ABSTRACT
The cow, the milking and milk handling procedures at the farm level expose the milk to potential risk of contamination with spoilage microorganisms. Milk contamination if not prevented will lead to milk losses along the dairy value chain. The objective of this study was therefore to identify the risk factors associated with contamination of milk with spoilage microorganisms at the farms in rural and peri urban in Nakuru County Kenya. A survey was conducted using a pre-tested semi structured questionnaires (250) and an observation checklist to identify the risk factors. A total of 560 samples obtained from the following identified contamination sources; the udder, hands, milking and bulking containers and water sources were analyzed for total viable counts (TVC), Coliform counts (CC), thermophillic bacteria counts (ThBC) and psychrophilic bacteria counts (PBC). The results from the survey showed that only 11% of rural farmers practiced hand and udder drying compared to 50% in peri-urban. Water treatment by either chlorination or boiling was done by 11% in rural and 30% in peri-urban respectively. Regression of risk factors versus farm gate milk from viable colony counts, showed that udder swabs were the highest source of contamination of milk (r =2.73). In the rural, hands of milking personnel recorded the highest for TVC (log10 3.7 CFU/ml). It is evident from the results that effective udder cleaning and observation of high personal hygiene by the hand milkers may reduce the risk of microbial contamination in both systems of milk production.
Key words: Risks, handling practices, contamination, rural, peri urban.
Livestock contributes about 50% to the agricultural Gross Domestic Product (GDP) in Kenya with dairy production contributing up to 33% of this (Lore et al., 2005). Milk production in Kenya is mainly from cattle, camel and goats. Dairy cattle however account for over 70% of national milk. The main dairy breeds include Friesian, Ayrshire, Jersey, Guernsey their crosses and indigenous cows. Smallholder dairy farmers dominate the dairy industry by accounting for over 75% at the production level (FAO, 2011).
Contamination of milk however begins at the farm during and after harvest (Kornacki and Johnson, 2001).Research has showed that most post- harvest milk losses are experienced in small scale dairy farms and at the farm level (Muriuki, 2003; Lore et al., 2005; FAO, 2011). Farm losses have been recorded to be highest in several countries. Kenya recorded the highest volume in losses at the farm in east Africa, standing at 54.2 million liters compared to 8.4 million liters, 28.6 million liters, 46.4 million liters in Uganda, Ethiopia and Tanzania respectively (Lore et al., 2005)
On farm dairy losses occur in three main forms; spillage spoilage and forced consumption. Spillage is caused by poor roads during transportation; spoilage is caused by spoilage microorganisms which produce lactic acid increasing the milk acidity above the accepted levels. When the milk fails the alcohol test it is rejected there after it is returned to the farm. At the farm the milk is forcefully consumed, thrown away, or sold at throw away prices leading to economic loss (Muriuki, 2003; FAO, 2011). Losses at the farm has been reported by World Bank to cost the farmers 2$ each month in developing countries (Bonfoh et al., 2003; Paola et al., 2013). Losses have also been attributed to by lack of adequate animal health control, inadequate training among farmers and farm employees on milk hygiene (Chye et al., 2004; Chizari et al., 2008; Paola et al., 2013).
Harvesting of milk which basically takes place at the farm faces many sources of contamination. The animal itself is a risk factor. If the cow is not healthy, then the milk is likely to be contaminated with microorganisms such as Staphylococcus, Streptococcus, enteric bacteria among others in cases of subclinical mastitis at the udder. Other commensal and pathogenic microorganisms have also been isolated from the udder. Traditional pre milking and post milking procedures used during harvesting are risk factors more so when milking is done in open fields in non-controlled environments. The milking environment in small scale farms are sometimes characterized by dust and faeces. The milking hands of personnel, milking containers and bulking containers are contact surfaces of the milk posing as risks for milk contamination. The water used at the farm during cleaning of the udder, hands and equipment has been considered a factor in milk contamination (Ingawa et al., 1992; Teka, 1997; Walstra et al., 1999; Kornacki and Johnson, 2001; Petrovick et al., 2006; Visser et al., 2007; Coorevitis et al., 2008; Kumar et al., 2012; Al-Hubeatyet al., 2013; Gleeson et al., 2013; Matofari et al., 2013). The major milk spoilage bacteria that have been isolated from raw milk include; coliforms, lactic acid bacteria (LABs), psychrotrophic bacteria (Pseudomonas spp.) and thermophillic bacteria (Bacillus spp.) (Griffiths and Phillips, 1990; Bareeda, 2012; Gleeson et al., 2013; Paola et al., 2013; Mesfine, 2015).
