Full Length Research Paper
ABSTRACT
Improving plant salt tolerance is thus a veritable challenge for breeders as salt stress is one of the most important factors that negatively affect plant growth and productivity worldwide. In this study, we evaluated the physiological strategies involved in the salt resistance of three salt resistant mutant lines. Three weeks old plants of the three mutant lines and that of the control cultivar Locale were submitted in pots to three NaCl concentrations (0; 100 and 200 mM) in a completely randomized design with three replications. Plant growth, ions and organic solutes contents were determined after two weeks of treatment. Plant growth reduction under salt stress was earlier and more accentuated in the control cultivar followed by line L2 than the salt resistant lines L18 and L23. The rate of Na+, proline and soluble sugars accumulation; that of K+ and Ca++ absorption and that of K/Na and Ca/Na reduction under salt stress varied greatly according to the population. The salt resistance of line L18 was due to Na+ accumulation in leaves associated to proline and soluble sugars accumulation, the maintenance of high absorption of Ca++, high K/Na and Ca/Na ratios whereas that of line L23 was due to Na+ exclusion from leaves associated to the maintenance of high absorption of high K+ and Ca++, high K/Na and Ca/Na ratios. Line L2 resist to salt stress via proline accumulation and the maintenance of high Ca/Na ratio. Thus, lines developped different salt resistance strategies according to their relative salt resistance level.
Key words: Amaranthus cruentus, mutant lines, salt resistance mechanisms.
INTRODUCTION
Soil salinity results from the accumulation of soluble salts containing chloride, sulphate, sodium carbonate, potassium, magnesium and calcium. Among them, NaCl and Na2SO4 are especially toxic for plant growth (Beghin, 2019). Unadequate agricultural practices, especially irrigation with poor quality water, are one of the major causes of soil salinization process (Ruan et al., 2010). A soil is considered as saline when the electrical conductivity of the soil exceeds 4 ds/m (Shrivastava and Kumar, 2015). Approximately 10% of the total agricultural lands (950 Mha) and 50% of the total irrigated area (230 Mha) in the world are encountering salt stress (Behra et al., 2022). Soil salinization is one of the major abiotic factors reducing plant growth at most developmental stage, the young seedlings and flowering stages is the most sensitive (Lutts et al., 1995; Khan et al., 2010).
Salt stress present two components: it acts through osmotic stress and compromises plant water uptake as a result of the lowering of external osmotic potential, and through an ionic stress resulting from either accumulation of toxic ions (Na+ and Cl-) or depletion in essential elements (potassium, calcium, manganese) (Behra et al., 2022). Plants display various strategies in order to cope with salt stress. One of them consists in reducing Na+ accumulation and enhancing K+ accumulation allowing tolower Na/K ratio mainly in photosynthetic leaves (Lutts et al., 1996; Almansouri et al., 1999; Wouyou et al., 2019). Another strategy consist in the biosynthesis of osmoprotectants and compatible solutes among which proline and soluble sugars are the most frequently reported (Gupta and Huang, 2014; Gouveitcha et al., 2021).
Amaranth (Amaranthus cruentus) is a pseudo-cereal which is mainly cultivated in Africa for its leaves rich in β-carotene, lipid, carbohydrate, calcium, iron, proteins, and vitamin C (Olaniyi et al., 2008; James et al., 2010; Achigan-Dako et al., 2014). It is also one of the traditional leafy vegetables produced in Benin, helping to fight against food insecurity and playing an important role in the economy (Dansi et al., 2008; Adjogboto et al., 2019). In Benin, an important part of the production zone of amaranth is prone to salinization by irrigation water (Gandonou, 2020). The majority of vegetable crop species are salt-sensitive and exhibit a very low salinity threshold (ECt, which ranged from 1 to 2.5 dS m-1 in saturated soil) (Behra et al., 2022). Several authors reported that salt stress reduces amaranth growth (Qin et al., 2013; Amukali et al., 2015; Lavini et al., 2016). This is especially the case for the most widely prized cultivar in Benin called Locale (Wouyou et al., 2017; Luyckx et al., 2021). Increasing salt tolerance in this amaranth cultivar will have a high benefical impact in nutritional and economic security in the country and thus appears as a major goal.
In a recent study, we developed some amaranth mutant lines from the sensitive cultivar Locale issued from γ-rays irradiation strategy and which exhibit impoved salt resistance comparatively to the initial cultivar (Atou et al., 2022). The underlying physiological and biochemical mechanisms involved in this improvement remain unidentified. The present study aims at evaluating the implication of sodium, potassium and calcium ions accumulation on one hand as well as proline and soluble sugars accumulation on the other hand in the salt resistance acquired by the salt resistant identified lines.
MATERIALS AND METHODS
Plant
Four populations of A. creuntus were considered in the present study. The first population corresponds to cultivar Locale from which mutant lines were generated. Locale was used in this study as control cultivar (CC). The three other populations were three mutant lines L2, L18 and L23 identified as salt-resistant in comparison with the control cultivar at the young plant stage as detailed in our previous report (Atou et al., 2022).
Experimental design and conditions
The experiment was carried out in a screening house of the International Institute of Tropical Agriculture (IITA/Benin) (N 6° 25’ 260’’ E 2° 19’ 682’’; 15 masl) in the City of Abomey-Calavi (Republic of Benin) located in the Gulf of Guinea which is characterized by a subequatorial bimodal climate with two dry seasons and two rainy seasons (Kinhoegbè et al., 2020). The annual rainfall varies between 1200 and 1500 mm/year and the temperature range from 24 to 30°C (Kinhoégbè et al., 2020). The seeds from the four amaranth populations were germinated in tubs filled with potting soil for two weeks. The young plants were then transferred to small pots (5.8 cm × 6 cm) containing a mixture of potting soil and sand (50:50) (one plant/pot) and grown for one week before stress application. Plants of the control cultivar Locale and of the three salt resistant mutant lines were subjected to salt stress in large earthen pots (11.3 cm × 14 cm) filled with 3 kg of the same mixture as before. Treatments consisted of watering the plants every other day with 100 ml/pot of water containing 0, 100 and 200 mM NaCl. The experimental set-up was a completely randomized design with two factors. The first factor is represented by the three saline treatments (T0 = 0 mM; T1 = 100 mM and T2 = 200 mM) and the second is represented by the four amaranth populations (the control cultivar, and salt-resistant mutant lines L2; L18 and L23) with three replicates.
