Full Length Research Paper
ABSTRACT
A 4000 L bio-digester was designed and fabricated using 5 mm mild steel plate with the main purpose of generating biogas and bio-fertiliser for Auchi Polytechnic Demonstration Farm. To achieve this, a varying ratio of 1.155 m3: 2.310 m3 mixtures of cow dung and water were anaerobically digested in the batch digester. The experiment lasted for 33 days during which the quantity of gas generated were evaluated. The volume of daily methane gas production ranged from 0 to 1.2 m3. The total volume of gas generated from the digestion was 21.68 m3 and comprised of 58% CH4, 25% CO2, 15% H2S and 2% other impurities. The results of physiochemical properties of the feedstock revealed a progressive increase of 6.0-6.2 in pH value from Day 4 after which it dropped sharply in value to 5.5 on the second week of fermentation. The minimum and maximum ambient temperatures of 24 and 30°C were observed while the slurry temperatures varied from 27 to 35°C. The average pressure built up of the digester was about 214,000 N/m2. The results further showed that biogas production is sensitive to pH and cannot produce optimally below the pH of 6.5. To achieve optimum biogas generation, a temperature range within mesophilic condition of 25 to 40°C and pH values of 7 should be maintained.
Key words: Biogas, mesophilic temperature, retention period, anaerobic digestion, digester.
INTRODUCTION
Nigeria is endowed with a lot of natural resources which comprises fossil fuel e.g. natural gas and coal that is, (petroleum) for power generation and other enormous purposes. The production and utilization of these resources is facing critical challenges. Nigerian government over the years have applied different technical approaches and as well invested huge amount of monies in the energy sector but substantial progress in solving the energy crisis in the country is yet to be made. The government of the day can no longer provide the required energy need and other agricultural input like fertilizers to her citizens. The rapid increase in population is suspected to have aggravated the situation.
Unlike other developed countries of the world, provision of adequate energy to her citizen is not a major problem. The major challenge is on how to harness the energy source which is environmental friendly and ecologically balanced. This need has forced the world to search for other alternative sources of energy since the energy sources like the solar, hydro, wind etc. require huge economical investment and technical power to operate, which is very difficult for the developing country like Nigeria.
According to Mshandete and Parawira (2009), Nigeria produces about 227,500 tons of fresh animal waste daily. This shows that Nigeria can potentially produce about 6.8 million m3 of biogas every day from animal waste only if properly managed. Mitel (1996) also reported that the sludge obtained from bio-fermentation process contains high concentration of nutrients and organic matter. The application of this sludge at the rate equivalent to traditional chemical fertilizer increase the yield of maize up to 35.7%, wheat 12.5%, rice 5.9%, cotton 27.5%, carrot 14.9% and spinach 20.6%.
In the present economic recession in the country, biogas energy can be one of the most reliable, easily available and economically feasible sources of alternative and renewable energy which can be managed by locally available materials and simple technology for both urban and rural dwellers. The biogas system also provides a barrier protecting ground water from contamination with untreated waste (Ocwieja, 2010). Furthermore, with the enormous cattle population in the country, millions of tonnes of dung released daily emit a lot of methane gas to the atmosphere, which is 320 times more harmful to human health than carbondioxide (Thakur, 2006). A biogas plant is an anaerobic digester that produces biogas and natural fertilizer from animal, food waste or plant waste. Although, biogas plant is not a new technology to many developed and some few developing countries of the world, in Nigeria the technology is still on skeletal basis. Biogas can provide a clean, easily controlled source of renewable energy from organic waste materials for a small labour input. This will go long way to replace firewood or fossil fuels which are becoming more expensive as supply falls below demand. Biogas is generated when bacteria degrade biological material in the absence of oxygen, in a process known as anaerobic digestion. Since biogas is a mixture of methane (also known as marsh gas or natural gas, CH4), hydrogen sulphide (H2S) and carbon dioxide. It is a renewable fuel produced from waste treatment.