There is a deficiency in information on the microbiological quality of these risk factors pointing out the most responsible source of contamination of milk after harvest at the farm. The aim of this study was to assess the risk factors in small scale farms associated with contamination of milk with spoilage microorganisms while at the same time profile the microbiological quality of these risk factors. The outcome of the study is expected to build on previous studies and be a useful source in developing mitigation measures to curb losses due to spoilage at the farm.
Study site
The study was carried out in Nakuru county Kenya where dairy farming is thriving. Nakuru county is a Kenyan highland found in rift valley where dairy milk production is highest in the country (Muriuki, 2003). Two locations were selected to capture rural and peri urban farm characteristics. Olenguruone sub-county is a rural setting which lies about 35°40’ 60’’E and 0°34’ 60’’S while Bahati-Wanyororo Sub-County is a peri-urban setting next to Nakuru town which lies about 36° 40’60’’E and 0°40’ 60’’N. Small scale dairy farmers were targeted because thy account for over 80% of milk producers in the country (FAO, 2011).
Conduct of survey
A cross sectional survey was done using a pretested questionnaires and observation checklist. In the rural setting 150 questionnaires were administered and 100 in the peri-urban. The questionnaire targeted farm characteristics highly associated with milk contamination with spoilage microorganisms. Some of the characteristics included; method of grazing, water sources, method of water treatment, waste disposal, training of farm personnel, method of cleaning milking and bulking containers, types of material for the containers. The observation checklist was used at milking time to assess the hygiene practices followed. Pre milking practices such as; udder and hand washing, pre-dipping, hand and udder wiping, type of material used for drying hands and udder. Post milking practices sought were; post dipping, and udder drying. Stratified random sampling was done in selection of farms for the survey since not all farmers had dairy cows and out of which only those willing to participate in the practice were selected.
Sample collection for microbiological analysis
Contamination sources identified from the survey (hands, udders, water source, milking and bulking containers) were sampled for microbiological analysis. Samples were collected early in the morning during milking time. Sample collection begun after the udder was cleaned and ready for milking and the milking personnel had done the necessary pre milking preparation. Sterile cotton swabs wrapped in splint wood sticks were used in swabbing hands and udder. A surface area of 38.5 cm2 from both hands of the milking personnel was swabbed. The swab was then immediately transferred into a sterile Stuart Transport Medium (Oxoid) in a screw cap Bijou bottle. The handle stick was broken while the swab remained in the transport medium. The cap of the bottle was then put back and transferred to the cool box. The teat of the udder was swabbed from the attachment of the teat to the udder downwards while avoiding contact with hair on the udder (Kumar, 2012). The four teats per cow were swabbed and transferred to the same bijou bottle.
Milk (50 ml) from all the quarters of the udder was collected in a 50 ml sterile sampling bottle. Milking containers and bulking containers at the farm gate were rinsed with 100 ml sterile water and the volumes of the containers were recorded. Milk at the farm gate in the bulking container was also sampled in sterile sampling bottles. Approximately 400 ml of water source used at the farm was sampled in a 500 ml sterile sampling bottle. The swabs, milk and water samples were transported in a cool box with ice bags at 8 to 10°C to the laboratory in six hours. In the peri urban, 30 farms were visited for sample collection which provided a total of 210 samples while in the rural, 50 farms were visited and this provided 350 samples making a total of 560.
Microbiological analysis
Examination of samples for total viable counts (TVC), coliform counts (CC), Thermophillic bacterial counts (ThBC) and psychrophilic bacterial counts (PBC) were done by standard procedures of International Dairy Federation (IDF), East African Standards of milk examination (EAC, 2006) and ISO (International Standard of Organisation). TVC was incubated at 32°C for 48 h (EAS 67:2000 (4.2.1) EAC 2006) in Plate count agar (OXOID). Colifrom counts were incubated at 30°C for 48 h (ISO, 2006) in MacConkey agar (OXOID). Psychrophillic bacteria was incubated aerobically at 6.5°C for 10 days (IDF) using Plate count Agar while thermophillic bacteria were incubated at 55°C for 48 h (Abdul-Hadi et al., 2014).
