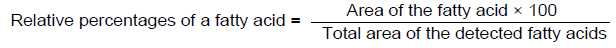ABSTRACT
Rapeseed oil is commonly used as cooking oil in Bangladesh especially in the rural areas and traditionally used with other food items in urban societies. This study evaluated the biochemical properties and chromatographic picturesque of various rapeseed oils namely mustard (wild and hybrid), rai (wild and hybrid) and canola oil in both raw and fried state. The qualitative tests such as acid value, iodine value, peroxide value, saponification value, unsaponifiable matter and Rechert-Meissl value were determined to differentiate the better quality of these consumable oils. Among five types of rapeseed oils, canola oil could be the best choice for consumption due to its lower erucic acid (6%) and high polyunsaturated fatty acids content, while the erucic acid content of mustard (wild), mustard (hybrid), rai (wild) and rai (hybrid) were 51.56, 64.82, 56.31 and 67.98% respectively. Canola oil also had the lowest level of acid value, peroxide value, saponification value and Reichert Meissl value but higher level of iodine value. On the other hand, rai (hybrid) fried oil was the lowest quality of consumable oil in comparison with other forms of rapeseed oils. Raw seed oils were also better than fried oils.
Key words: Rapeseed oils, qualitative test, gas-liquid chromatography (GLC).
Rapeseed oil which is popularly known as mustard oil in Bangladesh is obtained from the seeds of the plant Brassica spp. nowadays, most of the people of Bangladesh use rapeseed oil especially in frying and dressing of food stuffs. Two general types of rapeseed oils (Brussica campestris and Brussica juncea) are available in the subcontinent as well as Bangladesh. B. napus also found in USA, Canada and Europe (Sonntag, 1979). According to the USDA (2009), rapeseed oil was the second most important edible oil in the world but it contains high levels of erucic acid (51.56-67.98%), which is poorly metabolized and consequently fats could be accumulated in heart muscle and evidently causes multiple organ dysfunctions especially heart and liver (O'Brien, 2008; Sahasrabudhe, 1977; Rahman et al., 2016), besides some fatty materials could be deposited in the adrenal gland and ovarian tissues leading to some serious troubles on human (Charlton et al., 1975). Rapeseed oil also contains both omega-6 and omega-3 fatty acids in a ratio of 2:1 and trans fats (0.56 to 4.2%) (O'keefe et al., 1994; Ghafoorunissa, 1998, Rahman et al., 2014). Haliloglu et al. (2004) found high amounts of phytonutrients, particularly glucosinolates and minerals such as selenium and magnesium in rapeseed, which helps to protect against certain types of cancer (especially lung cancer), severity of asthma, to lower high blood pressure and macular degeneration (Haliloglu et al., 2004). In Bangladesh, people are habituated to consume soybean, palm and rice bran oil but they do not use olive, linseed, peanut, coconut or sunflower oil frequently. While, the modern trend of consuming rape seeds especially mustard oil in raw, fried and cooked form is increased day by day in the general people irrespective of economic status (Sarwar et al., 2014) but the nutritional quality of these oils are not assured. Marina et al. (2008) demonstrated the chemical characteristics of few convenient seed oils namely mustard, coconut, peanut, olive, linseed, sesame and sunflower oil but the detailed chemical characteristics of these oils remain obscure. Therefore, the objectives of this study were to publicize, determine and compare fatty acid profiles of different species of rapeseeds in both raw and fried form.
Different varieties of rape seeds both mustard and rai (wild, hybrid and fried formed) and Canola oil (origin Canada) were collected from different areas of Bangladesh.
The seeds were cleaned and sun dried to avoid contamination. Then the seeds were stored at 4ºC in refrigerator with sealed plastic packet to avoid the microbial contamination. Seeds were crushed and grinded by using electrical blender machine to obtain rapeseed oil.
Frying procedure
The oils were heated according to the method of Owu et al. (1998). About 500 ml oil was fired in a stainless steel pan for 10 min at 180°C and heated oil was obtained. The process was repeated two times to obtain three times heated oil with a cooling interval of at least three hours. Then the heated samples were analyzed after cooling for the chemical analysis to evaluate the changes caused by frying.