Plant growth determination
Plant height, shoot and root fresh and dry matters were measured after two weeks of treatment. For dry matter determination, fresh samples were transferred to an oven at 80°C for 72 h.
Shoot water content
Shoot water content was determined according to the formula: [(Shoot fresh mass – Shoot dry mass)/Shoot fresh mass] × 100 as used by Gouveitcha et al. (2021).
Ion concentrations
Ion determination was performed as reported by Henry et al. (2021). Leaves and roots were individually dried in an oven at 80°C for 72 h, ground in a mortar, and the powder was dried for additional 24 h. To determine the concentrations of Na+, K+ and Ca++, 20 mg of the leaf and root powders were placed in 10 ml jars and digested with nitric acid (68%) at room temperature. The solutions were filtered through Whatman paper (85 mm, Grade 1). The filtrate was used for the determination of cations (Na+, K+ and Ca++) using a flame spectrophotometer (Sherwood Model 360). Ion concentrations were expressed in mg g−1 DM (Dry Mass).
Extraction and determination of proline and soluble sugars
Proline and soluble sugars extraction and determination were performed as described by Henry et al. (2021). Samples of 100 or 200 mg of leaf fresh mass (youngest fully-unfolded leaf), or root fresh mass were used. Proline concentration was determined spectrophotometrically using the method of Bates et al. (1973) and obtained results were expressed as µmol proline g−1 FM (Fresh Mass). Standerd curve was established using proline (Sigma Aldrich) as standard. Total soluble sugars were estimated by the anthrone reagent method using glucose as the standard according to Yemm and Willis (1954) with slight modification (Manaa et al., 2014) using an UV-visible spectrophotometer (Jenway 7305). Soluble sugars concentration was expressed as µmol soluble sugars g−1 FM (Fresh Mass).
Statistical analyses
For all recorded parameters, the means and standard errors were calculated with three replications per treatment using the Excel spreadsheet. The results were subjected to one or two-ways analysis of variance (ANOVA) as appropriate and the means were compared with the Tukey-Kramer test. Analyses were performed using JMP Pro software (JMP Pro SAS Institute, 2009).
RESULTS
Effect of NaCl salt stress on plant growth
Salt stress reduced plant growth in mutant lines and the control cultivar (Table 1). NaCl induced a significant (p=0.001) decrease in plant growth at 100 and 200 mM NaCl for all growth parameters evaluated in the control cultivar. For lines L18 and L23, growth reduction under salt stress was non significant for all the growth parameters except for plant height and shoot fresh mass. In addition, plant height reduction under salt stress was significant at 100 and 200 mM NaCl for line L23 whereas it was significant only at 200 mM NaCl for line L18. This result indicated that growth of line L18 was less affected by NaCl than that of line L23. Line L2 has similar trend as the control cultivar but the root dry mass reduction under salt stress was not significant for line L2. Moreover, the relative shoot fresh mass reduction percentages under salt stress were lower for line L2 (40.81 and 54.08%) at 100 and 200 mM NaCl, respectively, than for control cultivar (56.84 and 63.04%). This confirms that growth reduction under salt stress was less accentuated in line L2 than in control cultivar and indicates that this line was intermediary between the control cultivar and lines 18 and L23.
Effect of NaCl on shoot water content
NaCl stress induced similar effect on shoot water content in the control cultivar and the three amaranth mutant plants with globally a slight non significant decrease (Table 2). Thus, shoot water content did not change significantly under salt stress in the four amaranth populations.
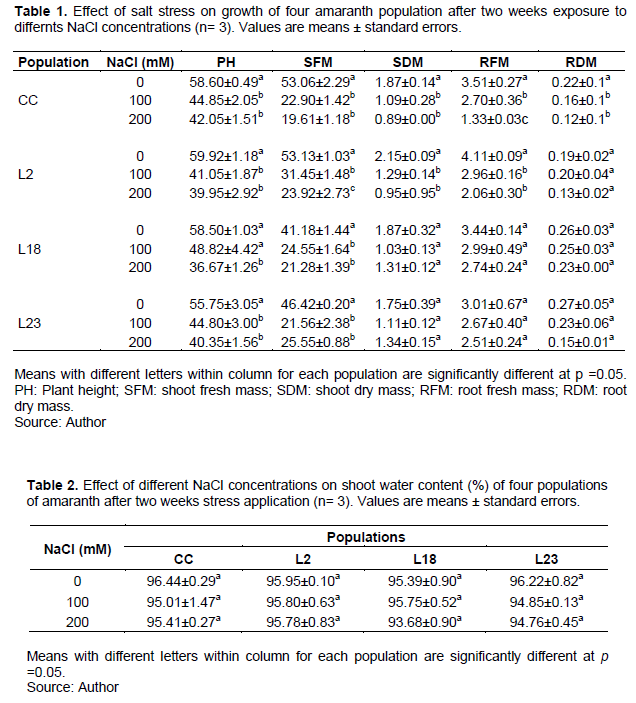
Effect of salt stress on plant ion contents
Two-ways ANOVA revealed a significant effect of NaCl only on leaf and root Na+ and K+ content while there was no significant impact of stress on Ca++ accumulation (Table 3). Moreover, a significant difference among populations was found for leaf Na+, roots K+ and leaf and roots Ca++. A significant interaction between NaCl stress and population was recorded for all parameters except for roots Na+. Thus, except for roots Na+, each population should be analyzed separately.
Effect of salt stress on leaf Na+ content
Salt stress induced a significant increase in Na+ content in leaves in the four considered populations (Table 4). This increase was significant only at 200 mM NaCl for the control cultivar and lines L2 and L23 but was already detected in response to 100 mM in L18. Thus, the rate of Na+ accumulation was more accentuated in leaves of line L18 than the control cultivar and the two other mutant lines. Moreover, Na+ accumulation rate in terms of percentages was lower in leaves for line 23 than in the control cultivar either at 100 mM (33.40/65.80) or at 200 mM NaCl (190.67/258.20). This is also valid for line L2 at 200 mM NaCl comparatively to control cultivar (192.35/ 258.20). Thus, line L18 accumulated more Na+ in leaves than the control cultivar whereas line L23, followed by line L2 (in a less extend) accumulated less Na+ in leaves than the control cultivar.