Data analysis
Data collected by survey questionnaires was used to determine risk factors. Data was analyzed using SPSS (Statistical Package for social scientists) version 20. A cross tabulation was done between the risk factors and location. Values obtained from the 100 ml rinse from milking and bulking containers was divided by the volume of the corresponding container to determine contamination (colony forming units per ml, CFU ml-1). The container volumes were not the same; however the bacterial counts were not corrected for this variation (Bonfoh et al., 2003). Microbial count data was first transformed to logarithmic values (log 10) before subjection to statistical analysis.The general linear model of SAS version 9.1.3 (SAS proc glm) was used to analyze milk microbial quality and the microbial quality of contamination sources. Mean comparison was done by the Fisher’s least significant difference (LSD) when analysis of variance showed significant difference in means. Statistical difference was determined at 95% confidence level. The microbiological quality of milk was regressed versus the risk factors determined (udder swabs, hand swabs, milking container, bulking container and water source) to identify the risk factor which contributes highest to farm gate milk quality.
Risk factors
From the survey questionnaire and observation checklist, none of the farms visited in both rural and peri urban practiced machine milking. Hand washing was practiced by all farmers in peri urban. Drying of hands and udder was practiced by 11% of farmers in rural and 50% in peri urban. Plastic milking containers were 60% in peri urban and 84% in rural location (Figure 1). Cross tabulation of risk factors practices between location showed that lack of hand drying was significantly different (P=0.007). Plastic milking and bulking containers were significantly different (p=0.04 and p=0.03 respectively) between locations. Lack of water treatment was practiced by 60% in rural and 80% in peri urban this was significantly different (p=0.008).
Microbiological quality of contamination sources
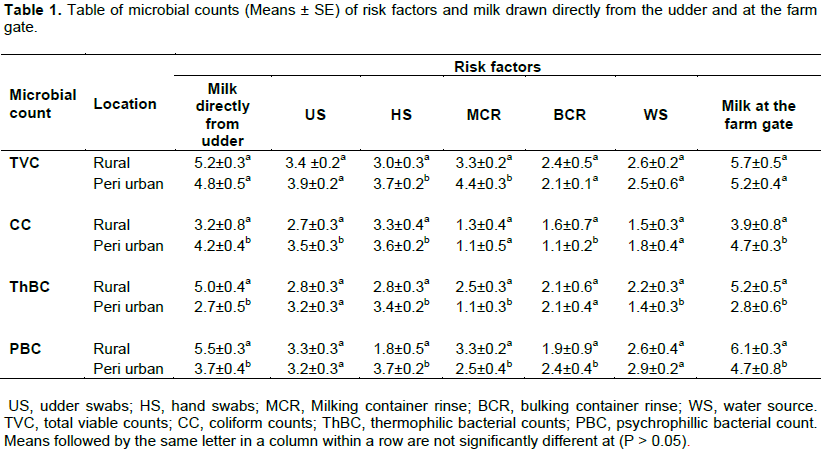
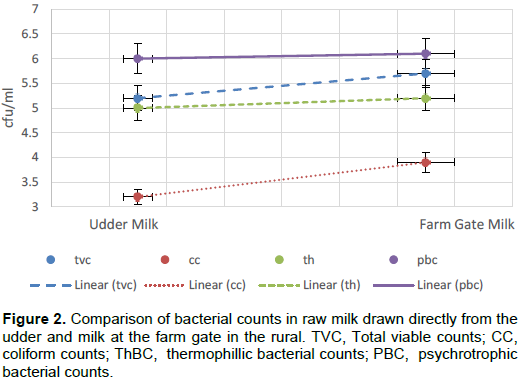
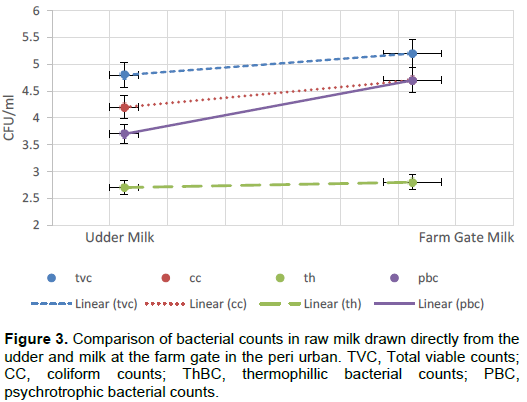
From the survey the risk sources of contamination to milk contamination with spoilage microorganisms included udder, hands, milking containers, bulking containers and water sources. Udder swabs recorded the highest counts in TVC (log 10 3.4 CFU/ml) in rural while milking container recorded the highest (log10 4.4 CFU /ml) in peri urban. Hands and udder recorded the highest counts in coliform for both locations. Water source recorded the highest in PBC in peri urban location while milking container rinses recorded a significantly (p≤0.05) lower value for ThBC in peri urban. There was a steady rise in microbial counts between the udder to the farm gate in all microbial counts evaluated (Table 1). Increase in TVC from the udder to the farm gate was 0.5 log cycle in rural (Figure 2). A significant increase in coliform count was recorded between the udder and farm gate in rural and peri urban milk (Figure 3).
