Full Length Research Paper
ABSTRACT
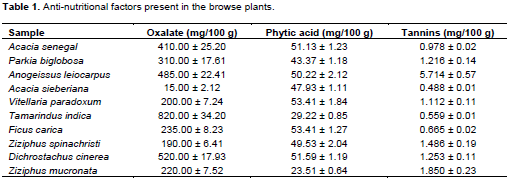
During the dry season, most plants and grasses dry up and ruminants are left with fewer plants to graze on. The plants available during such periods have relatively lower moisture contents and higher antinutritional factors because of the dry nature of the soil and prevalent atmospheric conditions. Increased levels of antinutritional factors above acceptable values, could have a detrimental effect on the metabolic and health status of animals grazing on such plants. This research work sought to determine the phytochemicals present and the levels of some antrinutritional factors in plants (Acacia senegal, Parkia biglobosa, Anogeissus leiocarpus, Acacia sieberiana, Vitellaria paradoxum, Tamarindus indica, Ficus carica, Ziziphus spinachristi Dichrostachus cinerea, Ziziphus mucronata) commonly grazed upon by ruminants in this region, to educate farmers and policy makers on the safety of these plants for ruminant nutrition. The plants were collected and identified, while phytochemical and anti-nutritional profiles were analyzed using standard spectrophotometric procedures. Quantitative antinutritional factors analysis showed that the level of oxalate varied from 15 to 180 mg/100 g, phytic acid from 23.51 to 53.41 mg/100 g and tannins from 0.486 to 1.850 mg/100 g, which are within internationally accepted permissible limits. Results of qualitative phytochemical analysis showed that flavonoids, tannin, cardiac glycosides and steroids were present in all the plants, while anthraquinones were absent. The results suggest that the browse plants in this location contain anti-nutrients at relatively low levels which make these plants safe for ruminant consumption.
Key words: Plants, antinutritional factors, phytochemicals, grazing ruminants
INTRODUCTION
METHODOLOGY
RESULTS AND DISCUSSION

CONCLUSION
CONFLICT OF INTERESTS
REFERENCES
|
Akande KE, Doma UD, Agu HO, Adamu HM (2010). Major antinutrients found in plant protein sources: Their Effect on Nutrition. Pak. J. Nutr. 9(8):827-832 |
|
|
Alabi DA, Akinsulire OR, Sanyaolu MA (2005). Qualitative determination of chemical and nutritional composition of Parkia biglobosa (jacq.) Benth. Afr. J. Biotechnol. 4:812-815. |
|
|
Babayemi OJ, Demeyer D, Fievez V (2004). Nutritional value of qualitative assessment of secondary compound in seeds of eight tropical browse, shrub and pulse legumes. Comm. Appl. Biol. Sci. 69(1):103-110. |
|
|
EL-Olemyl MM, AL-Muhtadi FJ, Afifi AA (1994). Experimental phytochemisry. A Laboratory manual College of Pharmacy, King Saud University. King Saud University Press. pp. 1-134. |
|
|
Franceschi VR, Nakata PA (2005). Calcium oxalate in plants: Formation and function. Annu. Rev. Plant Biol. 56:41-71. |
|
|
Francis G, Karem Z, Makkar HP, Becker K (2002). The biological action of saponins in animal systems: A review. Brit. J. Nutr. 88(6):587-605. |
|
|
Harbone JB (1973). Phytochemical Methods: A Guide to Modern Techniques of Plant Analysis. 3rd Charpman and Hall, London pp. 7- 13, 60, 89, 131-135, 186-188, 203, 279. |
|
|
Hoste H, Jackson F, Athanasiadou S, Thamsborg SM, Hoskin SO (2006). The effects of tannin-rich plants on parasitic nematodes in ruminants. Trends Parasitol. 22:253-261. |
|
|
Iwashina T (2000). The structure and distribution of the flavonoids in plants. J. Plant Res. 113:287-299. |
|
|
Jamur MC, Oliver C (2010). Permeabilization of cell membranes. Meth. Mol. Biol. 588:63-66. |
|
|
Ji XM, Peng XX (2005). Oxalate accumulation as regulated by nitrogen forms and its relation to photosynthesis in rice. J. Integr. Biol. 47:831-838. |
|
|
Kittakoop P, Mahidol C, Ruchirawat S (2014). Alkaloids as important scaffolds in therapeutic drugs for the treatments of cancer, tuberculosis, and smoking cessation. Curr. Top. Med. Chem. 14(2):239-252. |