Effect of salt stress on root Na+ content
As far as roots are concerned, salt stress induced a significant increase in Na+ content already in response to 100 mM NaCl with no difference among populations (Table 5).
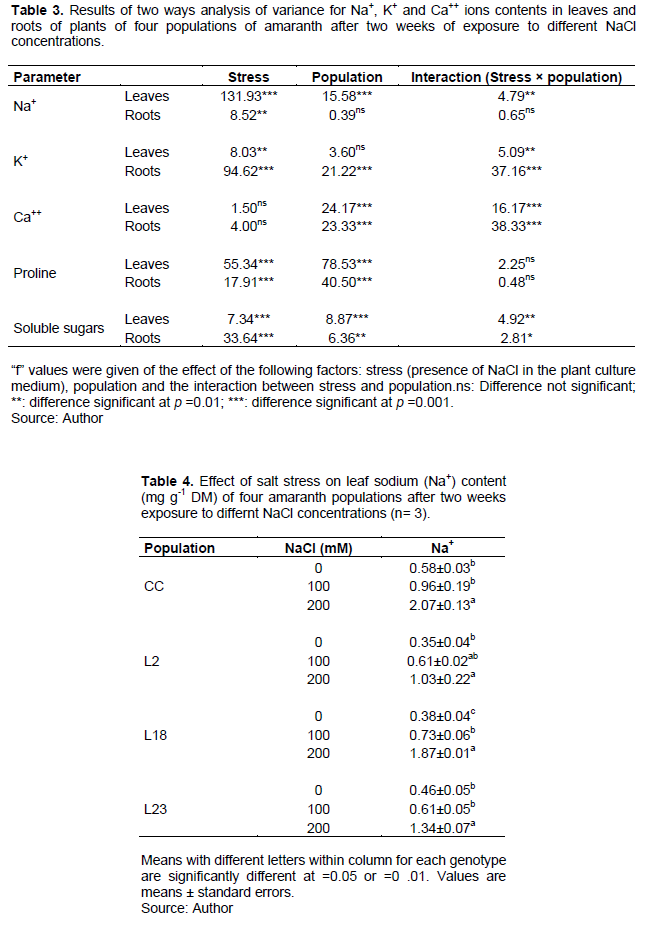
Effect of salt stress on leaf and roots K+ content
Salt stress induction had no significant effect on K+ content (Table 6) in leaves of the control cultivar but a slight increase at 100 mM NaCl followed by a high decrease at 200 mM NaCl was observed for line L18 (with a significant difference between 100 and 200 mM NaCl) whereas a significant decrease was observed in line L2 and a significant increase in line L23 at 100 mM NaCl (Table 6). In roots, a significant decrease in K+ content was observed in the control cultivar and line L18 at both 100 and 200 mM NaCl, while this effect was significant for line L2 at 200 mM NaCl only. No effect was observed in line L23. Thus, salt stress induced a decrease in leaves K+ content only in line L2 with an unexpected increase in line L23 at 100 mM NaCl. A decrease in root K+ was noticed for all population except line L23. It thus appears that L23 maintained an efficient K+ nutrition in both leaves and roots under salt stress in comparison with the control cultivar.
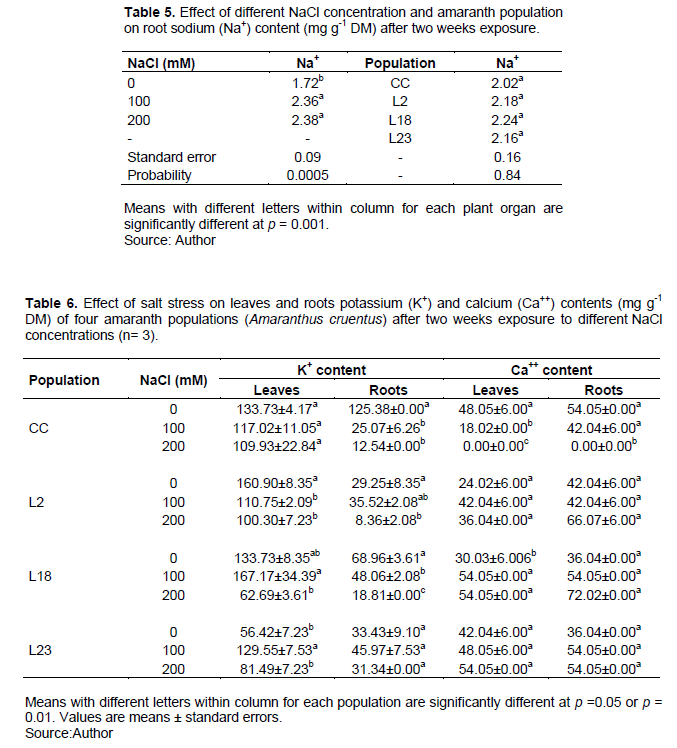
Effect of salt stress on leaf and roots Ca++ content
Salt stress induced a significant decrease in Ca++ content in leaves and roots for the control cultivar Locale, only (Table 6). No significant effect was observed for the three mutant lines except for line L18 in which a significant increase was observed in leaves. It thus appears that the three mutant lines maintained higher Ca++ absorption than the control cultivar in both leaves and roots under salt stress.
