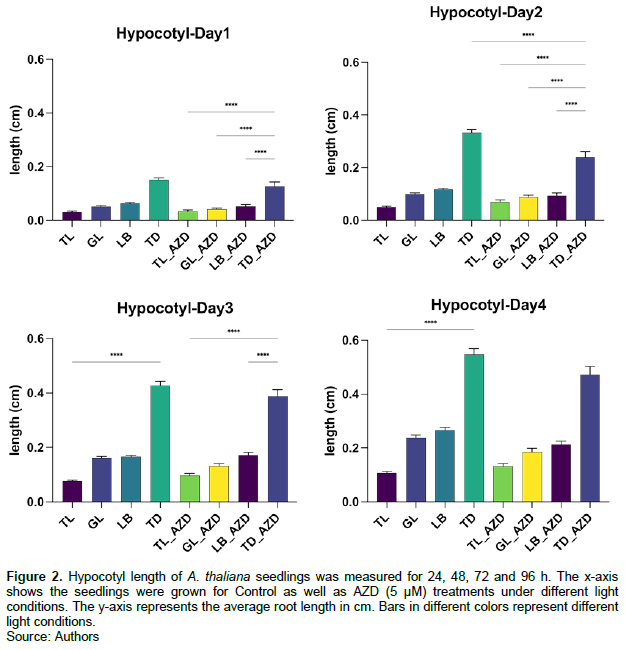
Hypocotyl growth under different light conditions
Shoot-shaded light conditions
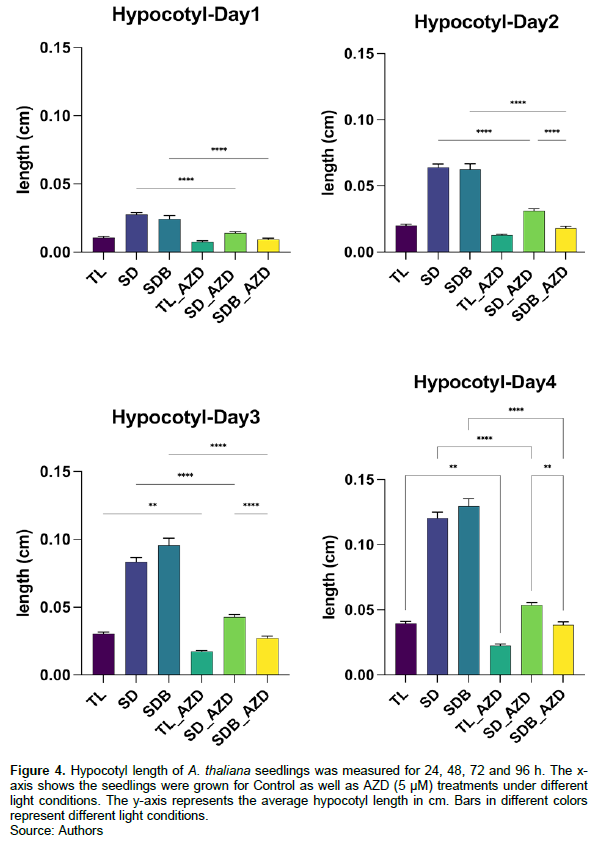
Seedling root and hypocotyl growth of the control group and AZD treatment group was compared under shoot-shaded light conditions (Figures 3 and 4). The root growth of the AZD treatment group was significantly slower than that of the control group. Moreover, root development of shoot dark (SD) condition was faster than shoot dark with light blocker (SDB) condition. Importantly, after 96 h of development, the root growth length in the AZD treatment group was less than 0.2 cm under different light conditions, far less than the control group (Figure 3).