Determination of biochemical properties of rapeseed oil
Iodine value
The iodine value of rapeseed oil was measured by the method of Hansberry et al. (1947).
Saponification value
Rapeseed oils were saponified with a known amount of potassium hydroxide, excess of which was determined by titration (Viswanathan et al., 1999).
Acid value
A weighed amount of materials were titrated with a suitable solvent in aqueous sodium hydroxide solution under specific conditions, which did not saponify the neutral portion (Hansberry et al., 1947).
Peroxide value
The peroxide value is defined as the milli equivalents of peroxide oxygen combined in a kilogram of oil. Peroxide value determined by the standard method (Das, 1989).
Unsaponifiable matter
The unsaponifiable matter is a fraction of fat or oil that remains insoluble after saponification of the fat sample by alkali which was determined by titration (Hilditch, 1949).
Rechert-Meissl value
This is the volume of 0.1 N aqueous alkali required to neutralize the water soluble but volatile acids obtained from exactly 5 g of the oil. Rechert-Meissl value was determined by the standard method (Roger castle griffin, 2nd edition, 84: 299).
Analysis of fatty acid methyl esters (FAME) by gas-liquid chromatography (GLC)
The fatty acid methyl esters were analyzed by Gas-Liquid Chromatography (GLC) method. The model of GLC machine was 9A, Shimadzu, Japan, 2010. The column was AT-5; Capillary length was 30 m, internal diameter (ID): 0.25 mm packed with 12 to 15% (w/w) ethylene glycol succinate liquid phase coated on 100/200 mesh Gas-chrom P was used. The injector temperature was 275°C and the detector temperature was 280°C respectively. Nitrogen gas (N2) was used as the carrier gas at a flow rate of 1.5 ml/min. Split ratio was 90:10. The temperature of the column was programmed initially at 120°C for 2 min, and then it allowed rising to 270°C at a rate of 7°C/min and the isothermal final period was 10 min. Thermal conductivity detector was excellent. Standard fatty acid methyl esters were used for the identification of the sample fatty acid peaks. The following fatty acids were used: The methyl esters of C 8:0, C 10:0, C 12:0, C 14:0, C 16:0, C 16:1, C 18:0, C18:1, C 18:2, C 20:0, C 22:0, C 22:1 and C24:0. The peak area of each component was measured automatically by chromatograph machine. The total mm of all peak areas were taken as 100% and the percent population of a given fatty acid peak was calculated accordingly. The fatty acids were expressed as weight percentages of total fatty acids.
Determination of fatty acid profile of rapeseed oils by gas-liquid chromatography (glc) method
Gas Chromatography was conducted with a gas liquid chromatography (GLC-2010 series, Shimadzu Co Japan). Each FAME in extract was identified by comparing retention time with those of known standard FAME (LIPID STANDARD, SIGMA Chemical Co., St. Loius, USA).
The area of fatty acids was measured with GLC solution 2010 software. The results were expressed as relative percentage of fatty acids. The relative percentages of fatty acids were calculated by the formula:
Statistical analysis
Statistical analyses were performed with SPSS for windows, version 11.5. Data were expressed as mean±SD. Significance was accepted at the p<0.05 and highly significance was accepted at the p<0.01 levels.
This study showed the biochemical properties of both raw and fried rapeseeds (Table 1). Acid value, iodine value, peroxide value, saponification and Reichert Meissl value act as a parameter to expose the better quality of fats and oil. The high quality of fats and oils is reciprocal with the acid value. The higher level of acid value indicates the more free fatty acids (FFA) in the oil and thus increased oxidation and rancidity. The normal acid value of oil ranges between 0.36 and 1.5 (Lange, 1944). Among five types of rapeseed oil, Rai (hybrid) had the highest acid value in both fried (0.932) and raw (0.622) oil while canola oil had the lowest one (0.376 and 0.329, respectively). In comparism with the acid value of these oils, raw oils were better than fried oils.