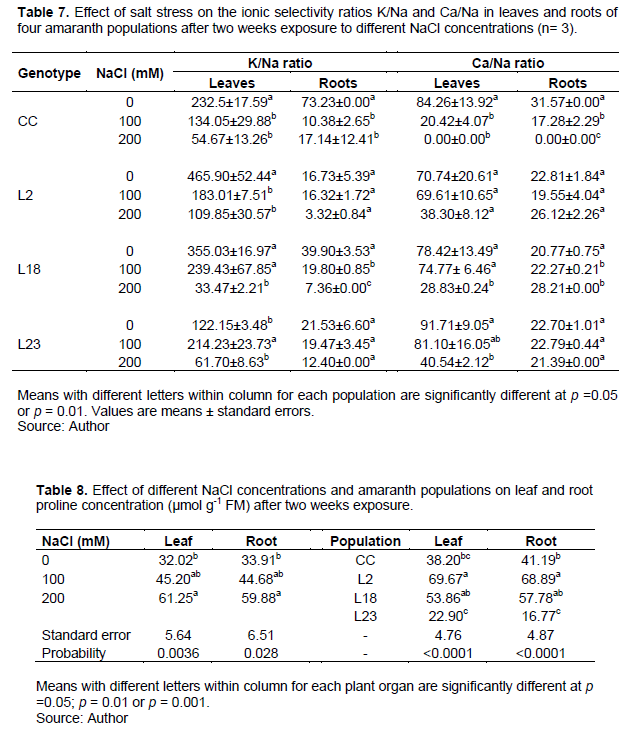
Effect of salt stress on ionic selectivity ratio
Salt stress induced a significant decrease on leaf K/Na ratio for the control cultivar and line L2 at all NaCl dose, whereas the decrease was significant only at 200 mM NaCl for line 18 (Table 7). No significant effect was observed for lines L23 at 200 mM but a significant increase was observed at 100 mM NaCl. In roots, a decrease in K/Na ratio at 100 mM NaCl was significant only for the control cultivar and line L18 (Table 7). Thus, salt stress induced a decrease in leaves K/Na ratio in all populations except for line L23 with an increase at 100 mM NaCl. In roots, no significant decrease in K/Na ratio was observed for line L2 and L23. It thus appears that line L23 maintained higher K/Na ratio in both leaves and roots than the control cultivar, followed by line L18 in leaves and by L2 in roots.
Salt stress induced a significant decrease on Ca/Na ratio in leaves for the control cultivar already at 100 mM NaCl, whereas the decrease was significant only at 200 mM NaCl for lines L18 and 23. No significant effect was observed for line L2 (Table 7). In roots, a decrease in Ca/Na ratio was significant only for the control cultivar; a significant increase was even observed for L18.
Thus, salt stress induced a decrease in leaves Ca/Na ratio for all population except for line L2. In roots no significant decrease was observed for the salt resistant mutant lines with a surpising increase for line L18. It appears that line L2 maintained higher Ca/Na ratio in leaves than the control cultivar, followed by lines L18 and L23. Line L18 maintained higher root Ca/Na ratio than the control cultivar followed by lines L2 and L23.
Proline and soluble sugars contents of leaves and roots
Two-ways ANOVA revealed a significant effect of NaCl and population on leaf and root proline as well as soluble sugars concentartions. We noticed a significant interaction between NaCl stress and population only for leaf and root soluble sugars content (Table 3). Thus, the effect of salt stress on leaf and root proline content must be analyzed independently from the population and vice-versa. However, that on leaf and root soluble sugars content must be analyzed for each amaranth population separately.
Proline content in leaves and roots
Salt stress induced an increase on leaf and root proline concentration which was significant at 200 mM NaCl (Table 8). Moreover, a significant difference was observed among populations for leaf and root proline concentrations and line L2 presented the highest concentrations either in leaf or in root followed by line L18; the lowest concentrations were observed in leaf and root of line L23. Thus, the concentrations of proline in roots and shoots were higher in L2 or L18 than the control cultivar indicating that lines L2 and L18 accumulated more proline in both leaves and roots than the control cultivar.
Soluble sugars content in leaves and roots
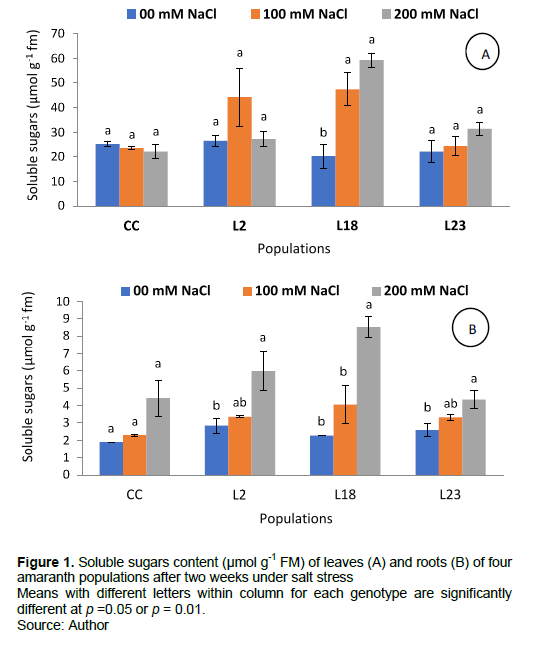
Salt stress induced a significant increase in soluble sugars content in leaves in line L18 only (Figure 1A) with the highest soluble sugars content. Thus, line L18 accumulated more soluble sugars in leaves under salt stress than the control cultivar and the two other mutant lines. In roots a significant increase was observed only in the three mutant lines at 200 mM NaCl but not at 100 mM NaCl (Figure 1B). Line L18 accumulated more soluble sugars in both leaves and roots under salt stress than the control cultivar, followed by lines L2 and 23 in roots.
DISCUSSION
Results of this study confirmed that growth reduction under salt stress was lower in lines L18 and L23, followed by line L2 than in the control cultivar Locale confirming the resistance status of these lines reported in our previous study (Atou et al., 2022). It is well known that the growth reduction of plants in response to salinity could be due to cell dehydration or to specific ionic toxicity effects, or to both parameters occurring simultaneously. The specific ionic toxicity included Na+ accumulation especially in leaves and a drastic decrease in the absorption of calcium and potassium, two major ions for an optimal cellular metabolism (Yildirim et al., 2006). No change in plant water content was observed for the four amaranth populations indicating that water content parameter is not the main aspect of salt stress effect in these populations as previously reported in amaranth cultivars (Wouyou et al., 2019).
Plants developed some physiological and biochemical mechanisms to survive, grow and produce in the presence of high salt concentrations including sodium ion exclusion and/or vacuolar compartmentation (Gouveitcha et al., 2021). The present study revealed that salt stress induced a significant increase of Na+ content in both leaves and roots in all the four evaluated populations. These results are consistent with those reported for other amaranth cultivars (Omani, 2005; Beghin, 2019; Wouyou et al., 2019; Luyckx et al., 2021; Atou et al., 2020; Estrada et al., 2021) and other plant species including pepper (Bouassaba and Chougui, 2018), tomato (Rodríguez-Ortega et al., 2019) and rice (Prodjinoto et al., 2021).