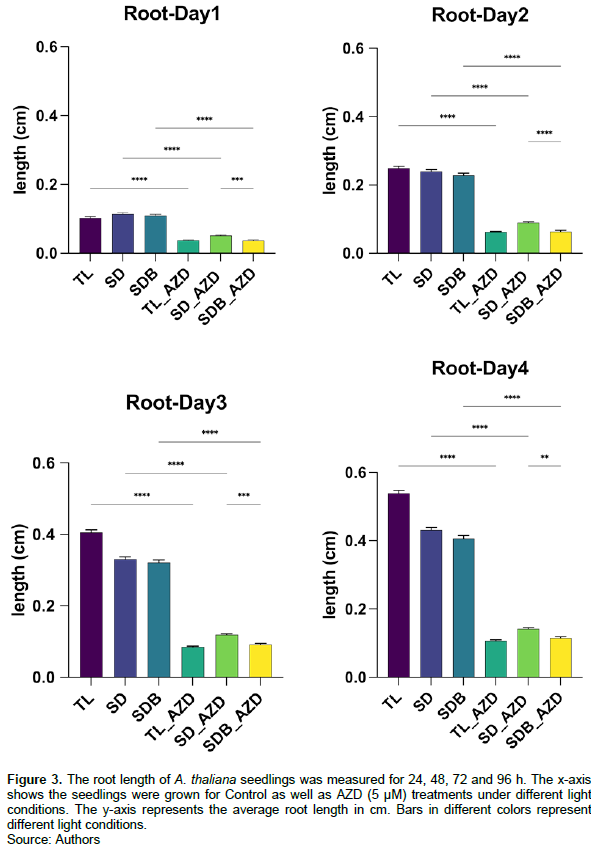
Root growth under different light conditions
In total light conditions (TL), hypocotyl development was slower than in the other two light conditions (SD, SDB). For the control treatment, the hypocotyl length increased more as the hypocotyl light intensity declined, as determined by comparing hypocotyl development under shoot dark (SD) and shoot dark with light blocker (SDB) conditions. For AZD treatments, it is worth mentioning that hypocotyl growth in the SD condition is faster than that in the SDB condition (Figure 4).
DISCUSSION
Arabidopsis was selected as a model plant about decades ago because of the unique traits that made it ideal for laboratory research. Arabidopsis has been cultivated on Petri dishes since then, and the vast majority of root biology research has been done with the root system exposed to light. Light appears to have a direct influence on root development and responses, according to recent research (Yokawa et al., 2014; Meng, 2015). Our results have revealed a role for light in both root growth and hypocotyl growth. Six different light conditions (total light, gradient light, light blocker, total dark, shoot dark, shoot dark with blocker) were set up since the influence of light on seedling growth and differentiation can be divided into direct and indirect aspects. The light conditions (shoot dark and shoot dark with blocker) are the indirect ways to explore the influence of light on root growth via changing the light intensity on the shoot. The investigations have found that the total root length under shoot dark (light intensity 7.91 µmol s-1 m-2) and shoot dark with blocker (light intensity 2.03 µmol s-1 m-2) circumstances was substantially less than under total light conditions. Within 24 and 48 h, there was no significant difference in root length across the three lighting conditions (SD, SDB and TL), but from 72 to 96 h, the total light condition had a considerably longer root length than the other two illumination conditions. This situation can be explained by the fact that photosynthesis mainly occurs in plant shoots, and plants are unable to produce enough organic matter (sucrose) to fulfill their growth requirements after a period of limited light. Yokawa et al. (2011) reported on the root-shoot ratio of Arabidopsis seedlings growing in the soil (whose roots are almost completely dark) is 1:1. The exposure of roots to light causes stress in the entire plant, and roots normally respond by increasing their growth. This indicates that illumination of the roots disturbs the balance of the root-shoot ratio, which is approximately 1:1 in a normal physiological situation. The analysis of our experimental data also supported this conclusion. The gradient light condition had a higher light intensity than the simulated natural condition (light blocker), and the gradient light condition had a longer root length than the light blocker condition. Meanwhile, in the gradient light condition, the hypocotyl length was less than in the light blocker condition. Most Arabidopsis studies are conducted out in transparent Petri dishes, ignoring the extra effects that the additional light may have on the roots. Results reported the root length under total light conditions was significantly shorter than that under gradient light and simulated natural conditions (light blocker), confirming the shortcomings of the traditional plant-growing (TPG) technique. Silva-Navas et al. (2015) demonstrated root illumination shortens root length and increases the early development of lateral roots, promoting root system expansion. Hypocotyl is a highly plastic organ whose length is controlled by a network of interacting elements including light and plant hormones (Vandenbussche et al., 2005). In continuous darkness, the process of hypocotyl elongation differs significantly from that in uniform light. TOR is critical for plant translational control (Méndez-Gómez et al., 2022). Translation re-initiation at upstream ORFs (uORFs) in genes that play crucial roles in stem cell control and organogenesis in plants is significantly reliant on TOR (Schepetilnikov et al., 2017). Many important proteins are encoded by uORF-mRNAs, including transcription factors, protein kinases, cytokines, and growth factors. The results from the study indicated that seedlings of A. thaliana treated with AZD (an ATP-competitive inhibitor of TOR) efficiently inhibited root and hypocotyl growth when compared to plants grown under control conditions. Our findings are consistent with previous research, which indicated that at the whole plant level, AZD treatment of A. thaliana delayed cotyledon and leaf development while also shortening root length (Montané and Menand, 2013). The situation is assumed to occur because AZD limits meristem activity in plants and may diminish the size of differentiated cells. Meanwhile, AZD may induce AtTOR haplo-insufficiency which results in reduced plant growth and stress resistance (Montané and Menand, 2013).