Regression coefficients were derived from the formula below to determine the most responsible sources of different microbial types in milk at the farm gate. Where Y represented each microbial type in farm gate milk (TVC/CC/ThBC/PBC) and was regressed against the microbial type of each risk factor (US, HS, MCR, BCR and WS) evaluated and X1 to X5 are the regression coefficients of the respective risk factors.
Bulking containers showed the highest regression coefficient value in peri urban TVC of farm gate milk. Hands, udder and water source were the highest contributors to coliform counts in rural farm gate milk. In peri urban udders were the highest contributors to coliform counts (Table 2).
The study established that farm practices which predisposed milk to microbial contamination included; lack of hand and udder washing, or washing without drying. A similar trend was observed in the peri-urban where 50% practice hand and udder drying (Figure 1). Without drying of hands and udder after washing becomes a risk because the water used in washing the udder and hands will drip in the milking container, mixing with the milk. The excess water from hands and udder if not dried off caries microorganisms from hands and udder in unhygienic conditions contributing to high microbial count in milk (Hogan et al., 1979; Gulton et al., 1984; Islam et al., 2009). It was reported that milking in a dry environment provides a significant reduction in microbial load in milk. Thus just washing hands and udder is not as effective as following the procedure with drying of the surfaces with a material like a towel (Islam et al., 2009).
Udder swabs in peri urban recorded high counts in TVC compared to their rural counterparts (Table 1). Due to the small pieces of land in peri-urban compared to the rural areas, most farmers opt to practice zero grazing. Zero grazed animals stay in one place the whole day and are likely to have dirty udders due to defaecation in the same spot they feed and spend the night. The proximity of the udder and the rectum of the cow cause easy cross contamination from faecal coliform and other bacteria (Islam et al., 2009). With these factors, compared to the rural where free range grazing was mostly practiced due to availability of land, the hygiene of the udder was better than in peri urban.
Water treatment by either boiling or chlorination was more common in the peri urban than in the rural location (Figure 1). The microbial load in milk from rural areas where minimal water treatment was done, reported high cumulative microbial counts (Figure 3) than peri urban water source. The microbiological quality of water used during milking, udder preparation, and equipment cleaning in the farm play an important part in microbial load of raw milk. This showed that water hygiene is an important aspect of microbiological quality of milk. This water easily contaminates the milk especially where udder and hands drying after washing is not practiced. Previous studies have reported the same findings (Ingawa et al., 1992; Visser et al., 2007; Matofari et al., 2013).
Plastic milking containers which predominated the rural farms contributed to high microbial counts in the rural areas than the peri urban farms. Rinses from milking containers in the rural recorded the highest in total viable counts (Table 1). Plastic containers have been proven to contain micro -pores which facilitate the formation of biofilms and are therefore difficult to clean and become sources of contamination especially for psychrotrophic and thermophillic bacteria (Bereda et al., 2012; Mesfine et al., 2015). Methods of containers cleaning also vary from use of hot water, scouring material and detergent types. Since no standards methods exist in cleaning these containers at the farm, the microbiological quality of the containers are not controlled and therefore remain risks to milk contamination (Wafula et al., 2016).
Bulking containers had a significant regression coefficient value in total viable counts in peri urban unlike milking containers in both locations (Table 2). Milking containers in both locations had a wide opening to reduce spillage during milking from the udder. This property also helps in reducing biofilms due to ease of cleaning. The cleaning material can easily reach all parts of the container effectively. However, the wide opening of the milking container poses a risk of contamination from the milking environment which is always contaminated with cow dung. Bulking containers are however placed away from the milking area and do not get contamination from this kind of environment. Bulking containers are characterized with small openings to reduce spillage during transportation; however this property makes them difficulty to clean since not all areas are easily reached by cleaning material. This characteristic is a risk factor since the containers become hard to clean and promote the development of biofilms (Kaindi et al., 2011).