|
|
McMahon LR, McAllister TA, Berg BP, Majak W, Acharya SN, Popp JD, Coulman BE, Wang Y, Cheng JK (2000). A review of the effects of forage condensed tannins on ruminal fermentation and bloat in grazing cattle. Can. J. Plant Sci. 80(3):469-485. |
|
|
Mouradov A, Spangenberg G (2014). Flavonoids: A metabolic network mediating plants adaptation to their real estate. Front Plant Sci. 5:620. |
|
|
Njidda AA (2010). Chemical Composition, Fibre Fraction and Anti-Nutritional Substances of Semi-arid Browse Forages of North-Eastern Nigeria. Nig. J. Basic Appl. Sci. 18(2):181-188. |
|
|
Okoli IC, Anunobi MO, Obua BE, Enemuo V (2003). Studies on selected browses of Southeastern Nigeria with particular reference to their proximate and some endogenous anti-nutritional constituents. Livest. Res. Rural Dev. 15(9). |
|
|
Podolak I, Galanty A, Sobolewska D (2010). Saponins as cytotoxic agents: A review. Phytochem. Rev. 9(3):425-474. |
|
|
Polshettiwar SA, Ganjiwale RO, Wadher SJ, Yeole PG (2007). Spectrophotometric estimation of total tannins in some ayurvedic eye drops. Indian J. Pharm. Sci. 69:574-576. |
|
|
Poulsen HD, Johansen KS, Hatzack F, Boisen S, Rasmussen SK (2001). Nutritional value of low-phytate barley evaluated in rats. Acta Agric. Scand. A Anim. Sci. 51(1):53-58. |
|
|
Raboy V (2007). The ABCs of low-phytate crops. Nature Biotechnol. 25(8):874-875. |
|
|
Roop-ngam P, Chaiyarit S, Pongsakul N, Thongboonkerd V (2012). Isolation and characterizations of oxalate-binding proteins in the kidney. Biochem. Biophys. Res. Commun. 3:424(3):629-634. |
|
|
Santhosh RS, Suriyanarayanan B (2014). Plants: A source for new anti-mycobacterial drugs. Planta Med. 80:9-21. |
|
|
Sarwar GG, Wu XC, Cockell KA (2012). Impact of antinutritional factors in food proteins on the digestibility of protein and the bioavailability of amino acids and on protein quality. Brit. J. Nutr. 108(2):315-332. |
|
|
Sendelbach LE (1989). A review of the toxicity and carcinogenicity of anthraquinone derivatives. Toxicology 57(3):227-240. |
|
|
Sidhu PK, Lamba JS, Kumbhar G, Sekhon GS, Verma S, Gupta MP (2014). Role of epidemiological factors in accumulation of oxalates in forage crops. Am. Int. J. Res. Formal. Appl. Nat. Sci. 7(1):48-52. |
|
|
Torres J, Dominguez S, Cerda MF, Obal G, Mederos A, Irvine RF, Diaz A, Kremer C (2005). Solution behavior of myo-inositol hexakisphosphate in the presence of multivalent cations. Prediction of a neutral pentainagnesium species under cytosolic/nuclear conditions. J. Inorg. Biochem. 99(3):828-840. |
|
|
Trease GE, Evans WC (1978). A textbook of Pharmacognosy 11th edition. Bailliere Tindall London. P 530. |
|
|
Tutelian VA, Lashneva NV (2013). Biologically active substances of plant origin. Flavonols and flavones: Prevalence, dietary sources and consumption. Vopr Pitan 82(1):4-22. |
|
|
Vetter J (2000). Plant cyanogenic glycosides. Toxiconomy 38:11-36. |
|
|
Wall M, Krider MM, Krewson CF, Eddy CR, Willaman JJ, Corell DS, Gentry HS (1954). Steroidal sapogeninins Vll. Survey of plants for steroidal sapogenins and other constituents. J. Am. Pharm. Ass. 63:1-7. |
|
|
Wischer G, Boguhn J, Steingab H, Schrollenberger M, Rodehutscord M (2013). Effects of different tannin rich extracts and rapseed tannin monomers on methane formation and microbial protein synthesis in vitro. Animal 7(11):1796-1805. |
|
|
Yankey RK, Debrah SK, Forson A, Ayivor JE, Buah-Kwofie A, Doe E, Blewu B, Bentil NO (2012). Oxalate, Cyanogenic Glycocide, iron and zinc contents of selected commercial brand baby food on the Ghanaian market. Elixir Food Sci. 46:8479-8482. |
|
Copyright © 2024 Author(s) retain the copyright of this article.
This article is published under the terms of the Creative Commons Attribution License 4.0