Moreover, the salt resistant line L18 accumulated more Na+ in the leaves than the control cultivar, suggesting that it is able to display a tolerance strategy towards toxic ions. One possible explanation could be that L18 efficiently compartmentalizes Na+ in the vacuole so that it cannot interfere with cytosolic enzyme activities (Chinnusamy et al., 2005). Sodium transfer to the vacuole involves numerous NHX transporters and requires additional activities of V-ATPase or PPi-ATPase (Zhang et al., 2022; Joshi et al., 2022). Mutation induced by γ-radiation may have modified regulation of gene expression coding for one of these proteins, allowing L18 to keep low cytosolic Na+ and explaining why L18 is a “sodium accumulating” line.
In contrast, another mutant line, L23 typically behaves as a sodium exclusion agent and avoid Na+ within the shoot part of the plant. According to Yang and Guo (2018) and Flowers et al. (2019), sodium exclusion is an alternative to Na+ vacuolar compartmentation. In some amaranth cultivars, Omami (2005) found that the salt tolerant population accumulated less Na+ ion in leaves than the sensitive genotype. It is now well established that Na+ enters in the root through non-selective cation channels such as NSCC. However, some specific transporters such as SOS1 may be involved in Na+ extrusion from the root to the surrounding medium. SOS1 activity is regulated by other proteins (SOS2 and SOS3): mutation occurring in genes coding for these proteins may have a positive effect on the exclusion efficiency (Malakar and Chattopadhyay, 2021). Other molecular target directly influencing Na+ translocation to the leaves could be some HKT protein involved in Na+ retrieval from the xylem sap, although such a strategy should lead to Na+ overaccumulation in the roots which was not observed in L23 in comparison to L2 or L18. It is noteworthy from our results that the amaranth salt resistant lines used in this study developed two opposite strategies (Na+ compartmentation versus Na+ exclusion) to cope with salinity. It is well known that for a considered plant species, two different salt-tolerant genotypes could use opposite strategies as reported in rice (Lutts et al., 1999) and sugarcane (Gandonou et al., 2011).
Results revealed that salt stress induced a decrease in leaves K+ content only in line L2 with surprinsingly an increase in line L23 at 100 mM NaCl. These results indicate that salt stress did not induce a systematic decrease in K+ content in leaves as generally reported in plants (Maggio et al., 2007; Shahid et al., 2011; Gouveitcha et al., 2021) and corroborated thus the report of Beghin (2019) who found that low doses of salt stimulate K+ uptake in the leaves of two varieties of amaranth. According to Gharbi et al. (2017) under low or moderate saline stress, halophyte species often tend to increase K+ uptake rather than reduce it, in order to ensure maintenance of the metabolic status of the plant. It appears from the present results that among the three salt-resistant lines, only line L23 maintained a high K+ accumulation in both leaves and roots under salt stress mainly in comparison with the control cultivar. Maintaining high K+ content in the presence of salt is a general response of the most salinity-tolerant genotypes in many plant species, and this ion is known to be a major component in osmotic adjustment under stress conditions (Wu et al., 1996). Similar results have been observed in other genotypes of amaranth (Omani, 2005; Wouyou et al., 2019; Estrada et al., 2021) and other plant species (Ibrahim et al., 2018; Gouveitcha et al., 2021). It has however to be mentioned that Na+ may also assume the function of a cheap osmoticum, thus lowering the cost of energy required for osmotic adjustment, provided Na+ is sequestered in the vacuoles (Ishikawa et al., 2022). In contrast to organic solutes, Na+ is available in the external medium and does not need to be produced. However, compartmentalization in vacuoles requires energy, although to a lower extent than proline synthesis (Munns and Gilliham, 2015). The Na+ and K+ contents alone are not an exhaustive characteristic: given the competition for transporters, salinity also induces a modification of the K+ contents. It is therefore the K/Na ratio that is an essential criterion for salt tolerance (Wu et al., 2018). The results of this study revealed that line L23 maintained higher K/Na ratio in both leaves and roots than the control cultivar, followed by line L18 in leaves and by L2 in roots indicating that the salt resistance of line L23 and L18 was due, at least partially, to their capacity to maintain high K/Na ratio in the presence of NaCl.
Salt stress induced a decrease in leaves and roots Ca++ content only in the control cultivar with surprinsingly an increase in leaves of line L18. The results of this study raise the following question: is the increase in Ca++ observed mainly in the salt-resistant Amaranthus cruentus mutant line L18 an indicator of the halophytic nature of this species as suggested by Gharbi et al. (2017) concerning K+? A decrease in Ca++ content is a general trend in plants exposed to salt stress (Rahimi and Biglarifard, 2011; R’him et al., 2013; Köster et al., 2018) and salt resistant genotypes generally maintain high supply of Ca++ under salt stress. It appears from the results that the three mutant lines maintained higher Ca++ concentration than the control cultivar in both leaves and roots under salt stress indicating that the salt resistance of these lines was due in part to their capacity to maintain high absorption of Ca++ in the presence of NaCl. A decrease in Ca/Na is a general trend in plants exposed to salt stress (R'him et al., 2013) and according to Cramer et al. (1985) in the presence of NaCl, NaCl displaces Ca++ from the plasmalemma of the root cells, which causes an increase in the membrane permeability resulting in an alteration of the selectivity ratio. This observation is of primary importance in relation to the crucial roles assumed by Ca++ in plant response to environmental stresses: it indeed assumes key function in a myriad of signal transduction pathways and is also directly influencing the hormonal status of the plant (Ma et al., 2022). Hence, maintenance of Ca++ in the presence of salt may be a tremendous advantage for the mutant lines.