The micro-flora of milk at the farm gate is as a result of the contamination it acquires the moment it leaves the udder. Milk drawn directly from the udder had lower readings of TVC compared to the farm gate translating to 8.3% a percentage increase of in the rural which is a 0.5 log cycle. Hygiene in the peri-urban area was generally high compared to rural hence the high increase in microbial load in milk between the udder and the farm gate. This is because hand washing, udder washing and drying of the same was mostly practiced in the peri-urban compared to rural. Water treatment by boiling and chlorination was also practiced more in peri-urban compared to the rural counterparts. Other studies have reported lower counts in milk where proper pre milking and post milking practices were carried out targeting hands and udder (Hogan et al., 1979; Gulton et al., 1984; Islam et al., 2009; Odongo et al., 2016)
From the regression, the highest source of contaminant was the personnel followed by udder and bulking containers (Table 2). Lack of hand drying, zero grazing and proximity of the udder to the rectum are reasons for the high correlation between hands, udder and the microbiological quality of milk Hands and udder hygiene are majorly affected by pre-milking procedures and water quality which have shown in this study as being substandard. Mitigation measures in reducing microbial load and improving farm hygiene should target the practices associated with personnel activities, pre and post milking practices udder washing and drying before milking and using boiled or treated water or detergents to wash hands udder and containers. The microbiological quality and safety of milk is determined by handling and hygiene practices of the farm and the milk. Hygiene milking and post milking practices will ensure a low microbial count in milk with a longer shelf life (Petrovick et al., 2006; Kornacki and Johnson, 2001; Walstra et al., 1999).
Failure to observe high hygiene during milking and milk handling will expose milk to potential risk of microbial contamination. Udder of the cow is the highest source of contributor to milk contamination immediately it leaves the animal. It is evident from the study that effective udder cleaning and observation of high personal hygiene of the milking hands may reduce the risk of microbial contamination in both systems of milk production. Total microbial load in raw milk at the farm is highly contributed to by hands of milking personnel, udder swabs and bulking containers. Coliform counts are contributed to majorly by water source at the farm and milker’s hands. Thermophilic bacterial counts are highly contributed to by hands majorly while psychrophilic bacterial counts are significantly affected by milking containers. The study was limited to microbial groups and did not identify specific microorganisms in terms of species. In future studies this aspect is recommended.
Most important is the need to train farmers and farm employees on the importance of farm hygiene especially where hand milking is involved. Resources should be directed towards increasing the knowledge base of farmers on the significant influence of microbial contamination at the farm to overall hygiene within the rest of the value chain in terms of safety and shelf life. The farmers should be encouraged to carry out outlined hygienic practices which include effective cleaning of hands, udder followed by proper drying. Water used at the farm should undergo treatment before use for milking preparations. The farmer should milk in a clean environment free of cow dung. Avoiding of calf suckling and tying the cow’s tail during milking are practices which would reduce contamination of milk.
The authors have not declared any conflict of interests.
REFERENCES
|
Abdul-Hadi H, Nadia I, Aysar S (2014). Prevalence of Thermophiles and Mesophiles in Raw and UHT milk. Int. J. Anim. Vet. Adv. 6(1):23-27.
|
|
|
|
Bereda A, Yilma Z, Nurfeta A (2012). Hygienic microbial quality of raw whole cow's milk produced in Ezhadistrict of the Gurage zone, Southern Ethiopia. Wudpecker J. Agric. Res. 1(11):459-465.
|
|
|
|
|
Chizari M, Jannat S, Abbasi S (2008). Role of extension in developing dairy farmers knowledge toward milk quality in Golpayegan township, Iran. American- Eurasian J. Agric. Environ. Sci. 3: 333-338.
|
|
|
|
|
Chye F, Abdullah A, Ayob MK (2004). Bacteriological quality and safety of raw milk in Malaysia. Food Microbiol. 21:535-541.
Crossref
|
|
|
|
|
East African Community (EAC) (2006). ICS 67-2000 – East African Standards. Raw cow Milk Specification.
|
|
|
|
|
Gleeson D, O'Connell A, Jordan K (2013). Review of potential sources and control of thermoduric bacteria in bulk-tank milk. Irish J. Agric. Food Res. 217-227.
|
|
|
|
|
Griffiths MW, Phillips JD (1990). Incidence, source and some properties of psychrotrophic Bacillus spp found in raw and pasteurized milk. Int. J. Dairy Technol. 43(3):62-66.
Crossref
|
|
|
|
|
Hogan H, Galton WA, Jr. OM, Adkinson RE, Pankey JW (1979). Effect of Pre-milking Udder Preperation on Milk Quality. Dairy Sci. Abs. 41:869.
|
|
|
|
|
Hutchison ML, Thomas DJI, Moore A, Jackson DR, Ohnstad I (2005). An evaluation of raw milk microorganisms as markers of on-farm hygiene practices related to milking. J. Food Prot. 68(4):764-772.
