ABSTRACT
The study investigated the potentials of alkaloidal fraction of leaf and stem of Crotalaria retusa with a view to understanding its mechanism of allelopathy in bean (Phaseolus vulgaris) seedlings. The study involved collection of C. retusa (leaves and stems), identification, dried, pulverized and extracted with methanol to yield methanolic extract (MECR). Crude alkaloid fraction was prepared from MECR by a procedure that consisted of acidification, basification and extraction with chloroform. Brown beans were grown in varying concentrations (0, 50 and 100 µg/ml) of crude alkaloid fraction with strychnine (10 µg/ml) as reference alkaloid. Leaves and stems of the seedlings were collected for biochemical analyses which included determination of percentage germination and evaluation of biochemical parameters. The results showed that percentage germination of bean seeds reduced with increasing concentrations of alkaloid fraction. The activities of antioxidant enzymes (superoxide dismutase and catalase) increased with alkaloid concentration in the leaves and stems of bean seedlings. The levels of metabolites (proline, reduced glutathione and ascorbic acid) increased significantly (p ≤ 0.05) in the stems and leaves of treated bean seedlings. However, there was reduction in the total protein and sugar contents of the leaves and stems of bean seedlings which implied stress. Alkaloidal fractions of C. retusa elicit significant allelopathic effects on germination of bean seedlings by reducing its germination rate. The alkaloid also affected some metabolites in bean seedlings which are markers of environmental stress. The study revealed that the mechanisms of action of the alkaloid fraction of C. retusa involved the induction of oxidative stress that resulted in the generation of reactive oxygen species (ROS) that caused metabolic derangement in the bean seedlings.
Key words: Alkaloidal fraction, allelopathy, strychnine, basification, extract, antioxidant enzymes.
Lambers et al. (1998) defined allelopathy as the growth suppression of one plant species by another due to the release of toxic compounds. Kohli et al. (1998) and Singh et al. (2001) opined that allelopathy refers to any direct or indirect effect of plants on other plants through the release of chemicals and plays an important role in many agro-ecosystems. Allelopathy is a form of positive and negative interaction among organisms that is caused by the action of chemical compounds referred to as allelochemicals (Rice, 1984). These compounds are produced mainly as a result of the secondary metabolism of plants and microorganisms (bacteria, viruses and fungi) and can influence several processes in ecosystems and agroecosystems (Olofsdotter et al., 2002). Allelochemicals can lead to different mechanisms of action in plants. An allelopathic effect is mainly referred to as a type of negative interaction (Radosevich et al., 2007; De Albuquerque et al., 2011), but positive interactions have also been reported, depending on the allelochemical considered, the target plant and the concentration tested (Eichenberg et al., 2014). Chemicals that impose allelopathic influences are called allelochemicals and included alkaloids, benzoxazinones, cinnamic acid and its derivatives, cyanogenic compounds, ethylene, bioregulators and flavonoids which had been isolated from over 30 families of terrestrial and aquatic plants and possess actual or potential phytotoxicity (Putnam, 1988). Rainfall causes the leaching of allelopathic substances from leaves that fall to the ground during period of stress, leading to inhibition of growth and germination of crop plants (Rice, 1974; Mann, 1987; Duke et al., 2000).
Other mechanisms through which allelopathic substances are released to the environment include root exudation, volatilization, residue decomposition, and other processes in both natural and agricultural systems (Rathinasabapathi et al., 2005). The readily visible effects of allelochemicals on the growth and development of plants include inhibition or retardation of germination rate; darkened and swollen seeds; reduced root or radicle and shoot or coleoptiles extension, swelling or necrosis of root tips, curling of the root axis, discoloration, lack of root hairs; increased number of seminal roots, reduced dry weight accumulation, and lowered reproductive capacity (Rice, 1974). Allelochemicals reduce or inactivate the physiological activity of plant hormones, which then inhibit the normal physiological process of plants. He and Lin (2001) found that hydroxyl benzoic acid, polyphenols and other compounds could affect the decomposition process of indole acetic acid and gibberellins. In addition, aqueous extracts of Oryza sativa increase indole acetic acid oxidase activity of receptor plants, and then reduce the level of indole acetic acid (Zeng et al., 2001). Crotalaria retusa Linn is an annual herbaceous plant growing up to 1 m tall. It produces bright yellow flowers that are borne on an upright spike above a mass of jade green leaves (Plate 1). The seeds become loose in the pod as they mature, and rattle when the pod is shaken (Wagner et al., 1999). It grows in various places around the world (600 or more species of Crotalaria are described worldwide, mostly from the tropics; at least 500 species are known from Africa).