This system produces two extremely useful products from the waste: biogas and slurry. The use of biogas for cooking and lighting reduces the strain on the environment by decreasing the use of biomass and the production of greenhouse gases. The objective of this study was to design, fabricate and evaluate batch biogas digester for generation of Biogas and natural fertilizer from cow dung for utilization in Auchi Polytechnic Agricultural Engineering Technology Demonstration Farm.
MATERIALS AND METHODS
Selection of site and fabrication materials for the digester
The project site was carefully selected, designed and constructed based on factors affecting digester installation and optimum gas generation among others according to the guidelines documented by Republic of Rwanda (2012). The material selected for the fabrication of the digester was a 5 mm galvanized steel sheet because it is cost effective and as well absorbs heat easily when compared to cement and brick. The material was folded and arc welding was carried out in order to fabricate fermentation digester and other component parts. The tank was pressure tested before taken to site for use, after coating with a black paint to aid heat retention within the walls of the digester. The major components of the digester (Plate 1) include:
Inlet chamber: It is a 75 mm diameter 5 mm galvanize steel pipe having half metre in length, which was fixed at an angle that allows the feedstock to move into the digester. A pipe reducer having an inner diameter of 8 mm and outer diameter of 150 mm was used as funnel.
Outlet chamber: This is the chamber through which the slurry after the digestion is moved out. It was made by a 75 mm diameter 5 mm galvanize steel pipe and placed at an angle that allows the slurry come out easily.
Digester body: This is the place where the anaerobic digestion takes place. The properly mixed feedstock was fed into the digester body and after the digestion process; slurry goes outside through the outlet or effluent chamber. The digester body was made of a 5 mm thick galvanized steel material.
Gas holder: The biogas formed after the anaerobic digestion was collected on the top of the plant, called gas holder. This was also made of the same material with the body of the digester.
Gas outlet: The biogas which was present in the gas holder was taken using the gas outlet, which was a gas valve connected with a reducer. The gas valve is opened when it is to be used. The gas valve was made of brass material.
Stirrer: It was fixed inside the digester through the top of gas outlet for intermittent stirring of the slurry to speed up fermentation and gas production.
Compost pits: These pits were also constructed to remove the spent slurry from the digester tank to the outlet chamber where it was finally used as bio fertilizer for crop use.
Potassium hydroxide (KOH) and Potassium permanganate (KMnO4): Both were used for absorbing carbon dioxide (CO2) and Hydrogen Sulphide (H2S) contained in the biogas respectively.
Gas cylinder: It was used for collection of purified methane gas.
Thermometer: The thermometer was employed to measure ambient and slurry temperature variations during digestion of the substrate.
Pressure gauge: It was used to measure pressure built-up during gas fermentation.
pH meter: The instrument was used for measuring pH values.
Design calculation
Amount of Total Solid (TS) in the slurry
TS= 8.5% of slurry (1)
Amount of Volatile Solid (VS) in the slurry
VS= 0.8TS (2)
Where TS = amount of Total Solid in the slurry
Substrate input (Sd)
Sd = B + w (m3/d) (3)
Where Sd = Substrate input
B = Biomass (organic matter)
w = water
Hydraulic retention time (HRT)
HRT was determined by chosen/given digesting temperature. Since the temperature of the environment varies from 25 to 35°C (mesophilic digestion). Therefore, HRT of 33 days was chosen. In a cattle-dung plant, the retention time was calculated by dividing total volume of the digester by volume of input added daily.
Digester volume (Vd)
Vd = Sd x RT (m3/day x number of days) (4)
Where Sd = daily substrate input quantity, RT= chosen retention time.
Daily gas production, G
The amount of gas generated each day, G (m3gas/d), was calculated on the basis of the specific gas yield, Gy of the substrate and the daily substrate input, Sd. The calculation was based on standard gas yield values per cattle unit
where G = Daily gas production, x = No. of cow that generate the biomass (organic matter) for the study, y = average wt. of dung (organic matter) estimated to be produced by each cow on daily basis (that is, cow produce 10 kg of manure).
Specific gas production Gp
It was calculated according to the following equation
Where Gp = Specific gas production, G = daily gas production, Vd = Digestive volume.