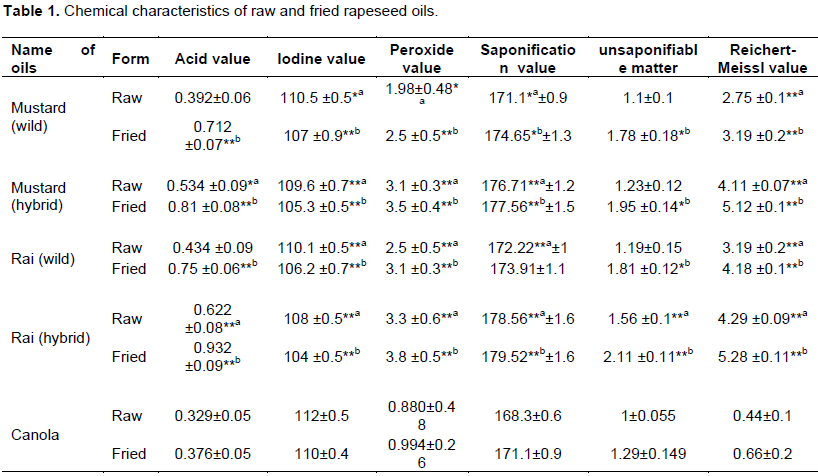
Iodine value is a measure of degree of unsaturation of oil. Edible oil with higher iodine values is always recommended for consumption, because of its more double bonds which are readily broken down by β oxidation and contains essential fatty acids but lower levels of cholesterol. On the contrary, edible oil with lower iodine value is less stable and more susceptible to oxidation and rancidity. In general, iodine value of oil ranges between 94 and 105. Table 1 also indicated that iodine values for mustard (wild and hybrid) and Rai (wild and hybrid) were 104 to 110 whereas canola oils were 110 (raw) and 112 (fried). Therefore, all rapeseeds in Bangladesh showed lower iodine values than canola. Frying process decreased iodine value in all cases. Among the rapeseeds, Rai (hybrid) was the lowest iodine values (108 and 104 for raw and fried, respectively). Peroxide value is one of the most widely used tests for oxidative rancidity in fats and oils. It is a measure of the concentration of peroxides and hydroperoxides formed in the initial stages of lipid oxidation and also useful for predicting shelf life. High peroxide values are a definite indication of a rancid fat, but moderate values may be the result of depletion of peroxides after reaching high concentrations. So, oils with low peroxide values are more preferable and good indication for better shelf life. The normal peroxide value of oil is max 1.0 whereas, the peroxide values of mustard and rai (both wild and hybrid) were 1.98, 3.1, 2.5, 3.3 for raw and 2.5, 3.5, 3.1, 3.8 for fried samples respectively. On the other hand, canola oil showed the lowest peroxide value for raw (0.88) and fried (0.994) formed than other rapeseeds in Bangladesh.
Saponification value is inversely proportional to the average molecular weight or chain length of the fatty acids present in the fat or oil. The saponification value of selected rapeseeds oil at different form (raw and fried) was analyzed by titrimetric analysis and the results were summarized in Table 1. This study found the saponification values of mustard (wild), mustard (hybrid), rai (wild), rai (hybrid) were 171.1, 176.71, 172.22 and 178.56 for raw and 174.65, 177.56, 173.91 and 179.52 for fried samples respectively while canola oils were found to have the lowest value, that is, 168.3 (raw) and 171.1 (fried) which significantly differ from all verities of rapeseeds and indicates better quality of oil. General saponification value of oil is ranged between 168 and 179. Saponification value is also higher in fried samples than raw form.
Unsaponifiable matter is a fraction of fat or oil that remains insoluble after saponification of the fat by alkali. Unsaponifiable of 0.5 to 2.0% in oil represents the presence of mixture of several lipids like sterols, tocopherols, hydrocarbons, higher aliphatic fatty acids and terpinoids alcohols (Jacobs, 1962). In this study, unsaponifiable matters present in rapeseeds were 1.1, 1.23, 1.19 and 1.56% for raw and 1.78, 1.95, 1.81 and 2.11% for fried samples of mustard (wild and hybrid) and rai (wild and hybrid) respectively. Among these rapeseeds, rai (hybrid) was the highest unsaponifiable matter while canola was the lowest ones (1.0% for raw and 1.29% for fried). Lower percentage of unsaponifiable matter in canola ensures the better quality in comparison with other varieties of rapeseeds in Bangladesh.