As a legume, it supports nitrogen fixing bacteria and is considered a “soil builder.” However, it is poisonous to cattle (like many legumes) (McMullen, 1999). The common names of the plant include rattle weed, shak-shak, and rattlebox, rattle pods, and wedge-leaf. C. retusa has been used medicinally for a number of purposes. The seeds are used in ethno-medicine for treatment of fever, as vermifuge and as antispasmodic agent (Nuhu et al., 2009). The leaves are excellent remedies for the treatment and management of Ptyalism (excessive secretion of saliva during pregnancy), diarrhea, scabies and impetigo. The powdered seeds boiled with milk are used for enhancing body strength, curing skin diseases, leprosy, flatulence and fever (Warrier et al., 1994). Monocrotalline, an alkaloid isolated from C. retusa elicits anti-tumor, anti-neoplastic, cardio depressant, hypotensive properties and serves as a defensive agent against predators (Morris et al., 1999, Umerie et al., 2010).Studies have revealed that the plant grows very rapidly and suppresses nearby plants which is a form of allelopathy. The study is designed to investigate the mechanism of suppression of the growth of other nearby plants, with a view to identifying the chemical(s) responsible for suppression and its toxicity.
Collection and identification of plant materials
Fresh leaves and stems of C. retusa were collected from Ajebamidele Area, beside Henry Alex- Duduyemi Memorial College (HAMC), Old Ibadan Road, Ile Ife, Osun State. Nigeria. The plant was identified and authenticated at IFE Herbarium, Department of Botany, Obafemi Awolowo University, Ile-Ife, Nigeria where specimen copy was deposited and specimen identification number IFE 17302 was obtained.
Reagents and chemicals
All the reagents used in the study were of analytical grade, adrenaline, trichloroacetic acid, 5, 5'-dithiobis-2-nitrobenzoic acid, toluene and hydrogen peroxide were obtained from Sigma-Aldrich Laboratories, Saint Louis, Switzerland. Phenol-crystals and sodium dihydrogenorthophosphoric acid were obtained from British Drug House (BDH), Chemicals Limited, Poole, England.
Collection of brown beans (Phaseolus vulgaris)
Brown beans (P. vulgaris) used were obtained from Sabo Market in Ile Ife, Osun State, Nigeria.
Preparation of C. retusa methanolic extract (CRME)
The methanolic extract of C. retusa was prepared by soaking 350 g of the powdered plant material in 800 ml of 70% (v/v) methanol for 48 h. The methanolic extract was collected by decantation and the residue was drained. The residue was subjected to further extraction 5 times using the same solvent and period until the extract became colourless. The supernatants were combined, filtered and centrifuged at 2,500 rpm for 15 min on a Bench Centrifuge (Model 800D Microfield Instrument, Essex, England). The combined extract was concentrated to dryness at 35°C under reduced pressure on Edward High Vacuum Pump, (Model ED-100 Edward Vacuum, Crawley, England). The dried residue termed (CRME) was stored in the desiccator until analyzed further.
Extraction of C. retusa alkaloidal fraction
The extraction of alkaloid mixture from the methanolic extract was carried out according to the procedure described by Djilani et al. (2006) as reported by Fasaanu et al. (2013) with slight modifications. The extraction involved dissolution of 95 g of CRME in 200 ml of 10% (v/v) HCl, this was filtered and basified with 1 M sodium hydroxide solution to a pH of 9. The resulting solution was extracted with chloroform (100 ml x 4) until the aqueous layer tested negative for alkaloid. The chloroform fractions were pooled and washed with distilled water to neutral pH (7) to remove non-freebase materials. The pooled chloroform fractions were dried over anhydrous sodium sulphate and concentrated to dryness at 35°C on a rotary evaporator. The residue which was termed crude alkaloid mixture was collected, weighed and stored in the dessicator until further analyzed.
Bean seeds germination and analysis
Bean seeds were grown, harvested and analyzed according to the procedure of Fasaanu et al. (2013) with Strychnine (10 µg/ml) as reference alkaloid. Typically, 1 g alkaloid was dissolved in a total of 50 ml distilled water to give final concentration of 20 mg/ml. The working solutions were prepared and diluted to give concentrations of 50 and 100 µg/ml. Bean seeds were sterilized with 2% (v/v) sodium hypochlorite for 15 min at room temperature and then washed with distilled water three times. Fifteen uniform seeds were grown in 800 ml capacity plastic containers filled to three quarter with loamy soil for each treatment. The planting was carried out in triplicate for all the four groups and doused with varying concentrations of alkaloid mixture. The planting was carried out two times. The reference alkaloid (strychnine, 10 µg/ml) was used and distilled water in control. After four days of planting, germination percentages were calculated. The stems and leaves were collected on the 4th, 5th and 15th day of growth and stored in the freezer for subsequent analyses.