Digester loading, Ld
The digester loading, Ld was calculated from the daily total solid input:
Where LdT =Digester Loading,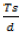 = daily total solids input, Vd = digestive volume.
= daily total solids input, Vd = digestive volume.
Volume of gas holder Vg
To minimize the size and to keep the cost as low as possible, the gasholder was not built to accommodate a full day gas production on the basis that the gas will be used throughout the day and the gasholder will never be allowed to reach full capacity. The gasholder was designed to hold between 60 to 70% of the volume of the total daily gas production, G. For the purpose of this research, the volume of gasholder, Vg designed to hold the gas was 65% × daily gas production.
Gas holder capacity (C)
Where C = Gas holder capacity, Vg = Gasholder volume, G = daily gas production.
Biogas yield
Biogas yield was determined using the equation reported by Arthur (2004), expressed as:
Where Gy = Biogas yield 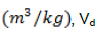 ,= Digestive volume
,= Digestive volume 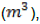 , and FS= Mass of feed stock (kg)
, and FS= Mass of feed stock (kg)
Volumetric capacity Vd
Where: Vd = volume (m3), r = radius (m), h = height (m) and 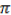 = 3.142
= 3.142
Cylindrical volume of digester
Conical volume of digester (Cd)
Where: r = radius (m), h = height (m) and 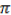 = 3.142
= 3.142
pH
Report from literature showed that a pH range of 6.8 to 7.2 gives optimum yield of biogas (Olaoye et al., 2014; Dobre et al., 2014; Mateescu, 2016). For the purpose of this research a pH value of 7 was maintained.
Temperature regulation
The temperature range between the mesophilic temperatures (20 to 40°C) is the best range for producing biogas (Olaoye et al., 2014; Sibiya et al., 2014; Ukpai et al., 2015; Mir et al., 2016). For the purpose of this research, a temperature range of 20 to 35°C was maintained throughout the research to avoid the effect of ambient temperature influencing the temperature of the slurry. Summary of design calculation for the digester plant is shown in Table 1.
Sample collection and experimental details
A total quantity of 1155 kg (1.155 m3) fresh cow dung was collected from Aviele and Auchi abattoirs in sealed drums respectively for the experiment. This was further mixed with water in the ratio of 1:2 in the mixing tank to make an approximately final 3465 kg (3.465 m3) slurry that was fed into the digester through the inlet chamber as shown in Plate 2. When the digester was filled to 80% of its total volume, the introduction of slurry was stopped after mixing 5 kg of seeders (anaerobic bacteria) to speed the rate of fermentation. The plant was closed and then the slurry was stirred with the manual stirrer incorporated in the digester plant on a daily basis to speed up the gas production. The experiment was allowed to run for 33 days in batch fermentation during and after which the following were carried out:
(i) Volume of gas produced was recorded daily.
(ii) The temperature of the digester content was taken once daily.
(iii) The pH of the digester content was taken once daily.
(iv) Measurement of the retention time (time between the commencement of gas production and termination of the experiment).
(v) Measurement of the amount of gas produced daily during the experiment.
(vi) Analysis of the gas to separate it to its different components.
Analysis of gas to evaluate its contents
A gas delivery pipe fixed with rubber hose was connected from the digester to two 1000 ml gas absorbers flask containing 25 g each of potassium hydroxide and potassium permanganate (KMnO4) dissolved in 250 ml distilled water to obtain a 10% solution for CO2 absorption and H2S respectively along the pipe. The gas collection bag was connected to the moisture trap container containing water for gas collection over water which was finally connected to gas cylinder to collect methane gas under high pressure. The digester was maintained at room temperature and pH was monitored with a pH meter connected to a sampling point. Weights of both flasks and gas cylinder were then measured, using an electronic scale on daily basis.
