This study was conducted in order to investigate the dietary inclusion effects of a commercially available probiotic mixture and an antibiotic on the growth performance, cecal microbiota and small intestinal morphology in broiler chickens. A total of 100 broiler chickens (Cobb 500) were subjected to 35-days of study period. The broiler chickens were randomly divided into four groups named T1 (control group), T2 (antibiotic fed group, amoxicillin 200 gm/ton of feed), T3 (probiotic fed group, Pro.B 250 g/ton of feed) and T4 (probiotic fed group, Pro.B 500 g/ton of feed), respectively. Body weight gain and carcass yield were measured by electrical balance at seven-days-interval. The role of the used probiotic and antibiotic on intestinal microflora were evaluated by the total E. coli count (TEC), total Salmonella count (TSC), total Lactobacillus count (TLC) and total Bacillus count (TBC) whereas the intestinal morphology was determined by histological examination. The results of this study revealed that the obtained live weight gains were significantly (p<0.01) higher in both probiotic fed groups during the periods of 3rd, 4th and 5th week of age as compared to the control and antibiotic fed groups. Data analysis from the cecum samples showed that the TEC was significantly higher (p>0.01) in the control group as compared to both probiotic fed groups. On the other hand, the TLC and TBC were significantly higher (p>0.01) in both probiotic fed groups as compared to the control and antibiotic fed groups. Both probiotic fed groups showed strong evidence in increasing the length of jejunal villi compared to the control and antibiotic fed groups. Among the probiotic fed groups, T4 group showed a better response in every evaluated parameter included in this study. The results of this study thus revealed that the probiotic supplementation used on the T4 group promoted the most significant and beneficial influence on the growth performance, carcass yield, bacterial antagonism and intestinal morphology.
Nowadays, the efficiency of poultry to convert the feed into meat plays a key role in the poultry industry worldwide (Arthur et al., 2003). In recent years, concerns about antimicrobial resistance have grown, but the main concerns have been focused specifically on antimicrobial resistance within the food supply (Cui et al., 2005). The use of antibiotics, including chlortetracycline as growth promoters to increase production performance and to decrease mortality, was recommended to be banned by the European Union (Perreten, 2003). This increases the microbial resistance to antibiotics and residues in chicken meat products which can be harmful to consumers (Nonga et al., 2009). Due to their several negative effects, antibiotics have gradually been replaced by other products such as probiotics in order to control intestinal pathogenic bacteria and become an alternative as growth promoters (Fuller, 1992). Probiotics are defined as live microbial supplements which beneficially affect the intestinal microbial balance of the animal host (Fuller, 1989). One of their main roles is to control the cholesterol and triacylglycerol serum levels (Lin et al., 1989; Taranto et al., 1998). Indeed, there are some evidences suggesting the reduction of the cholesterol and fatty acid composition of the broiler chicken serum by the Lactobacillus feed supplementation (Kalavathy et al., 2003). Due to indiscriminate use of antibiotics in the poultry industry, some bacteria have developed resistance to commonly used antibiotics that is being considered as a vital threat to the poultry production of Bangladesh (Akond et al., 2008; Hasan et al., 2011). To combat this problem probiotic can be used as a growth promoter and alternative to antibiotic in poultry industry of Bangladesh (Kabir et al., 2005; Kabir, 2009). The objectives of this study were to investigate the effects of a commercially available probiotic mixture diet supplementation on the growth performance, cecal microbiota, carcass yield and intestinal morphology of broiler chickens.
Experimental birds
A commercially available probiotic mixture named Pro.B® (marketed by PVF Agro Limited, Bangladesh and manufactured by K.M.P Biotech Co. Limited, Thailand) was used in this study. A total of one hundred day old broiler chicks (Cobb 500 strain) were obtained from a local sale centre (CP-Bangladesh Ltd, Mymensingh, Bangladesh). Broiler chickens were divided into four groups named group T1, T2, T3 and T4 at the beginning of the experimental study. The four groups were composed of 25 broiler chickens each. The group T1 was fed without the addition of antibiotics or probiotics and was identified as the control group. The group T2 was identified as the antibiotic (Amoxicillin 200 g/ton feed) fed group. Finally, both groups T3 and T4 were identified as the probiotic (Pro.B® 250 gm/ton of feed and Pro.B® 500 gm/ton of feed, respectively) fed groups.