Preparation of homogenates of leaf and stem of bean seedlings
Homogenates of leaf and stem of bean seedlings used for the biochemical estimations were as follows:
(i): Superoxide dismutase (SOD) activity: 0.2 g of leaf and stem were homogenized separately in 5 ml of ice cold phosphate buffer (0.1 M, pH 7.4), centrifuged at 1,500 rpm at room temperature for 10 min in a Model 92 Bench centrifuge. The supernatant was collected into clean vial bottles for SOD assay.
(ii): Catalase activity: 0.5 g of leaf and stem were separately homogenized in 5 ml of 0.1 M phosphate buffer (pH 7.0), centrifuged at 1,500 rpm at room temperature for 10 min.
(iii): Proline estimation: 0.5 g of leaf and stem were separately homogenized with pre-washed sand using mortar and pestle with 5 ml 3% (w/v) sulphosalycylic acid. The homogenates were centrifuged at 4,000 rpm for 10 min. The supernatant was collected and used for the analysis.
(iv): Reduced glutathione estimation: The homogenates of stem and leaf of bean seedlings were prepared by separately homogenizing 0.2 g of each in 2.5 ml of 5% (w/v) TCA. The homogenates were centrifuged at 1000 rpm for 10 min. The supernatant (0.1ml) was used for the estimation of GSH.
(v): Protein concentration estimation: Leaf (500 mg) and stem (500 mg) were separately weighed and homogenized in 10 ml of 0.1 M phosphate buffer (pH 7.4), centrifuged at 4,000 rpm for 10 min. The supernatants were collected and used for protein estimation.
(vi): For total soluble sugar concentration estimation, leaf (500 mg) and stem (500 mg) were separately homogenized with 10 ml of 80% (v/v) ethanol. The homogenates were centrifuged at 2000 rpm for 20 min.
Biochemical analyses
Biochemical analyses on the homogenates were carried out as follows: Estimation of total proteins (Lowry et al., 1951), total sugars (Dubois et al., 1956); ascorbic acid (Omaye et al., 1979); reduced glutathione (Moron et al., 1979); proline (Bates et al., 1973); and anti-oxidant enzyme activities assays superoxidase (Misra and Fridovick, 1972); catalase (Claiborne 1985).
Statistical analysis
All data were expressed as mean ± SEM, n=5. Statistical analysis of data was performed using a one-way analysis of variance (ANOVA) followed by Duncan Multiple Range Test (Tallarida and Murray, 1981). P values <0.05 were considered statistically significant.
The study reported the allelopathic potential of alkaloid fraction of C. retusa with a view to investigating the mode by which the plant affects the nearby plants and the likely involvement in the suppression activity of the plant on neighboring plants. Crude alkaloid of C. retusa was extracted by a combined procedure of acidification, basification and chloroform extraction. Moreover, chemical tests and thin layer chromatography (TLC) analysis of crude C. retusa alkaloid revealed only one or single spot on the chromatogram which implied that the plant might contain only one type of alkaloid. Moreover, physical analysis with Erhlich reagents revealed the alkaloid was a typical pyrrolizidine type in nature (Mattocks and Jukes, 1986; Witabouna and Brahima, 2012). Germination of P. vulgaris was inhibited by the alkaloid fraction of C. retusa (Figure 1). The results revealed that percentage seedling germination was concentration dependent. It decreases with increasing concentrations of alkaloid of C. retusa. In the control, the percentage germination was 100%, in group II (50 µg/ml) 69.3%; in group III (100 µg/ml) 40%; while the reference alkaloid (Strychine, 10 µg/ml) gave 73.3% germination, respectively.
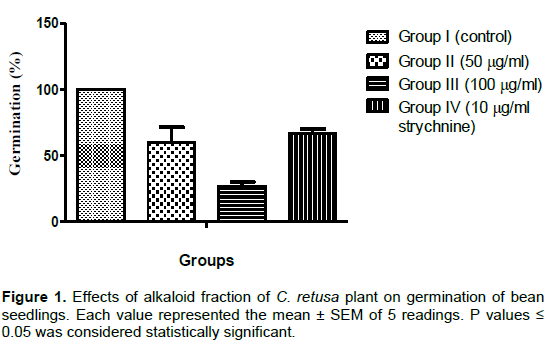
Germination is a complex process which involves imbibition, that is, uptake of water and oxygen for activation of nutrients, enzyme systems and oxidative phosphorylation, formation of enzyme systems, metabolism of stored compounds, radical emergence and seedling growth (Yoshida and Hirasava, 1997; Raven et al., 2005). C. retusa alkaloid might affect one or more of these processes thereby causing derangement in the germination of bean seedlings. This observation agreed with the findings of Hussein et al. (2011) in which Lantana camara leaf extract decreased the germination of Bidens pilosa and that of Fasaanu et al. (2013) on the allelopathic activity of Dioscorea dometrum alkaloid on bean seedlings and tomato seeds. Antioxidant defenses are extremely important as they represent the direct removal of free radicals. Superoxide dismutase (SOD), is located in the chloroplasts, and catalyzes the conversion of superoxide anion, (O2-) to H2O2 and O2 and plays a key role in quenching reactive oxygen species (ROS) (Vijayavel et al., 2004). In the present study a dose-dependent increase in SOD activity in groups treated with alkaloid fraction of C. retusa was observed, as well as the group treated with strychnine (Tables 1 and 2). The increase in SOD activity might be linked to an increase in superoxide radical formation as well as to de novo synthesis of enzyme (Verma and Dubey, 2003), which in turn could be associated with an induction of SOD genes by superoxide-mediated signal transduction (Fatima and Ahmad, 2005). The up regulations of SODs is implicated in combating oxidative stress due to biotic and abiotic stresses and have a critical role in the survival of plants under stressful environment. Significant increase in SOD activity under salt stress has been observed in various plants viz. mulberry (Harinasut et al., 2003), Cicer arietinum (Kukreja et al., 2005) and Lycopersicon esculentum (Gapinska et al., 2008).
