Full Length Research Paper
ABSTRACT
This research investigates relationships between resistances with heat and work. It is completely proven that the ratio of work to temperature for the realistic process is no less than that for the reversible process. The equalities and inequalities on the heat, work and ratio of work to temperature could be applied to the gravitational field and chemical reactions. The relationships between path functions and state functions are studied in the chemical reactions. Some criteria for spontaneous directions have been suggested such as the equalities and inequalities on the heat, work, and ratio of work to temperature, except the Clausius inequality and must be ordinarily obeyed in the spontaneous process.
Key words: First law of thermodynamics, Clausius inequality, thermodynamic functions, chemical reactions, gravitational field, resistances, ratio of work to temperature.
INTRODUCTION

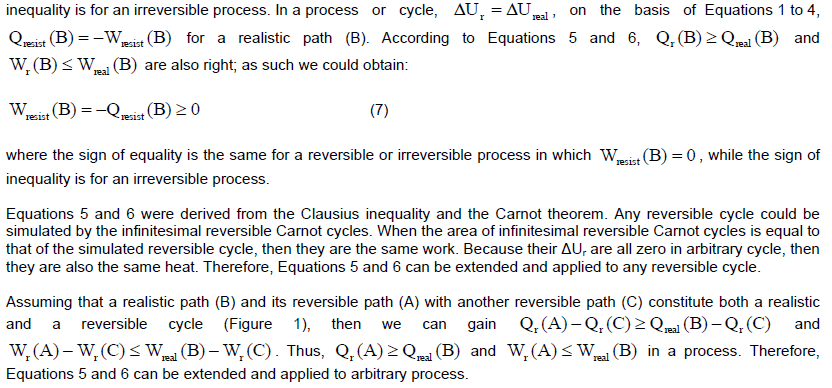
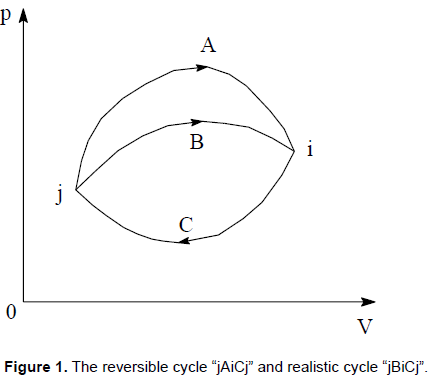
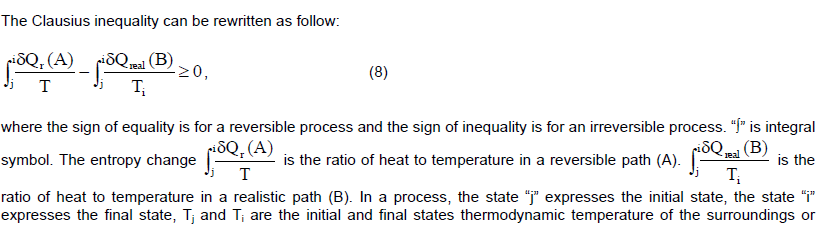



CONCLUSION
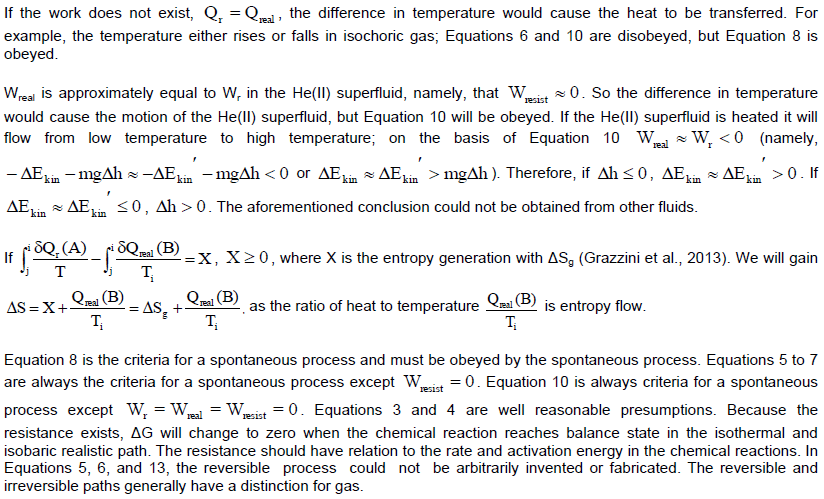
CONFLICT OF INTERESTS
REFERENCES
|
Atkins P, Paula J (2014). Atkins' physical chemistry, tenth ed. Oxford University Press, Oxford. pp. 45-130. |
|
|
Borgnakke C, Sonntag RE (2014). Fundamentals of thermodynamics. 8nd ed. John Wiley & Sons Singapore Pte. Ltd., Singapore. pp. 35-496. |
|
|
de Abreu R, Guerra V (2012). Introducing thermodynamics through energy and entropy. Am. J. Phys. 80(7):627-37. |
|
|
DeVoe H (2013). A comparison of local and global formulations of thermodynamics. J. Chem. Educ. 90(5):591-597. |
|
|
Gislason EA, Craig NC (2013). Criteria for spontaneous processes derived from the global point of view. J. Chem. Educ. 90(5):584-590. |
|
|
Grazzini G, Borchiellini R, Lucia U (2013). Entropy versus entransy. J. Non-Equilib. Thermodyn. 38:259-271. |
|
|
Jacobs P (2013). Thermodynamics. Imperial College Press, London. pp. 1-52. |
|
|
Jin C (2016). The inequality of work derived from Clausius inequality and Carnot theorem. 2016 International Conference on Energy, Power and Electrical Engineering. Atlantis Press, Paris. pp. 315-317. |
|
|
Lee S, Lee K, Lee J (2015). An alternative presentation of the second law of thermodynamics. J. Chem. Educ. 92(4):771-773. |
|
|
Marcus Y (2013). Internal pressure of liquids and solutions. Chem. Rev. 113:6536-6551. |
|
|
Muschik W (2014). Contact temperature and internal variables: A glance back, 20 years later. J. Non-Equilib. Thermodyn. 39(3):113-121. |
|
|
Nieto R, González C, Jiménez Á, López I, Rodríguez J (2011). A missing deduction of the Clausius equality and inequality. J. Chem. Educ. 88 (5):597–601. |
|
|
Sandler SI, Woodcock LV (2010). Historical observations on laws of thermodynamics. J. Chem. Eng. Data. 55(10):4485-4490. |
|
Copyright © 2024 Author(s) retain the copyright of this article.
This article is published under the terms of the Creative Commons Attribution License 4.0