Feeding and drinking
The evaluated broiler chickens had ad libitum feed and water and were exposed to 24 h of lighting during the study. The evaluated birds received a starter diet (Nourish Broiler Feed, Bangladesh) from 1 to 14 days of age and a grower diet (Nourish Broiler Feed, Bangladesh) from 15 to 35 days of age. Both diets were given in a mash form using round feeders from the first week onwards whereas drinking water was supplied two times daily. One feeder and one round drinker with eight litre capacity were provided in each pen. Routinely, feeders were cleaned once a day while drinkers were washed two times daily.
Management practices
The day-old broiler chickens were immediately weighed after its arrival and randomly distributed in each pen. The broiler chickens were provided with vitamin C (1 g/5 L water) to overcome transportation stress. The room of the experimental house was partitioned into four pens using wire-net where a group of 25 broiler chickens were randomly allocated to each pen. Therefore, floor space was about 1 sq. ft for each bird to ensure comfortness of the birds. One 100-watt hanging electric bulb at the bird level for each pen was used to maintain brooding temperature. The brooding temperature and humidity was measured four times per day by an automatic digital thermo-hygrometer. A strict biosecurity program was maintained inside and outside of the experimental sheds as an effective part of the disease prevention program. A foot bath was maintained at the gate of the shed where TH4+ solution (Sogeval, France) and Povisep (Jayson Pharmaceuticals Ltd., Bangladesh) was alternatively used as disinfectants. Regarding the health status of the evaluated animals, immunizations were performed by the use of inactivated vaccines against BCRDV and Gumboro disease on 6th and 11th day of age and booster doses on 24th and 21st day of age respectively.
Pro.BÒ
Pro.B® marketed by PVF Agro Limited, Bangladesh and manufactured by K.M.P Biotech Co. Limited, Thailand was used in this study. A minimum of 1.0 × 1010 colony forming units (CFU)/L were present in Pro.BÒ. Pro.BÒ product is a powder preparation containing the live viable strains of naturally occurring microorganisms such as Bacillus subtilis, Bacillus licheniformi and Bacillus pumilus.
Antibiotic
Amoxicillin (Renamox® 30% vet, Renata Ltd., Bangladesh) which is a broad spectrum antibiotic widely used against several bacterial diseases of poultry was included in this study. Each milligram powder contains amoxicillin 300 mg as amoxicillin trihydrate BP.
Body weight
The broiler chickens were weighed by group at the beginning of the study and every seven days intervals until the end of the study. The weights were taken at the morning. The average live weight and the live weight gains of the broilers chickens on different dietary treatments were calculated on a weekly basis and at the end of the study.
Calculation of average daily growth, feed efficiency, feed conversion ratio (FCR) and mortality rate
Average daily growths (ADG) were calculated as the unit of body weight gain per day per broiler. The amount of feed consumed by the experimental broiler chickens of different treatment groups were calculated in a every week basis by deducting the amount retained from the amount supplied in that week.
The feed conversion ratio (FCR) was calculated as the unit of feed consumed per unit of body weight gain. The mortality rate of the broiler chickens were recorded per group and their survivability was also calculated.
Preparation of samples for bacteriological studies
During the course of the experimental study five birds from each group were randomly selected at 21 and 35 days of age and portions of cecum with their contents were obtained aseptically with a sterile scalpel and forceps for determining bacteriological status. These portions were homogenized uniformly using a mortar and pestle. From the homogenized mass a 1 g portion was transferred to a sterile tube containing 9 ml of 0.1% peptone water. Thus, 1: 10 dilution of the sample was obtained. Then, serial dilutions of each of the samples in 0.1% peptone water were made following the recommendations of the International Organization for Standardization (ISO, 1995).