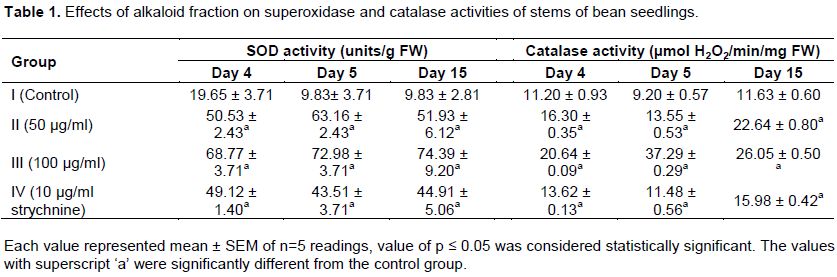
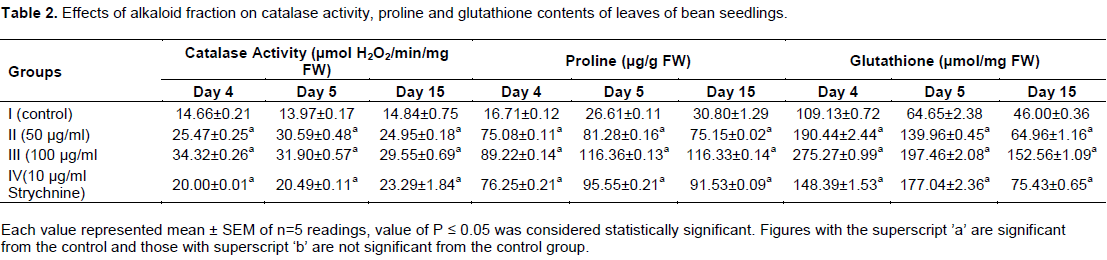
Catalase (CAT) is an enzyme that scavenges the active oxygen species in plant cells. It participates actively in the main defense system against accumulation and toxicity of hydrogen peroxide and plays the role of controlling hydrogen peroxide level in cells (Zhang et al., 2007). Catalase detoxifies hydrogen peroxide and converts it to water and oxygen. Catalase activity increased in a dose-dependent manner upon application of the alkaloid fraction of C. retusa in this study (Tables 1 and 2), this might be due to corresponding generation of hydrogen peroxide in the different plant tissues. Similar results (Xiao-Jun et al., 2013) were obtained when stem and root of aqueous extracts of Paeonia decomposita were applied to growing wheat seedlings. Reduced glutathione (GSH) is one of the crucial metabolites in plants which are considered an important intracellular defense against ROS-induced oxidative damage. It plays a central role in several physiological processes, including regulation of sulphate transport, signal transduction, conjugation of metabolites, detoxification of xenobiotics and the expression of stress-responsive genes (Xiang et al., 2001; Mullineaux and Rausch, 2005). In this present study, increased glutathione content was obtained in both the leaves and the stems of bean seedlings upon alkaloid fraction application (Tables 2 and 3). This could be due to oxidative stress imposed as a result of treatment. This result was similar to the observation of Pietrini et al. (2003) who showed that enhanced antioxidant activity in the leaves and chloroplast of Phragmites australis Trin. (cav.) ex Steudel was associated with a large pool of GSH which resulted in protecting the activity of many photosynthetic enzymes against the thiophilic bursting by Cadmium (Bharwana et al., 2013).