Crossref
|
|
|
|
|
Ingawa KH, Adkinson RW, Gough RH (1992). Evaluation of a Gel Teat Cleaning and Sanitizing Compound for Premilking Hygiene 1, 2. J. Dairy Sci. 75(5):1224-1232.
Crossref
|
|
|
|
|
Islam MA, Islam MN, Khan MAS, Rashid MH, Obaidullah SM (2009). Effect Of Different Hygenic Condition During Milking On Bacterial Count Of Cows' Milk. Bangladesh J. Anim. Sci. 38(1-2):108-114.
|
|
|
|
|
ISO (2006). Microbiology of food and animal feeding stuffs. Horizontal method for the enumeration of coliforms. Colony-count technique. ISO 4832:2006. International Organization for Standardization, Geneva.
|
|
|
|
|
Kaindi DWM, Schelling E, Wangoh J, Imungi JK, Farah Z, Meile L (2011). Microbiological Quality of Raw Camel Milk across the Kenyan Market Chain. Global Science Books. (1):79-83.
|
|
|
|
|
Kalogridou-Vassiliadou D (1992). Biochemical activities of Bacillus species isolated from flat sour evaporated milk. J. Dairy Sci. 75(10):2681-2686.
Crossref
|
|
|
|
|
Kornacki JL, Johnson JL (2001). Enterobacteriaceae, coliforms, and Escherichia coli as quality and safety indicators. Compendium of methods for the microbiological examination of foods. 4:69-82.
|
|
|
|
|
Kumar AV, Rao LV, Kumar MK, Srinu B, Rao TM (2012). Efficacy of udder disinfectants on reduction of bacterial load and certain pathogens of public health significance. J. Microbiol. Biotechnol. Res. 2(1):147-151.
|
|
|
|
|
Matofari JW, Shalo PL, Younan M, Nanua JN, Adongo A, Qabale F, Misiko BN (2013). Analysis of microbial quality and safety of camel (Camelus dromedarius) milk chain and implications in Kenya. J. Agric. Ext. Rural Dev. 5(3):50-54.
|
|
|
|
|
Mesfine S, Feyera T, Mohammed O (2015). Microbiological Quality of Raw Cow's Milk from Four Dairy Farms in Dire Dawa City, Eastern Ethiopia. World J. Dairy Food Sci. 10(1):09-14.
|
|
|
|
|
Muriuki H (2003). Milk and Dairy Products, Post-harvest Losses and Food Safety in Sub-Saharan Africa and the Near East: A Review of the Small Scale Dairy Sector–Kenya. Rome, Italy: Food and Agricultural Organization.
|
|
|
|
|
Odongo NO, Lamuka PO, Matofari JW, Abong GO (2016). Risk factors associated with the post-harvest loss of milk along camel milk value chain in isiolo county Kenya. Afr. J. Agric. Res. 11(8):674-682.
Crossref
|
|
|
|
|
Paola B, Anna FAC, Simone S, Sara B, Casimiro C (2013). Microbiological survey of milk and dairy products from a small scale dairy processing unit in Maroua (Cameroon). J. Food Control Elsevier. 32:366-370.
Crossref
|
|
|
|
|
Petrović M, PaviÄić Ž, Tomašković A, Cergolj M (2006). Effect of milking hygiene to the number of bacetria in cow milk. StoÄarstvo 60(6):403-411.
|
|
|
|
|
Teka G (1997). Food hygiene principles and food borne disease control with special reference to Ethiopia. Faculty of Veterinary Medicine, Department of Community Health, Addis Ababa University.
|
|
|
|
|
Vissers MMM, Driehuis F, Te Giffel MC, De Jong P, Lankveld JMG (2007). Short communication: quantification of the transmission of microorganisms to milk via dirt attached to the exterior of teats. J. Dairy Sci. 90(8):3579-3582.
Crossref
|
|
|
|
|
Wallace RL (2008). Bacteria Counts in Raw Milk.
|
|
|
|
|
Walstra P, Geurts TJ, Noomen A, Van Boekel M (1999). Principles of Milk Properties and Processes. Dairy Technol. 1:149-170.
|
|
|
|
|
Wafula W, Wafula JM, Masani JN (2016). Effectiveness of the Sanitation Regimes used by dairy actors to control microbial contamination of plastic jerry cans surfaces. Int. J. food Contamination 3(9):16-28.
|
|