Reichert-Meissl (RM) values represent the amount of volatile and water soluble acids components of an oil and fat. The lowest level of this value indicates the better quality of oil. Table 1 also showed that RM values of various rapeseeds were 2.75, 4.11, 3.19, and 4.29 for raw and 3.19, 5.12, 4.18 and 5.28 for fried samples of mustard (wild and hybrid) and rai (wild and hybrid) respectively. Thus, rai (hybrid) was the highest RM value of both raw and fried samples while canola oil had the lowest level of RM values (0.44 and 0.66 for raw and fried oils, respectively). This table also indicated that fried samples were more hazardous than raw oils.
Identification and estimation of the fatty acid percentage composition of rapeseed oils by gas liquid chromatography-flame ionization detector (GLC-FID)
Fatty acid in the rapeseed oil usually begin with the saturated acid C10:0 (capric acid). In all the rapeseeds samples palmitic, stearic and behenic acids are represented as a minor constituent. Bulk of the unsaturated fatty acid (Table 2) is presented as C22:1 or erucic acid. However, palmitic acids or C16:0 was the major saturated of the total fatty acids. The erucic acid (C22:1) had the highest proportion of the total mixture. Oleic acid (C18:1) and Linoleic acid (C18:2) were the next two fatty acids in most of the rapeseed oils. However linolenic acids (C18:3) did not contribute in the mixture. The most significant fatty acid like erucic acid (C22:1) had the attention of several workers all over the glove because of its complication of several diseases. Figure 1 represents the standard fatty acid methyl esterspattern developed by gas-liquid chromatographic techniques whereas Figures 2 to 8 indicated the patterns of fatty acid methyl esters of various rapeseed oils such as mustard (wild), mustard (hybrid), fried mustard (wild), rai (wild), rai (hybrid), fried rai (wild) and canola oils respectively. As many as thirteen major and minor peaks were observed in the chromatograph. Due to unavailability of some standard fatty acids, all the fatty acids in the sample oils could not be identified. The chromatograph provide information related to separation of individual esters of fatty acids and their respective retention time, printed on each individual peak of the fatty acid methyl esters.
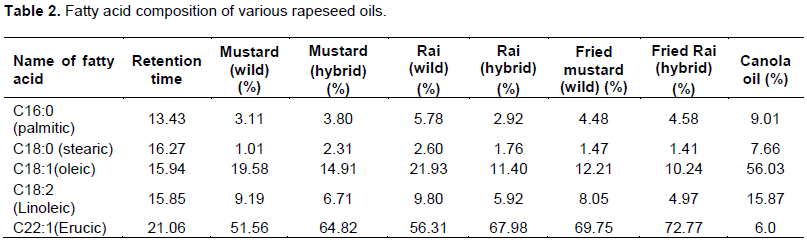
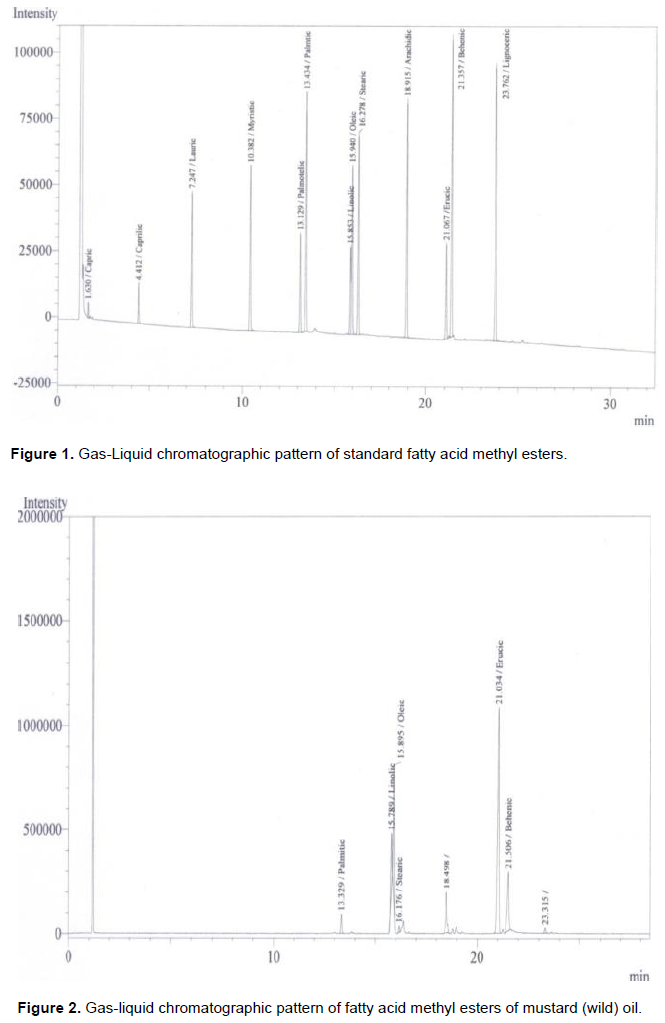
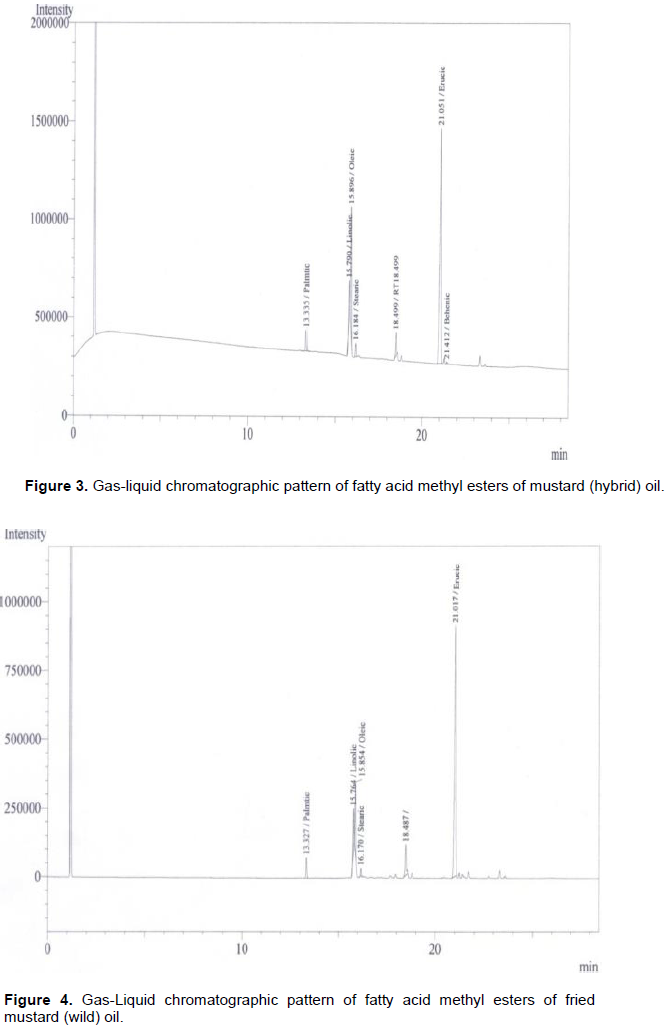
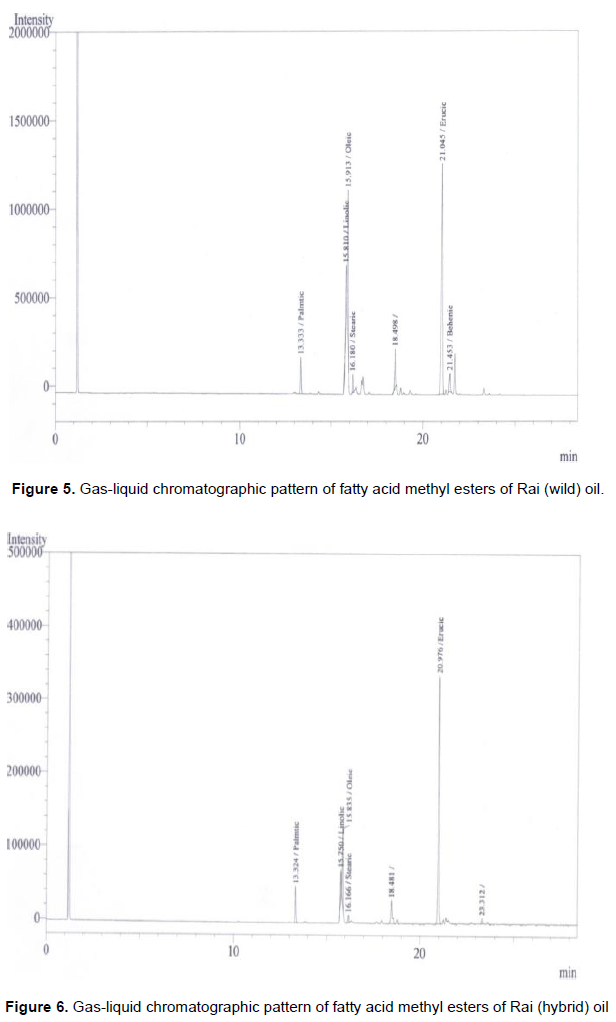
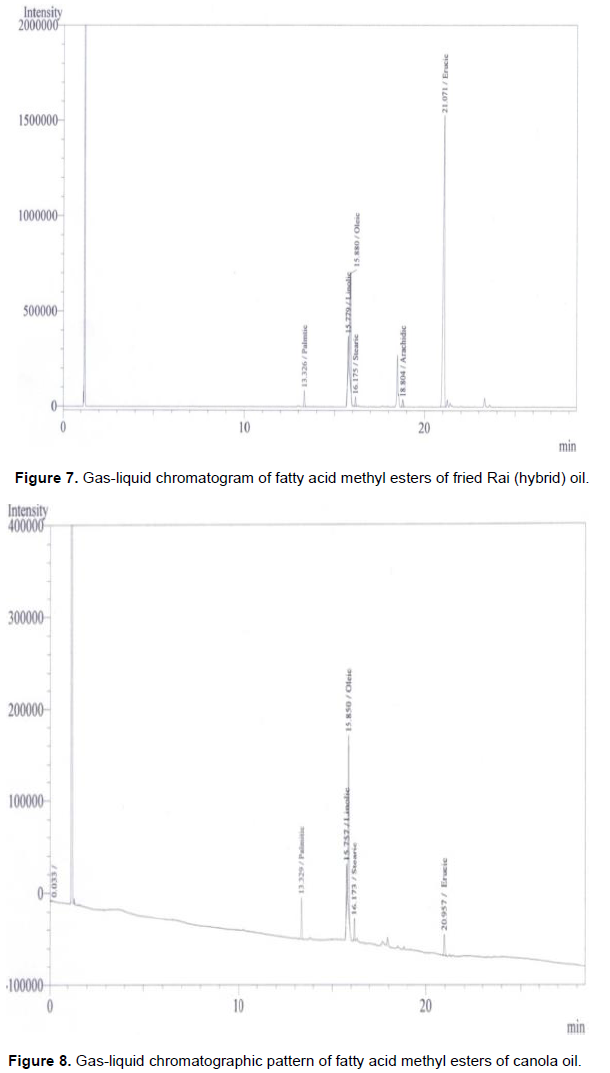
In Figure 2, total saturated fatty acids observed was 4.12%, and palmitic acid, the major saturated fatty acid was found to be 3.11%. Whereas, stearic acid was 1.01% in the mustard (wild) oil. Among monoenoicfatty acid, erucic