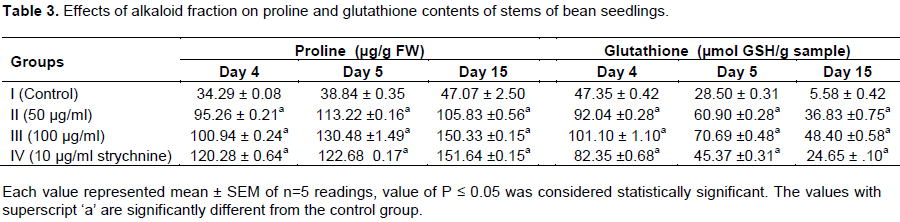
Proline, an imino amino acid, is well known to accumulate in wide variety of organisms ranging from bacteria to higher plants on exposure to abiotic stress (Ahmad et al., 2006). Proline also acts as a major reservoir of energy and nitrogen, which could be used in resuming the growth after stress removal (Chandrashekhar and Sandhyarani, 1996). It has been proposed to act as an osmo protectant, a protein stabilizer, a metal chelator, an inhibitor of lipid peroxidation, and OH.and1O2 scavenger (Ashraf and Foolad, 2007; Trovato et al., 2008), it was also shown to stabilize the structure of macromolecules and organelles (Mallika and Tinkari, 2012). In this study, a dose – dependent accumulation of proline was observed in the leaves and stems of the bean seedlings upon the application of alkaloid fraction of C. retusa (Tables 2 and 3). This might be due to increased synthesis of proline or its decreased degradation. Enhanced synthesis of proline under drought or salt stress has been implicated as a mechanism to alleviate cytoplasmic acidosis and maintain NADP+: NADPH at values compatible with metabolism (Hare and Cress, 1997; Gusti et al., 2012). It was noted that alkaloid fraction of C. retusa exhibited significant allelopathic effects on the germination and growth of bean seedlings. Accumulation of proline in the plant seedling might have been due to induction of oxidative stress resulting from the allelopathic effects of alkaloid of C. retusa. Moreover, the study showed a dose-dependent reduction in the protein content of both the leaves and stems of P. vulgaris upon application of the alkaloid fraction of C. retusa (Tables 4 and 5). The reduction in protein content might be due to dysfunction of protein synthesis machinery caused by oxidative damage. It was shown that phenolic acids, an allelochemical decreased the incorporation of certain amino acids into proteins and thus affected the rate of protein synthesis (Baziramakenga et al., 1997).
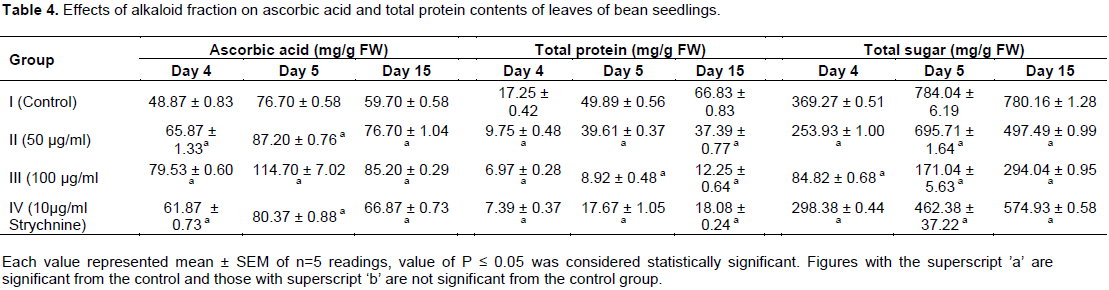
Mersie and Singh (1993) also demonstrated that ferulic acid, inhibited protein synthesis and reduced the incorporation of (14C) leucine. Protein synthesis involves three stages namely initiation, elongation and termination of the polypeptide chain (Alberts, 2008). The blocking of any one of these stages by an allelochemical would cause inhibition of protein and nucleic acid syntheses (Hassall, 2003). Ascorbic acid is the most abundant, powerful and water soluble antioxidant which directly scavenged superoxide, hydroxyl radicals, singlet oxygen and converts H2O2 to water via ascorbate peroxidase reaction (Smirnoff, 2005; Athar et al., 2008). In the present study, ascorbic acid contents increased under alkaloid fraction treatment. Ascorbic acid occurs in all plant tissues, being higher in photosynthetic cells, meristems and some fruits. Its concentration is reported to be highest in mature leaves with fully developed chloroplasts and chlorophylls. It has been reported that ascorbic acid mostly remains available in reduced form in leaves and chloroplast under normal physiological conditions (Smirnoff, 2000). The observed increase in ascorbic acid contents of leaves and stems in this study (Tables 4 and 5) could be due to the oxidative stress imposed by the alkaloid fraction. Yang et al. (2008) reported that high light condition and drought significantly increased the ascorbic acid content in Picea asperata seedlings. The total soluble sugar contents were reduced in all the treated groups (Tables 4 and 5) which could be due to derangement of the photosynthetic apparatus of the plant. The results are in agreement with the observations of Masoodi et al. (2013) that aqueous extracts of Populus deltoides and Ulmus wallichiana reduced the soluble sugar contents of maize, beans and sunflower. Conclusively, alkaloidal fractions of C. retusa elicit significant allelopathic effects on germination of bean seedlings by reducing its germination rate. The alkaloid also affected some metabolites in bean seedlings which are markers of environ-mental stress. The study revealed that the mechanisms of action of the alkaloid fraction of C. retusa involved the induction of oxidative stress that resulted in the generation of reactive oxygen species (ROS) that caused metabolic derange-ment in the bean seedlings.