As the biogas flowed, potassium hydroxide solution absorbed CO2, while potassium permanganate (KMnO4) absorbed H2S. The remaining unabsorbed gas was collected as methane. After, a period of 33 days the pressure continues to remain unchanged, the set up was disconnected and weights of flask with their solutions were again taken. The difference in weights of (flask + solution) from the initial readings gave the mass of H2S and CO2 absorbed; while the increase in mass of the collecting bag indicated the mass of methane in the gas. The procedure was repeated twice, in each case; fresh solutions of potassium permanganate (KMnO4) and potassium hydroxide were prepared. The sketch for the setup is shown in Figure 1 whereas the results of gas analysis are presented in Table 2.
RESULTS AND DISCUSSION
The analysis of the compositions of biogas generated comprised of 58% CH4, 25% CO2, 15% H2S and 2% other impurities as presented in Table 1. The compositions of the biogas is in collaboration with work of Dahunsi and Oranusi (2013) who reported biogas compositions of CH4, CO2, H2S and other impurities to be 58, 24 and 18% respectively. Figure 2 depicts the results of biogas generated during the fermentation, pressure built-up and pH values whereas Figure 3 shows the relationship between volume of daily methane gas production and temperatures (ambient and slurry) during and after the organic fermentation.
Volume of biogas produced during and after the fermentation
Methane gas production started on 10th day of detention producing 0.7 m3 of methane biogas as shown in Figure 2. The methane gas production followed an increasing trend on the 11th day of organic digestion with the value of 1.02 m3 and reduced to 0.84 m3 on the 12th day after which the methane gas production continued to increase reaching the peak range values of 1.17, 1.18 and 1.2 m3 on the 21st, 22nd and 23rd day respectively. Thereafter the volume continued to drop gradually for the rest of the study period until it finally fell back to 0.08 m3.The delay in the production of gas till the 10th day asserts that cow dung contains fibrous materials that takes time to degrade which is in line with the report of Babatola (2008).
From the analyses, the increase in methane gas production per unit time from the 21st day onwards to 23rd can be attributed to the effects of a more settled bacteria culture due to increase in ambient and operating temperatures. The breaks or nonlinearity of gas production in some days during the fermentation period showed that there may be possibility of unfavorable ambient condition and temperature fluctuation among others that influenced methane producing bacteria which is a major factor in biogas yield (CSANR, 2012; Ubwa et al., 2013). The lower biogas yield at the beginning and at the end of digestions is attributed to the fact that biogas production rate in batch condition directly corresponds to time and specific growth rate of methanogenic bacteria in the bio-digester (Gupta et al., 2009; Rabah et al., 2010). More so, about 70% of the methane is produced from acetate-consuming organisms that are slow-growing and highly sensitive to changes in the environment.
Relationship between volume of daily gas production and pH value against detention time
The initial pH was 5.8 with a decrease in value of 5.5 to 5.4 on the second and third day of fermentation as shown in Figure 2. A progressive increase in pH value was observed from Day 4 after which a sharp drop in value was noticed on the second week of organic fermentation. The highest pH value was recorded on the 17th day of the experiment as 7.3 when gas production has started yielding. A final pH value of 6.34 was recorded at the end of the experiment. The effect of increase in pH value of organic matter in the digester was that it reduced the growth of microbes which resulted to lower gas production. When the digester pH value is 7.2 or lower, NH4+ is favoured. When the digester pH value is greater than 7.2, NH3 is favoured. Ammonia-nitrogen concentration beyond 1500-3000 mg/L is not only inhibitory; but creates an additional problem of foam and scum generation. On the other hand, presence of ammonia facilitates the regulation of pH and may by that means prevent volatile fatty acids (VFA) inhibition which can lead to system failure (Vidal et al., 2000).
The initial drop in pH values for second and third day of detention may have influenced the activities of aerobes and facultative aerobes to produce relevant acidic metabolites, which are acted on by methanogenic bacteria to produce methane. The highest biogas yields were observed at digester pH value of 7.2 which is in the close range of the findings of Report No. ETSU B 1118, (1986); Mahanta et al. (2004) and Wise (1987), who stated that an optimum biogas production is achieved when the pH value of input mixture in the digester is the range of 6.25 and 7.50.