Results of this study revealed that salt stress induced an increase in leaves and root proline, and that the concentrations of proline in roots and shoots were higher in L2 or L18 than the control cultivar, and that of line L23 was lower that of the control cultivar. Proline accumulation under salt stress is a common behaviour in plants (Mishra and Saxena, 2009; Bouassaba and Chougui, 2018) and it was considered to be involved in salt resistance strategy (Ehsanpour and Fatahian, 2003; Bouassaba and Chougui, 2018). The fact that the proline accumulation was higher in both leaves and roots of mutant lines L2 and L18 than in the control cultivar indicated that the salt resistance of these lines was due at least partially to proline accumulation. Besides its role in osmotic adjustment, proline was reported to assume a plethora of functions in stressed plants and was considered as an efficient antioxidant, a regulator of cytosolic pH, a protecting coumpounds acting to preserve cellular structures, a storage form of N for growth resumption after stress relief (Zhao et al., 2021).
Deciphering the precise roles of proline in our mutant line still require additional experiments, especially considering that glycine betaïne, which was not quantified in the present study, is another osmoticum able to accumulate to high concentrations in the plants from the Amaranthaceae family (Munns et al., 2020).
Salt stress induced an increase in soluble sugars contents in leaves and roots of all populations which is a common behaviour in plants exposed to salt stress. It has been postulated that growth may be more inhibited than photosynthesis on a short term basis, thus leading to passive sugar accumulation (Van Zelm et al., 2020). If this hypothesis is valid, it implies that the most sensitive plants must contain the highest sugar level. However, salt-resistant plants are known to accumulate high concentartions of soluble sugars mainly in the leaves (Bouassaba and Chougui, 2018; Kpinkoun al., 2019). The fact that the salt resistant line L18 accumulated more soluble sugars in both leaves and roots than the control cultivar and the two other mutant lines indicate that the salt resistance of this line could be related to high soluble sugars accumulation. Soluble sugars may be involved in osmotic adjustment and protection of cellular structures but this is true for non-reducing sugars such as sucrose but not for reducing ones such as hexose which induce Maillard’s reaction at high concentration (Ma et al., 2022). Hence, identification of accumulated sugars would provide interesting data regarding their roles in salt resistance of mutant lines.
The two most resistant lines on the basis of growth, namely L18 and L23, have associated at least five different strategies of resistance to salinity, while line L2 developed only two resistance strategies. These findings confirm our previous report about the relative salt resistance level of the three mutant lines (Atou et al., 2022).
Further studies are needed to better understand the response of these mutants lines to salt stress based on important physiological parameters such as photo-synthesis related parameters, stomatal conductance, transpiration, lipid peroxidation, antioxidants accumulation and activities, sodium and chloride effects discrimination.
CONCLUSION
Salt stress caused globally an increase in sodium ions, free proline and soluble sugars content and a decrease in plant growth, K/Na and Ca/Na ratios in both leaves and roots in the four amaranth populations evaluated with a significant variability among the bahaviour of the population. The response of K and Ca contents to salt stress varied greatly according to the population. The overall response of the resistant mutant lines revealed a variability among strategies used by lines to resist salt stress; the salt resistance of line L18 was due to Na+ accumulation in leaves associated to proline and soluble sugars accumulation and the maintenance of high centent of Ca++, high K/Na and Ca/Na ratios; whereas that of line L23 was due to Na+ exclusion from leaves associated to the maintenance of high centent of K+ and Ca++, high K/Na and Ca/Na ratios. Line L2 resists to salt stress via proline accumulation and maintain high Ca/Na ratio. Thus, the two most salt-resistant lines (L18 and L23) used more different strategies of resistance to salinity than line L2.
CONFLICT OF INTERESTS
The authors have not declared any conflict of interests.
REFERENCES
|
Achigan-Dako EG, Sogbohossou OED, Maundu P (2014). Current knowledge on Amaranthus spp: research avenues for improved nutritional value and yield in leafy amaranths in sub-Saharan Africa. Euphytica 197:303-317. |
|
|
Adjogboto A, Likpètè DD, Akponikpe PBI, Djenontin AJ, Baco MN, Sossa-Vihotogbe CNA, Agbossou EK (2019). What application method would be appropriate in fertilizer microdosing of traditional leafy vegetables in West Africa? Acta Horticulturae pp. 11-20. |
|
|
Almansouri M, Kinet JM, Lutts S (1999). Compared effects of sudden and progressive impositions of salt stress in three durum wheat (Triticum durum Desf) cultivars. Journal of Plant Physiology 154:743-752. |
|
|
Amukali O, Obadoni BO, Mensah JK (2015). Effects of different NaCl Concentrations on germination and seedling growth of Amaranthus hybridus and Celosia argentea. African Journal of Environmental Science and Technology 9:301-306. |
|
|
Atou A, Tonouewa G, Wouyou A, Kpochemè E, Missihoun AA, Montcho D, Ahoton L, Agbangla C, Gandonou CB (2022). Screening of Amaranth (Amaranthus cruentus L.) mutant lines for salinity tolerance. International Journal of Plant and Soil Science 34:785-797 |
|
|
Atou R, Henry E, Mensah ACG, Gouveitcha BG, Loko B, Wouyou AD, Assogba Komlan F, |
|
|
Gandonou CB (2020). Effet améliorateur d'un apport extérieur de calcium et de potassium sous différentes formes sur la tolérance à la salinité de l'amarante (Amaranthus cruentus L.). Journal of Applied Biosciences 146:15025-15039. |