The authors have not declared any conflict of interest.
REFERENCES
|
Ahmad P, Sharma S and Srivastava PS (2006). Differential physio biochemical responses of high yielding varieties of mulberry (Morus alba) under alkalinity (Na2CO3) stress in vitro. Physiol. Mol. Bio. Plants 12:59-66.
|
|
|
|
Alberts B (2008). Molecular Biology of the Cell, 5e. New York: Garland Sci.
|
|
|
|
|
Ashraf M, Foolad MR (2007). Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 59:206-216.
Crossref
|
|
|
|
|
Athar HR, Khan A, Ashraf M (2008). Exogenously applied ascorbic acid alleviates salt-induced oxidative stress in wheat J. of Environ. Exp. Botany 63: 224-231.
Crossref
|
|
|
|
|
Bates SR, Waldran RP, Teare ID (1973). Rapid Determination of Free Proline for Water Stress Studies. Plant and Soil 39:205-208.
Crossref
|
|
|
|
|
Baziramakenga R, Leroux GD, Simard RR, Nadeau P (1997). Allelopathic effects of phenolic acids on nucleic acid and protein levels in soybean seedlings Canad. J. Bot. 75:445-450.
Crossref
|
|
|
|
|
Bharwana SA, Farooq MA, Abbas F, Ahmad MSA (2013). Alleviation of Lead Toxicity by Silicon is Related to Elevated Photosynthesis, Antioxidant Enzymes Suppressed Lead Uptake and Oxidative Stress in Cotton. J. Bio. Bio. 4172:2155-6199
|
|
|
|
|
Chandrashekhar KR, Sandhyarani S (1996). Salinity induced chemical changes in Crotalaria striata DC. Ind. J. Plant Physiol. 1:44-48.
|
|
|
|
|
Claiborne A (1985). Catalase activity. In: Handbook of Methods for Oxygen Radical Research (R. A. Greenwald,Ed). Boca Raton, FL, pp. 283-284.
|
|
|
|
|
De Albuquerque MB, Santos RC, Lima LM, Melo Filho PA, Nogueira RJMC, Da Câmara CAG and Ramos AR (2011). Allelopathy, an alternative tool to improve cropping systems. A review. Agron Sustain Dev. 31:379-395.
Crossref
|
|
|
|
|
Djilani A, Legseir B, Soulimani R, Dicko A, Younos C (2006). New Extraction Technique for Alkaloids. J. Braz. Chem. Society 17:513-520.
Crossref
|
|
|
|
|
Dubois M, Gilles KA, Hamilton JK, Rebers PA and Fred S (1956). Colorimetric methods for determination of sugars and related substances. J. Anal. Chem. 28: 350-356.
Crossref
|
|
|
|
|
Duke SO, Dayan FE, Romagni JG, Rimando AM (2000). Natural products as sources of herbicides: current status and future trends. Weed Research 40: 99–111.
Crossref
|
|
|
|
|
Eichenberg D, Ristok C, Kroeber W, Bruelheide H (2014). Plant Polyphenols-Implications of Different Sampling, Storage and Sample Processing in Biodiversity-Ecosystem Functioning Experiments. Chem Ecol. 30:676–692.
Crossref
|
|
|
|
|
Fasaanu OP, Oziegbe M, Oyedapo OO (2013). Investigations of Activities of Alkaloid of Trifoliate Yam (Dioscoreadumetorum) (Kunth) Pax. Ife J. Sci.15:251-262.
|
|
|
|
|
Fatima RA, Ahmad M (2005). Certain antioxidant enzymes of allium cepa as biomarkers for the detection of toxic heavy metals in wastewater. Sci. of the Total Environ. 346:256-273.
Crossref
|
|
|
|
|
Gapinska M, Sklodowska M, Gabara B (2008). Effect of short- and long-term salinity on the activities of antioxidative enzymes and lipid peroxidation in tomato roots Acta Physiol. Plant 30:11-18.
Crossref
|
|
|
|
|
Gusti MA, Didik I, Prapto Y, Bambang DK, Rukmini K (2012). Physiological Responses of Jatrophato Drought Stress in Coastal Sandy Land Conditions. Makara J. of Sci. 16/2:115-121.
|
|
|
|
|
Hare PD, Cress WA (1997). Metabolic implications of stress-induced proline accumulation in plants. J. Plant Growth Regulation 21:79-102.
Crossref
|
|
|
|
|
Harinasut P, Poonsopa D, Roengmongkol K, Charoensataporn R (2003). Salinity effects on antioxidant enzymes in mulberry cultivar. Sci. Asia 29:109-113.