acid (C22:1) was the major component (51.56%) and oleic acid (C18:1) was 19.58%. The unsaturated linolic acid (C18:2) was 9.19% in mustard (wild) oil. The percentage of fatty acids in the mustard (hybrid) was presented in Figure 3. The total saturated fatty acids observed was 6.11%. Palmitic acid, the major saturated fatty acid was found to be 3.80%. The second major saturated fatty acid stearic acid was 2.31% in the mustard (hybrid) oil. Among monoenoic fatty acid, erucic acid (C22:1) was the major component, which was observed to be 64.82% and oleic acid (C18:1) was 14.91%. The unsaturated linolic acid (C18:2) was 6.71% in mustard (hybrid) oil. The total saturated fatty acid of rai (wild) was 8.38% and palmitic acid, the major saturated fatty acid was found to be 5.78% (Figure 5). The second major saturated fatty acid, stearic acid was 2.60% while the monoenoic fatty acid, erucic acid (C22:1) was the major component (56.31%). Oleic acid (C18:1) content was 21.93% and polyunsaturated fatty acid, Linolic acid (C18:2) was 9.80% in rai (wild) oil. On the other hand, Figure 6 showed that rai (hybrid) had 4.68% total saturated fatty acids and 2.92% palmitic (saturated) fatty acid. The major component, erucic acid (C22:1) was 67.98% and stearic acid, oleic acid (C18:1) and linolic acid (C18:2) were 1.76, 11.40 and 5.92% respectively in rai (hybrid) oil.