|
|
Bates IS, Waldern RP, Teare ID (1973). Rapid determination of free proline for mater stress studies. Plant and Soil 39:205-207. |
|
|
Beghin C (2019). Étude de l'effet de la salinité du sol sur la valeur nutritionnelle des feuilles de Amaranthus cruentus. Faculté des Bioingénieurs, Université Catholique de Louvain, MSc Thesis. View |
|
|
Behra TK, Krishna R, Ansari WA, Aamir M, Kumar P, Kashyap SP, Pandey S, Kole C (2022). Approaches involved in the vegetable crops salt stress tolerance improvement: Present status and way ahead. Frontiers in Plant Science 12:787292. |
|
|
Bouassaba K, Chougui S (2018). Effet du Stress salin sur le comportement biochimique et anatomique chez deux variétés de piment (Capsicum annuum L.). European Scientific Journal 14:159-174. |
|
|
Chinnusamy V, Jagendorf A, Zhu JK (2005). Understanding and improving salt tolerance in plants. Crop Science 45(2):437-448. |
|
|
Cramer GR, Lauchli A, Polito VS (1985). Displacement of Ca2+ by Na+ from the plasmalema of root cells. A primary response to salt stress. Plant Physiology 79(1):207-211. |
|
|
Dansi A, Adjatin A, Adoukonou-Sagbadja H, Fa-ladé V, Yedomonhan H, Odou D, Dossou B (2008). Traditional leafy vegetables and their use in the Benin Republic. Genetic Resources and Crop Evolution 55(8):1239-1256. |
|
|
Ehsanpour A, Fatahian N (2003). Effects of salt and proline on Medicago sativa callus. Plant Cell, Tissue and Organ Culture 73(1):53-56. |
|
|
Estrada Y, Fernández-Ojeda A, Morales B, Egea-Fernández JM, Flores FB, Bolarín MC, Egea I (2021). Unraveling the strategies Used by the Underexploited Amaranth Species to Confront Salt Stress: Similarities and Differences with Quinoa Species. Frontiers in Plant Science 12:604481. |
|
|
Flowers TJ, Glenn EP, Volkov V (2019). Could vesicular transport of Na+ and Cl- be a feature of salt tolerance in halophytes? Annals of Botany 123(1):1-18. |
|
|
Gandonou CB (2020). Situation de reference de la salinité de l'eau d'irrigation utilisée dans la zone d'intervention du projet, Rapport d'activité, projet PADMAR-Bénin 24 p. |
|
|
Gandonou CB, Bada F, Abrini J, Skali Senhaji N (2011). Free proline, soluble sugars and soluble proteins concentrations as affected by salt stress in two sugarcane (Saccharum sp.) cultivars differing in their salt tolerance. International Journal Biological and Chemical Sciences 5:1488-1493. |
|
|
Gharbi E, Martínez JP, Benahmed H, Hichri I, Dobrev PI, Motyka V, Quinet M, and Lutts S (2017). Phytohormone profiling in relation to osmotic adjustment in NaCl-treated plants of the halophyte tomato wild relative species Solanum chilense comparatively to the cultivated glycophyte Solanum lycopersicum. Plant Science 258:77-89. |
|
|
Gouveitcha MBG, Mensah ACG, Montcho Hambada D, Assogba Komlan F, Gandonou CB (2021). Réponse au stress salin de quelques cultivars de gombo (Abelmoschus esculentus L. Moench) produits au Bénin au stade jeune plant. Journal of Applied Biosciences 161:16616-16631. |
|
|
Gupta B, Huang B (2014) Mechanism of salinity tolerance: physiological, biochemical and molecular characterization. International Journal of Genomics, Article ID: 701596. |
|
|
Henry EY, Kinsou E, Mensah ACG, Komlan AF, Gandonou CB (2021). Réponse des plantes de tomate (Lycopersicon esculentum Mill.) cultivées sous stress salin à une application exogène de calcium et de potassium. Journal of Applied Biosciences 159:16363-16370. |
|
|
Ibrahim MEH, Zhu X, Zhou G, Ali AYA, Ahmad I, Farah GA (2018). Nitrogen fertilizer alleviated negative impacts of NaCl on some physiological parameters of wheat. Pakistan Journal of Botany 50(6):2097-2104. |
|
|
Ishikawa T, Shabala L, Zhou M, Venkataraman G, Yu M, Sellamuthu G, Chen Z-H, Shabala S (2022). Comparative analysis of root Na+ relation under salinity between Oryza sativa and Oryza coartata. Plants 11:656. |
|
|
James B, Atcha-Ahowé C, Godonou I, Baimey H, Goergen H, Sikirou R, Toko M (2010). Integrated pest management in vegetable production: A guide for extension workers in West Africa. IITA. |
|
|
JMP Pro SAS Institute, (2009). JMP® 8. User Guide, Second Edition. Cary, NC: SAS Institute Inc. Cary, NC, USA. |
|
|
Joshi S, Nath J, Singh AK, Pareek A, Johi R (2022). Ion transporters and their regulatory signal transduction mechanisms for salinity tolerance in plants. Physiologia Plantarum 174:e13702. |
|
|
Khan MN, Siddiqui M, Mohammad F, Naeem M, Khan MM (2010). Calcium chloride and gibberellic acid protect linseed (Linum usitatissimum L.) from NaCl stress by inducing antioxidative defence system and osmoprotectant accumulation. Acta Physiologiae Plantarum 32:121-132. |
|
|
Kinhoégbè G, Djèdatin G, Loko LEY, Favi AG, Adomou A, Agbangla C, Dansi A (2020). Onfarm management and participatory evaluation of pigeonpea (Cajanus cajan [L.] Millspaugh) diversity across the agroecological zones of the Republic of Benin. Journal of Ethnobiology and Ethnomedicine 16:1-21. |
|
|
Köster P, Wallrad L, Edel KH, Faisal M, Alatar AA, Kudla J (2018). The battle of two ions: Ca2+ signalling against Na+ stress, Plant Biology 21(S1):39-48. |
|
|
Kpinkoun KJ, Zanklan AS, Assogba-Komlan F, Mensah CGA, Montcho D, Kinsou E, Gandonou GB (2019). Évaluation de la résistance à la salinité au stade jeune plant de quelques cultivars de piment (Capsicum spp.) du Benin. Journal of Applied Biosciences 133:13561-13573. |
|
|
Lavini A, Pulvento C, d'Andria R, Riccardi M (2016). Effects of saline irrigation on yield and qualitative characterization of seed of an amaranth accession grown under Mediterranean conditions. Journal of Agricultural Science 154:858-869. |
|
|
Lutts S, Bouharmont J, Kinet JM (1999). Physiological characterization of salt-resistant rice (Oryza sativa) somaclones. Australian Journal of Botany 47:835-849. |
|
|