Crossref
|
|
|
|
|
Hassall, KA. (2003). The Biochemistry and Uses of Pesticides. Food. Sci. and Nut. 47:231-258.
|
|
|
|
|
He HQ, Lin WX (2001). Studies on Allelopathic Physiobiochemical Characteristics of Rice. China J. Eco-Agric. 9:56-57.
|
|
|
|
|
Hussein F, Ghulam S, Sher Z and Ahmad B (2011). Allelopathy by Lantana camara (L), Pakis. J. Bot. 43(5):2373-2378.
|
|
|
|
|
Kohli RK, Batish D, Singh HP (1998). Allelopathy and its implications in agroecosystems. J. Crop Prod. 1:169–202.
Crossref
|
|
|
|
|
Kukreja S, Nandval AS, Kumar N, Sharma SK, Sharma SK, Unvi V, Sharma PK (2005). Plant water status, H2O2 scavenging enzymes, ethylene evolution and membrane integrity of Cicerarietinum roots as affected by salinity. Plant Bio. 49:305-308.
Crossref
|
|
|
|
|
Lambers H, Chapin III, FS, Pons TL (1998). Plant Physiological Ecology. Springer-Verlag, Berlin pp. 231-243.
Crossref
|
|
|
|
|
Lowry OH, Farr AL, Randall RJ (1951). Protein Estimation with Folin Ciocalteau Reagent. J. Biol. Chem. 193:265-275.
|
|
|
|
|
Mallika M, Tinkari D (2012). Early Seedling Growth and Accumulation of Proline and Phenol in Trigonellafoenum-graecum Under Heavy Metal Stress. Int. J. Sci. Res. (3):2319-7064.
|
|
|
|
|
Mann SY (1987). Influence of axis removal on amino-, carboxy-, and endopeptidase activities in cotyledons of germinating Vignamungoseeds. Plant Cell Physiol. 25:547-554.
|
|
|
|
|
Masoodi TH, Islam MA, Wani MA, Mir SA and Gangoo SA (2013). Allelopathic effect of Populusdeltoidesand Ulmuswallichianaon biochemical constituentsof maize, bean and sunflower. Karnataka J. Agric. Sci. 26(2):265-267.
|
|
|
|
|
Mattocks AR and Jukes P (1986). Chemistry of Sulphur-Bound Pyrrolic Metabolites in the Blood of Rats Given Different Types of Pyrrolizidine Alkaloids. Nat. Tox. 1:89-95.
Crossref
|
|
|
|
|
McMullen CK (1999). Flowering Plants of the Galapagos. Ithaca, New York, USA: Comstock Publisher Association., 370pp.
|
|
|
|
|
Mersie W, Singh M (1993). Phenolic acids affect photosynthesis and protein synthesis by isolated leaf cells of velvet-leaf. J. Chem. Ecol. 19: 1293-1301.
Crossref
|
|
|
|
|
Misra HP, Fridovich I (1972). The role of Superoxide anion in the auto-oxidation of epinephrine and a simple assay for Superoxide dismuthase. Biochem. J. 247:3170-3175.
|
|
|
|
|
Morris JB (1999). Perspectives on New Crops and New Uses. Edited (Janicle, J.). (ASHS, Press Alexandria, VA, USA). pp. 196-201.
|
|
|
|
|
Moron MS, Depierre JW, Manneryik B (1979). Levels of Glutathione, reductase and glutathione –S-transferase activities in rat lung and liver. Biophysics Acta 582:67-78.
Crossref
|
|
|
|
|
Mullineaux PM, Rausch T (2005). Glutathione, photosynthesis and the redox regulation of stress-responsive gene expression. Photosynthesis Res.86:459-474.
Crossref
|
|
|
|
|
Nuhu H, Abdurrahman EM, Shok M (2009). Ethnomedical Studies of Crotalaria Species found in Zaria, Northern Nigeria. Nig. J. Pharm. Sci. 8(2):54-58.
|
|
|
|
|
Olofsdotter M, Jensen LB, Courtois B (2002). Improving crop competitive ability using allelopathy – an example from rice. Plant Breeding. 121:1-9.
Crossref
|
|
|
|
|
Omaye ST, Tornbull TO, Sauberlich HE (1979). Selected Methods for the Determination of Ascorbic Acids in Cell, Tissue and Fluids. Methods Enzymol. 62:3-11.
Crossref
|
|
|
|
|
Pietrini F, Iannelli MA, Pasqualini S, Massacci A (2003). Interaction of cadmiumplant abiotic stress resistance Environ. Exp. Bot. 59:206-216.
|
|
|
|
|
Putnam AR (1988). Allelochemicals from plants as herbicides. J. Weed Technol. 2:510-518.