Enumeration of total E. coli count (TEC)
In order to determine the TEC, each sample was taken in a sterile test tube and diluted with 10 ml of 0.1% peptone water. Another tube containing 9 ml of 0.1% peptone water was taken and 1 ml diluted sample from the first test tube was added and mixed well and repeated it from the first to last tube. Finally 1 ml was discarded from last tube. One hundred μl of the diluted sample from 10 fold dilution was then inoculated into two MacConkey agar (Hi-Media, India) plates and spread with a sterile glass spreader. Then, the plates were then kept in an incubator at 37°C for 24 to 48 h. Following incubation, agar plates exhibiting colonies were counted. The average number of colonies was multiplied by the dilution factor to obtain the total E. coli count. The total E. coli count was calculated according to the ISO (1995). The results of the total bacterial count were expressed as the number of colony forming units per gram (CFU/gm) of samples.
Enumeration of total Salmonella count (TSC)
The procedures of sampling, dilution and streaking for the determination of TSC were similar to those followed in TEC. Only in case of TSC, xylose lysine deoxycholate (XLD) agar (Hi-Media, India) was used. The calculation for TSC was similar to that of total viable count.
Enumeration of total Lactobacillus count (TLC)
The procedures of sampling, dilution and streaking for the determination of TLC were also similar to those followed in TEC. Only in case of TLC, Man, Rogosa and Sharpe (MRS) agar (Hi-Media, India) was used. The calculation for TLC was similar to that of total viable count.
Enumeration of total Bacillus count (TBC, spore forming Bacillus)
To determine the total Bacillus count (TBC, spore former Bacillus), each sample was taken in a sterile test tube and diluted with 10 ml of 0.1% peptone water to make a 10 fold dilution. The diluted sample was then placed in water bath for 30 m at 80°C temperature. Then the sample was cooled and inoculated on nutrient agar (Hi-Media, India) plate and then followed the procedure of total viable count.
Preparation of samples for histological studies
During the course of the histological study five birds from each group were randomly selected at 21 and 35 days of broiler age. The portions of jejunum were collected and fixed in the Bouin’s fluid for fixation of tissues for histological studies. The tissues were then dehydrated in the graded alcohol, cleared in xylene, embedded in paraffin and finally the sections were cut at 6 µm thickness by a rotary microtome (AO Spencer No. 820 Precision Rotary Microtome, USA). The sections so prepared were stained with standard the hematoxylin and eosin method (Gridley, 1960).
Statistical analysis
The data of the experimental study on live weight and carcass yield were analized by applying one-way ANOVA followed by Boneferonni and Duncan tests using IBM SPSS statistics software (version 20.1).
The results of the live weight gains were found significantly (p>0.01) higher (Table 1) in experimental birds of the group T3, and group T4 as compared to the control and antibiotic fed groups during 3rd, 4th and 5th weeks of age. The average carcass weight was found to have yielded more in the group T4 birds than the group T3 birds when they were reached 3rd, 4th and 5th week of age.