Lutts S, Kinet JM, Bouharmont J (1995). Changes in plant response to NaCl during development of rice (Oryza sativa L.) varieties differing in salinity resistance. Journal of Experimental Botany 46:1843-1852. |
|
|
Lutts S, Kinet JM, Bouharmont J (1996). Effects of salt stress on growth, mineral nutrition and proline accumulation in relation to osmotic adjustment in rice (Oryza sativa L.) cultivars differing in salinity resistance. Plant Growth Regulation 19:207-218. |
|
|
Luyckx A, Beghin C, Quinet M, Achadé B, Prodjinoto H, Gandonou CB, Lutts S (2021). Salinity differently affects antioxidant content and amino acid profile in two cultivars of Amaranthus cruentus differing in salinity tolerance. Journal of the Science of Food and Agriculture 101:6211-6219. |
|
|
Ma L, Liu X, Lv W, Yang Y (2022). Molecular mechanisms of plant responses to salt stress. Frontiers in Plant Science 13:934877. |
|
|
Maggio A, Raimondi G, Martino A, De Pascale S (2007). Salt stress response in tomato beyond the salinity tolerance threshold. Environmental and Experimental Botany 59:276-282. |
|
|
Malakar P, Chattopadhyay D (2021). Adaptation of plants to salt stress: the role of ion transporters. Journal of Plant Biochemistry and Biotechnology 30:668-683. |
|
|
Manaa A, Gharbi E, Mimouni H, Wasti S, Aschi Smiti S, Lutts S, Ben Ahmed H (2014). Simultaneous Application of Salicylic Acid and Calcium Improves Salt Tolerance in Two Contrasting Tomato (Solanum lycopersicum) Cultivars. South African Journal of Botany 95:32-39. |
|
|
Mishra N, Saxena P (2009). Effect of salicylic acid on proline metabolism in lentil grown under salinity stress. Plant Science 177:181-189. |
|
|
Munns R, Gilliham M (2015) Salinity tolerance and crops - what is the cost? New Phytologist 208(3):1-11. |
|
|
Munns R, Passioura JB, Colmer TD, Byrt CS (2020). Osmotic adjustment and energy limitations to plant growth in saline soil. New Phytologist 225:1091-1096. |
|
|
Olaniyi JO, Adelasoye KA, Jegede CO (2008). Influence of nitrogen fertilizer on the growth, yield and quality of grain amaranth varieties. World Journal of Agricultural Sciences 4:506-513. |
|
|
Omami EN (2005). Response of Amaranth to salinity stress, Ph. D Thesis, University of Pretoria, South Africa 235 p. |
|
|
Prodjinoto H, Irakoze W, Gandonou C, Lepoint G, Lutts S (2021). Discriminating the impact of Na+ and Cl− in the deleterious effects of salt stress on the African rice species (Oryza glaberrima Steud.). Plant Growth Regulation pp. 1-19. |
|
|
Qin L, Guo S, Ai W, Tang Y, Cheng Q, Chen G (2013). Effect of salt stress on growth and physiology in amaranth and lettuce: Implications for bioregenerative life support system. Avances in Space Research 51(3):476-482. |
|
|
R'him T, Tlili I, Hnan I, Ilahy R, Benali A, Jebari H (2013). Effet du stress salin sur le comportement physiologique et métabolique de trois variétés de piment (Capsicum annuum L.). Journal of Applied Biosciences 66:5060-5069. |
|
|
Rahimi A, Biglarifard A (2011). Influence of NaCl and different substracts on plant growth, mineral nutrient assimilation and fruit yield os strawberry. Notulae Botanicae Hoti Agrobotanica Cluj-napoca 39:219-226. |
|
|
Rodríguez-Ortega WM, Martínez V, Nieves M. (2019) Agricultural and physiological responses of tomato plants grown in different soilless culture systems with saline water under greenhouse conditions. Scientific Reports 9:6733. |
|
|
Ruan CJ, Silva JAT da, Mopper S, Qin, P, Lutts S (2010). Halophyte improvement for a salinized world. Critical Reviews in Plant Sciences 29:329-359. |
|
|
Shahid MA, Muhammad A, Pervez MA, Balal RM, Ahmad R, Ayyub CM, Abbas T, Akhtar N (2011). Salt stress effects on some morphological and physiological characteristics of okra (Abelmoschus esculentus L.). Soil and Environment 30(1):66-73. |
|
|
Shrivastava P, Kumar R (2015). Soil salinity: A serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi Journal of Biological Sciences 22(2):123-131. |
|
|
Van Zelm E, Zhang Y, Testernik C (2020). Salt tolerance mechanisms in plants. Annual Review of Plant Biology 71:403-433. |
|
|
Wouyou A, Gandonou CB, Assogba Komlan F, Montcho D, Zanklan SA, Lutts S, Gnancadja SL, (2017). Salinity resistance of five amaranth (Amaranthus cruentus) cultivars at young plants stage. International Journal of Plant and Soil Science 14:1-13. |
|
|
Wouyou A, Prodjinoto H, Zanklan AS, Vanpee B, Lutts S, Gandonou CB (2019). Implication of ions and organic solutes accumulation in amaranth (Amaranthus cruentus L.) salinity resistance. American Journal of Plant Sciences 10(12):2335-2353. |
|
|
Wu SJ, Ding L, Zhu JK (1996). SOS 1, a Genetic Locus Essential for Salt Tolerance and Potassium Acquisition. Plant Cell 8:617-627. |
|
|
Wu H, Zhang X, Giraldo JP, Shabala S (2018). It is not all about sodium: revealing tissue specificity and signalling roles of potassium in plant responses to salt stress. Plant and Soil 431(1):1-17. |
|
|
Yang Y, Guo Y (2018). Unraveling salt stress signaling in plants. Journal of Integrative Plant Biology 60:796-804. |
|
|
Yemm W, Willis AJ (1954). The Estimation of Carbohydrates in Plant Extracts by Anthrone. Biochemical Journal 57:508-514. |
|
|
Yildirim E, Taylor AG, Spittler TD (2006). Ameliorative effects of biological treatments on growth of squash plants under salt stress. Sciencia Horticulturae 111:1-6. |
|
|
Zhang J, Xu T, Liu Y, Chen T, Zhang Q, Li W, Zhou H, Zhang Y, Zhang Z (2022) Molecular insights into salinity responsiveness in contrasting genotypes of rice at the seedling stage. International Journal of Molecular Science 23:1624. |
|
|
Zhao S, Zhang Q, Liu M, Zhou H, Ma C, Wang P (2021). Regulation of plant responses to salt stress. International Journal of Molecular Sciences 22(9):4609. |
|
Copyright © 2024 Author(s) retain the copyright of this article.
This article is published under the terms of the Creative Commons Attribution License 4.0