Crossref
|
|
|
|
|
Radosevich SR, Holt JS, Ghersa C (2007). Ecology of weeds and invasive plants: relationship to agriculture and natural resource management. New York: Wiley; P 454.
Crossref
|
|
|
|
|
Rathinasabapathi B, Ferguson J, Gal M (2005). "Evaluation of Allelopathic Potential of Wood Chips for Weed Suppression in Horticultural Production Systems." Hort. Sci. 40:711-713.
|
|
|
|
|
Raven PH, Ray F, Evert SE (2005). Biology of Plants, 7Th Edition. New York: Freeman WH and Company Publishers. Pp 504-508.
|
|
|
|
|
Rice EL (1974). Allelopathy. Academic Press, New York, 353pp.
|
|
|
|
|
Rice EL (1984). Allelopathy, 2nd edition. Rice, E.L., editor. Orlando: Academic Press; P 422.
|
|
|
|
|
Singh HP, Kohli RK, Batish DR (2001). Allelopathy in agro-ecosystems: an overview. J. Crop Prod. 4:1-41.
Crossref
|
|
|
|
|
Smirnoff N (2000). Ascorbic Acid: Metabolism and Function of a Multifaceted Molecule, Plant Biol. 3:229-235.
|
|
|
|
|
Smirnoff N (2005). Ascorbate, tocopherol and carotenoids: metabolism, pathway engineering and functions. In Smirnoff,N. (Ed.), Antioxidants and Reactive Oxygen Species in Plants, Blackwell Publishing Ltd., Oxford, UK, pp. 53-86.
Crossref
|
|
|
|
|
Tallarida, R, Murray RB (1981). Manual of Pharmacologic Calculations. Springer-Verlag New York. pp. 206-210.
Crossref
|
|
|
|
|
Trovato M, Mattioli R, Costantino P (2008). Multiple roles of proline in plant stress tolerance and development. RendicontiLincei 19:325-346.
Crossref
|
|
|
|
|
Umerie SC, Okonkwo IF, Nwadialor NA, Okonkwo JC (2010). Studies on the Oil and Nutritive Value of Seeds of Crotalaria retusa L. (Fabaceae). Pak. J. Nutr. 9:912-914.
Crossref
|
|
|
|
|
Verma S, Dubey RS (2003). Lead Toxicity induces Lipid Peroxidation and alters the Activities of Antioxidant Enzymes in Growing Rice Plants. Plant Sci. 164:645-655.
Crossref
|
|
|
|
|
Vijayavel K, Gomathi RD, Durgabhavani K, Balasubramanian MP (2004). Sublethal effect of naphthalene on lipid peroxidation and antioxidant status in the edible marine crab Scylla serrata. J. Marine Pollution Bull. 48:429-433.
Crossref
|
|
|
|
|
Wagner WL Herbst DR, Shomer SH (1999). Manual of the Flowering Plants of Hawaii, Honolulu, Hawaii USA. Bishop Museum Press pp. 1919-1925.
|
|
|
|
|
Warrier PK (1994). Pyrrolizidine Alkaloids in Plants. J. of Ind. Phytochem. 2:123-130.
|
|
|
|
|
Witabouna MK, Brahima K (2012). Qualitative Analysis of the Pyrrolizidine Alkaloids from 11 Asteraceae and Boraginaceae Used in Traditional Medicine in Cote d'Ivoire. Res. J. Phytochem. 6:75-83.
Crossref
|
|
|
|
|
Xiang C, Werner BL, Christensen EM, Oliver DJ (2001). The biological functions of glutathione revisited in Arabidopsis transgenic plants with altered glutathione levels. Plant Physiol.126:564-574.
Crossref
|
|
|
|
|
Xiao-Jun Y, Hui-Xing S, Guang-Li L, Qi-Bing C (2013). Allelopathic Effects of Paeoniadecompositaon Seed Germination and Protective Enzymes Activities of Wheat. J. Med. Plants Res. 7(16):1057-1062.
|
|
|
|
|
Yang Y, Han C, Liu Q, Lin B, Wang J (2008). Effect of drought and low light on growth and enzymatic antioxidant system of Piceaasperata seedlings. Acta Physiologiae Plantarum 30:127-134.
Crossref
|
|
|
|
|
Yoshida I, Hirasawa E (1997). Gibberellin induces endopeptidase activity in detached cotyledons of Pisum sativum L. Plant Growth Reg. 19:55-63.
Crossref
|
|
|
|
|
Zeng RS, Luo SM, Shi YH (2001). Physiological and biochemical mechanism of allelopathy of secalonic acid on higher plants. Agron. J. 93: 72-79.
Crossref
|
|
|
|
|
Zhang LZ, Wei N, Wu QX, Ping ML (2007). Anti-oxidant response of CucumissativusL. to fungicide carbendazim. Pesticide Biochem. Physiol.89:54-59.
Crossref
|
|