Figure 4 also showed that the fried mustard (wild) oil contain total saturated fatty acids (5.95%), palmitic acid (4.48%), stearic acid (1.47%), erucic acid (69.75%), oleic acid (12.21%) and polyunsaturated linolic acid (8.05%). While the fatty acid compositions of fried rai (hybrid) oil were shown in Figure 7. This gas liquid chromatography (GLC) pattern indicated the highest mono unsaturated fatty acid was erucic acid (72.77%). Others fatty acid such as total saturated fatty acids, palmitic acid, stearic acid, oleic acid and linoleic acid were 5.99, 4.58, 1.41, 10.24 and 4.97%, respectively. Although all the experimental rapeseed oils contain higher percentage of erucic acid, canola oil had only 6.0% erucic acid (Figure 8).
Erucic acid rich oil may affect many organs (e. g. heart, liver) and may induce myocardial lesion, necrosis (Hung et al., 1977), and cardiac lipidosis by accumulation of substantial fat in the heart muscle (Kramer et al., 1992). Chakraborty (2003) showed rapeseed oils contain 20 to 50% erucic acid but this study found 51.56 to 67.98% (in raw) and 69.75 to 72.77% in fried rapeseed oil. Erucic acid content of fried rapeseed oil was higher than the raw seed oil which was accorded with Chacko and Rajamohan, (2011). Figure 8 also emphasized that canola oil had total saturated fatty acids (16.67%), palmitic acid (9.01%), stearic acid (7.66%), oleic acid (56.03%) and linoleic acid (15.87%). Harris and Connor (1980) demonstrated that large amounts of polyunsaturated fat in the diet exhibit significant hypolipidemic effect (Harris and Connor, 1980). So, canola oil could be more preferable for consumption than other rapeseed oils.
Among different varieties of common rapeseed oils like mustard, rai and canola (both raw and fried) in Bangladesh, canola oil might be the most favorable oil for consumption because of its low erucic acid and high polyunsaturated fatty acids content.
Canola oil also had the lowest acid value, peroxide value, saponification and Reichert Meissl value but higher iodine value which indicated better quality of consumable oil. While rai (hybrid) fried oil was lower grade oil in compare with other form of rapeseed oils. Raw seed oils were also better than fried oils.
The authors have not declared any conflict of interests.
REFERENCES
|
Chacko C, Rajamohan T (2011). Repeatedly heated cooking oils alter platelet functions in cholesterol fed Sprague dawley rats. Int J Biol Med Res. 2(4):991-997.
|
|
|
|
Chakraborty MM (2003). Safety of specific acids in chemistry and technology of oils and fats. Allied publishers Pvt. Limited. New Delhi. P 121.
|
|
|
|
|
Charlton KM, Corner AH, Davey K (1975). Cardiac lesions in rats fed rapeseed oils. Can. J. Comp. Med. 39(3):261-269.
|
|
|
|
|
Das RK (1989). Industrial chemistry, part-2, pub. kalyani, Ludhiana-New Delhi. P 255.
|
|
|
|
|
Ghafoorunissa (1998). Requirment of dietary fats to meet nutritional needs and prevent the risk of atherosclerosis an Indian prespective. Indian J. Med. Res. 108: 191-202.
|
|
|
|
|
Haliloglu I, Bayir A, Sirkeci N (2004). Comparison of fatty acid composition in some tissue of rainbow trout living in seawater and freshwater. Food Chem. 86:55-59.