The control of root-to-shoot is a complex physiological process in plant. ROS-regulating factor was shown to play a key role in Arabidopsis (Jin et al., 2022). TOR signaling activity, which promotes growth and cell division, may be suppressed in QC. The presence of TOR in both the apical and basal meristems of the root shows that TOR is involved in both root proliferation and cell expansion (Montané and Menand, 2013). Furthermore, Barrada et al. (2015) stated that the TOR kinase is emerging as a key regulator of plant environmental and hormonal responses. TOR increases BR signaling, most likely via a signaling relay mediated by BIN2 substrates (Wu et al., 2019). Interestingly, by comparing the experimental data, it was discovered that hypocotyl length in the control group under shoot dark with blocker condition was larger than that under shoot dark condition; however, it was the opposite after AZD therapy. The outcome can be explained by the following reason: The use of AZD suppresses the activity of TOR, which indirectly affects the BR signaling, leading to a significant influence on the hypocotyl being almost completely dark condition (shoot dark with blocker condition).
CONCLUSION
A number of conclusions were drawn from this study: (1) That root growth speeds up if it grows within light-dark gradients and AZD shows a clear inhibitory role in root growth. (2) The length of hypocotyls under total dark conditions was much larger than that under other illumination conditions and that the length of hypocotyls under complete illumination was always the shortest. AZD has no significant influence on hypocotyl growth under the studied condition. (3) Root development of shoot dark (SD) condition was faster than shoot dark with light blocker (SDB) condition. Also, after 96 h of development, the root growth length in the AZD treatment group was less than 0.2 cm under different light conditions, far less than the control group (4). For AZD treatments, the hypocotyl growth in the SD condition is faster than that in the SDB condition. This study shows that AZD, a TOR inhibitor, drastically reduced root and hypocotyl length. The findings are consistent with previous research, which indicated that at the whole plant level, AZD treatments of A. thaliana delayed cotyledon and leaf development while also shortening root length (Montané and Menand, 2013).
CONFLICT OF INTERESTS
The authors have not declared any conflict of interest.
RECOMMENDATION
Further experiments are needed to pool-down other kinases that may be involved in this pathway.
ACKNOWLEDGMENTS
The authors Xingyu Yan and Felipe Yamashita acknowledge scholarships from Stiftung Zukunft (Germany).
REFERENCES
|
Baluška F, Mancuso S (2013). Root apex transition zone as oscillatory zone. Frontiers in Plant Science 4:354. |
|
|
Baluška F, Mancuso S, Volkmann D, Barlow PW (2010). Root apex transition zone: A signalling-response nexus in the root. Trends in Plant Science 15(7):402-408. |
|
|
Brand U, Hobe M, Simon R (2001). Functional domains in plant shoot meristems. BioEssays 23(2):134-141. |
|
|
Briggs WR, Olney MA (2001). Photoreceptors in plant photomorphogenesis to date. Five phytochromes, two cryptochromes, one phototropin, and one superchrome". Plant Physiology 125:85-88. |
|
|
Crang R, Lyons-Sobaski S, Wise R (2018). A concept-based approach to the structure of seed plants. Plant Anatomy. |
|
|
Deprost D, Yao L, Sormani R, Moreau M, Leterreux G, Nicolaï M, Bedu M, Robaglia C, Meyer C (2007). The Arabidopsis TOR kinase links plant growth, yield, stress resistance and mRNA translation. EMBO Reports 8(9):864-870. |
|
|
Ibrahim N, František B (2022). The role of N-terminal module of PhyB in modulating root and hypocotyl growth length in Arabidopsis. African Journal of Biotechnology 21(6):287-291. |
|
|
Jin T, Wu H, Deng Z, Cai T, Li J, Liu Z, Waterhouse PM, White RG, Liang D (2022). Control of root-to-shoot long-distance flow by a key ROS-regulating factor in Arabidopsis. Plant, Cell and Environment 45(8):2476-2491. |
|
|
Ju?rgens G, Mayer U, Ramon A TR, Berleth T, Mise?ra S (1991). Genetic analysis of pattern formation in the Arabidopsis embryo. Development 113:27-38. |
|
|
Kadereit J.W, Körner C, Kost B, Sonnewald U (2014). Strasburger - Lehrbuch Der Pflanzenwissenschaften. Berlin, Heidelberg: Springer Berlin Heidelberg. |