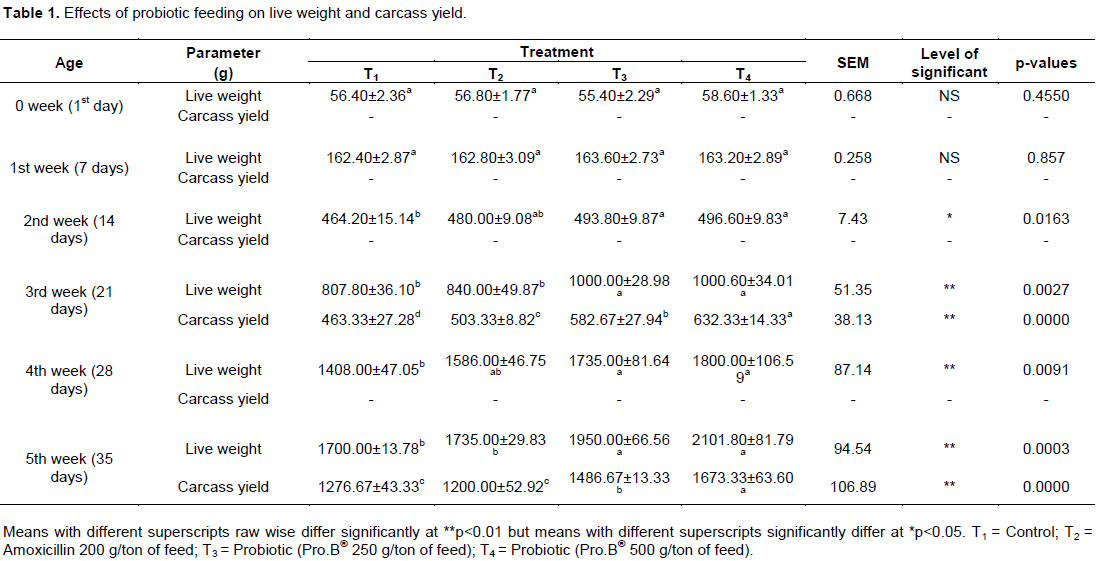
Table 2 shows that the average daily growth and feed conversion ratio were found significantly (p>0.01) higher in group T4 as compared to others. Data analysis from cecum samples (Tables 3 and 4) showed that the TEC was significantly higher (p>0.01) in the control group as compared to the probiotic fed groups. The TLC and TBC were significantly higher (p>0.01) in probiotic fed groups as compared to the control group. The histological study of the jejunal tissue was performed at 21 and 35 days of age. The jejunal glands (Figure 1) were much larger and prominent in Pro.B® fed broilers than the antibiotic and conventional fed broilers. The thickness of the jejunal wall was much wider in probiotic fed groups (T3 and T4) than control and antibiotic fed groups as presented in Figure 2. Among the probiotic fed groups (T3 and T4), T4 group gave better response in every cases in this study.
The evidence of data analysis had shown that the average live weight gains were found always on the increase in probiotic fed groups as compared to control and antibiotic fed birds on the 2nd, 3rd, 4th and 5th week of age. According to Table 2 there were significant differences (p<0.01) between the probiotic fed groups and the antibiotic and control groups in body weight gains (g) and FCR. This result is in agreement with the previously reported findings of several works. Higher body weight gains for probiotic fed broilers were also reported by Kamruzzaman et al.(2005),Kabiretal. (2005), Islam et al. (2014),Celik et al. (2007), Raceviciute et al. (2007), Kabir (2009), Toghyani et al. (2011) and Kral et al. (2012). Data analysis from cecum samples shown (Tables 3 and 4) that the TLC were significantly higher (p>0.01) in the probiotic fed groups as compared to the control and antibiotic fed groups. Data analysis for the TSC from cecum samples has shown no significant differences among four groups (Tables 3 and 4). The TBC were significantly higher (p>0.01) in the probiotic groups (Table 3 and 4) as compared to the control group. On the other hand, the TEC was significantly higher (p>0.01) in the control group as compared to the probiotic fed groups. The present findings support the previously described results by Edens (2003) and Kabir et al. (2005). The thickness of the jejunal wall was much wider in group T4 (Pro.B® 500gm/ton of feed) broilers than the conventional and antibiotic fed group broiler chicks at 21 days of age as presented in Figure 1. The villus growth was higher in the group T4 at 35 days of age as compared to the other groups. The present result supports the findings previously described by Samanya et al. (2002), Kabir et al. (2005), Rehman et al. (2007) and Islam et al. (2014).
From this study it is clearly revealed that probiotic supplementation (Pro.BÒ 500 g/ton of feed) promoted significant influence on growth performance, carcass yield, bacterial antagonism and morphological changes of intestinal wall.