Crossref
|
|
|
|
|
Hansberry R, Clausen RT, Norton LB (1947). Variations in the chemical composition and insecticidal properties of the yam bean (Pachyrrhizus). J Agric Res 74: 55-64.
|
|
|
|
|
Harris WS, Connor WE (1980). The effect of salmon oil upon plasma lipids, lipoproteins, and triglyceride clearance. Trans. Assoc. Am. Phys.43:148-55.
|
|
|
|
|
Hilditch TP (1949).The industrial chemistry of fats and waxes, 3rd edition, Bailliere tinadall and Cox. London. P 80.
|
|
|
|
|
Hung S, Umemura T, Yamashiro S (1977). The effects of original and randomized rapeseed oils containing high or very low levels of erucic acid on cardiac lipids and myocardial lesions in rats. Lipids12 (2):215-21.
|
|
|
|
|
Jacobs MB (1962). Chemical analysis of Foods and Foods products, 3rd edition, D.Van Nostrand Comp. Ltd. London. P 370.
|
|
|
|
|
Kramer JK, Sauer G, Wolyn MS (1992). Effects of dietary saturated fat on erucic acid induced myocardial lipidosis in rats. Lipids 27(8):619-623.
Crossref
|
|
|
|
|
Lange NA (1944). Handbook of chemistry. Handbook publishers Ins, Sandusky, Ohio.14th edition. P 678.
Crossref
|
|
|
|
|
Marina AM, Che Man YB, Nazimah SAH, Amin I (2008) Antioxidant capacity and phenolic acids of virgin coconut oil. Int. J. Food Sci. Nutr. 60(sup2):114-123.
|
|
|
|
|
O'Brien R (2008). Fats and Oils Formulating and Processing for Applications, Third Edition: Formulating and Processing for Applications. CRC Press. pp: 37-40.
Crossref
|
|
|
|
|
O'keefe SE, Gaskinsâ€Wright SA, Wiley V, Chen I (1994). Levels of trans geometrical isomers of essential fatty acids in some unhydrogentated U.S. vegetable oils. J. Food Lipids 1(3):165-176
Crossref
|
|
|
|
|
Owu DU, Osim EE, Ebong PE (1998). Serum liver enzymes profile of Wistar rats following chronic consumption of fresh or oxidized palm oil diets. Acta Trop. 69(1):65-73.
Crossref
|
|
|
|
|
Rahman H, Nasreen L, Habib K, Rahman N (2014). Effects of Dietary Coconut Oil on Erucic Acid Rich Rapeseed Oil-induced Changes of Blood Serum Lipids in Rats. Curr. Nutr. Food Sci. 10(4):302-307.
Crossref
|
|
|
|
|
Rahman MH, Habib K, Rahman SS, Nasreen L, Ud-Daula A (2016). Ameliorating Effect of Dietary Sesame Oil on High Erucic Acid Rapeseed Powder–Induced Changes of Blood Serum Lipids in Rats. J. Environ. Sci. Toxicol. Food Technol.10 (2):2319-2402.
|
|
|
|
|
Roger castle griffin (1921). Technical methods of analysis, Mac Graw-Hall Book Company, Inc. New York & London, 2nd edition. pp: 84:299.
|
|
|
|
|
Sahasrabudhe MR (1977). Crismer values and erucic acid contents of rapeseed oils. J. Am. Oil Chem. Soc. 54(8):323-324.
Crossref
|
|
|
|
|
Sarwar MT, Rahman MH, Raza MS, Rouf SM, Rahman MN (2014). Determination of Erucic Acid Content in Traditional and Commercial Mustard Oils of Bangladesh by Gas- Liquid Chromatography. Adv. Biochem. 2(1):9-13.
Crossref
|
|
|
|
|
Sonntag (1979). In Bailey's industrial oil and fat products. J. Wiley and Sons, New York, Toronto. P 43.
|
|
|
|
|
United States Department of Agriculture (USDA) (2009). Major Vegetable Oils: World Supply and Distribution at Oilseeds: World Markets and Trade Monthly Circular. FOP. pp:1-09.
|
|
|
|
|
Viswanathan MB, Thangadurai D, Vendan KT, Ramesh N (1999). Chemical analysis and nutritional assessment of Teramnus labialis (L.) Spreng. (Fabaceae). Plant Foods Hum. Nutr. 54:345-352.
Crossref
|
|