|
|
McNellis TW, Deng XW (1995). Light control of seedling morphogenetic pattern". Plant Cell 7(11):1749-1761. |
|
|
Menand B, Desnos T, Nussaume L, Berger F, Bouchez D, Meyer C, Robaglia C (2002). Expression and disruption of the Arabidopsis TOR (target of rapamycin) gene. Proceedings of the National Academy of Sciences of the United States of America 99(9):6422-6427. |
|
|
Méndez-Gómez M, Castro-Mercado E, Peña-Uribe CA, Reyes-de la Cruz H, López-Bucio J, García-Pineda E (2020). Target of rapamycin signaling plays a role in Arabidopsis growth promotion by Azospirillum brasilense Sp245. Plant Science 293:110416. |
|
|
Meng LS (2015). Transcription coactivator Arabidopsis ANGUSTIFOLIA3 modulates anthocyanin accumulation and light-induced root elongation through transrepression of Constitutive Photomorphogenic1. Plant Cell and Environment 38(4):838-851. |
|
|
Montané MH, Menand B (2013). ATP-competitive mTOR kinase inhibitors delay plant growth by triggering early differentiation of meristematic cells but no developmental patterning change. Journal of Experimental Botany 64(14):4361-4374. |
|
|
Petricka JJ, Winter CM, Benfey PN (2012). Control of Arabidopsis root development". Annual Review of Plant Biology 63:563-590. |
|
|
Qu Y, Liu S, Bao W, Xue X, Ma Z, Yokawa K, Baluška F, Wan Y (2017). Expression of root genes in Arabidopsis seedlings grown by standard and improved growing methods. International Journal of Molecular Sciences 18:951. |
|
|
Ren M, Venglat P, Qiu S, Feng L, Cao Y, Wang E, Xiang D, Wang J, Alexander D, Chalivendra S, Logan D (2013). Target of rapamycin signaling regulates metabolism, growth, and life Span in Arabidopsis. Plant Cell 24(12):4850-4874. |
|
|
Schepetilnikov M, Makarian J, Srour O, Geldreich A, Yang Z, Chicher J, Hammann P, Ryabova LA (2017). GTPase ROP2 binds and promotes activation of target of rapamycin, TOR, in response to auxin. The EMBO Journal 36(7):886-903. |
|
|
Silva?Navas J, Moreno?Risueno MA, Manzano C, Pallero?Baena M, Navarro?Neila S, Téllez?Robledo B, Garcia?Mina JM, Baigorri R, Gallego FJ, del Pozo JC (2015). D-Root: a system for cultivating plants with the roots in darkness or under different light conditions. Plant Journal 84(1):244-255. |
|
|
Su J, Liu B, Liao J, Yang Z, Lin C, Oka Y (2017). Coordination of cryptochrome and phytochrome signals in the regulation of plant light responses. Agronomy 7(1):25. |
|
|
Vandenbussche F, Pierik R, Millenaar FF, Voesenek LA, Van Der Straeten D (2005) Reaching out of the Shade. Current Opinion in Plant Biology 8(5):462-468. |
|
|
Verbelen JP, Cnodder TD, Le J, Vissenberg K, Baluška F (2006). The root apex of Arabidopsis thaliana consists of four distinct zones of cellular activities: meristematic zone, transition zone, fast elongation zone, and growth terminating zone. Plant Signaling and Behavior 1(6):296-304. |
|
|
Wu Y, Shi L, Li L, Fu L, Liu Y, Xiong Y, Sheen J (2019). "Integration of nutrient, energy, light, and hormone signalling via TOR in plants. Journal of Experimental Botany 70(8):2227-2238. |
|
|
Xu W, Ding G, Yokawa K, Baluška F, Li QF, Liu Y, Shi W, Liang J, Zhang J (2013). An improved agar-plate method for studying root growth and response of Arabidopsis thaliana. Scientific Reports 3:1273. |
|
|
Xu W, Ding G, Yokawa K, Baluška F, Li QF, Liu Y, Shi W, Liang J, Zhang J (2020). Light signaling and UV-B-mediated plant growth regulation. Journal of Integrative Plant Biology 62(9):1270-1292. |
|
|
Yokawa K, Fasano R, Kagenishi T, Baluška F (2014). Light as stress factor to plant roots-case of root halotropism. Frontiers in Plant Science 5:718. |
|
|
Yokawa K, Kagenishi T, Kawano T, Mancuso S, Baluška F (2011). Illumination of Arabidopsis roots induces immediate burst of ROS production". Plant Signaling and Behavior 6(10):1460-1464. |
|
|
Zeng Y, Schotte S, Trinh HK, Verstraeten I, Li J, Van de Velde E, Vanneste S, Geelen D (2022). Genetic Dissection of Light-Regulated Adventitious Root Induction in Arabidopsis thaliana Hypocotyls. International Journal of Molecular Sciences 23(10):5301. |
|
Copyright © 2024 Author(s) retain the copyright of this article.
This article is published under the terms of the Creative Commons Attribution License 4.0