According to de Mes et al. (2003), production rate of methane is lower for pH values outside the range of 6.5 to 7.5. If pH is maintained within the optimum range of 6.8-7.2, the percentage of methane in the produced biogas will be at its maximum (Ghaly, 1996). The pH value in a biogas digester is also a function of the retention time. In the initial period of fermentation, as large amounts of organic acids are produced by acid forming bacteria, the pH value inside the digester can decrease below 5. This hinders or even stops the fermentation process. Methanogenic bacteria are very sensitive to pH value and do not thrive below a value of 6.5.
Relationship between volumes of daily gas produced and Pressure built-up against detention time
The pressure built up of the digester (Figure 2) ranged from 0 to 480,000 N/m2 whereas the average pressure of the digester was 214,000 N/m2. The maximum methane gas was produced on the 23rd day of organic fermentation which is 132,000 N/m2. The maximum daily methane biogas production at pressure of 172,000, 170,000, 176,000 and 162,000 N/m2 were observed to be 1.16, 1.17, 1.18 and 1.20 m3 respectively. It was discovered that methane forming bacteria works best in the pressure of about 110,000 to 120,000 N/m2. The volume of methane gas obtained per day decreased with increasing pressure. The gas composition was also affected by increasing the digester pressure.
As also seen from Figure 2, the methane content increased daily reaching a maximum at the digester pressure of 162,000 N/m2, after which the methane content tended to have a constant value. This can be attributed to the increase in carbon dioxide dissolution in the liquid slurry with increasing pressure. Thus, the noted decrease in the amount of gas obtained was partially compensated for by the increase in the methane content. It should also be noted that high concentrations of pressure inside the digester, increases the dead slurry volume present in the outlet chamber. Since the gas generated from this portion of slurry was generally not collected, it would thus represent a loss and contribute to the prevailing decrease in gas production rate.
Relationship between volume of daily methane gas production and temperatures (ambient and slurry) during and after digestion of the organic matter
Figure 3 shows the ambient and slurry temperature variations for the detention period of 33 days. The minimum and maximum ambient temperatures of 24 and 30°C were observed while the slurry temperatures varied from 27 to 35°C which shows that both temperatures were within the mesophilic range (30 to 40°C). The result of the analysis shows that slurry temperatures were higher than ambient temperatures in most of the days.
Thus, the highest amount of daily methane biogas generated was 1.2 m3 on the 21st day of digestion at 35°C. The higher temperatures produced increased the rate of digestion of the slurry, thereby leading to increased gas generation. The gas yield depends on the ambient temperature and frequency of agitation of the substrate in the biogas plant. The higher the temperature, the shorter digestion time needed to attain a specific rate of biogas production. When the digester temperature is maintained at 25°C, it takes approximately 50 days for digestion of cattle waste. But, if the temperature ranges between 32 and 38°C is maintained, digestion is completed within 28 days (Babatola, 2008). Digestion at high temperature range (30 to 40°C) supports higher rates of biological degradation and biogas production (Itodo et al., 2002). But, raising the temperature above 40°C will lead to inhibition of methane production
(Rajeshwari et al., 2000). A shock change in temperature may also lead to pH drop in the reactor. This drop is due to temperature inhibition of methanogenesis, leading to accumulation of fermentation products (Leitão et al., 2005).
CONCLUSION AND RECOMMENDATIONS
It is evident from the study that the total volume of gas generated from the digestion was 21.68 m3 and comprised of 58% CH4, 25% CO2, 15% H2S and 2% other impurities. The optimal efficiency of anaerobic digestion and gas production depend on the intensity of bacterial activity, which is influenced by several factors such as ambient temperature, temperature of digester material, loading rate, hydraulic retention time and pH value of digester content. The results showed that upper limit of the mesophilic range gives a higher biogas yield. The optimum temperature observed from the experiment was 40°C. Therefore, to achieve optimal biogas production, it is expected that a high temperature range within mesophilic condition of 25 to 40°C be maintained.
Thirdly, it was observed that a pH of 7 gave favourable condition for bacterial growth and better biogas yield in the digester when compared to other pH values. Evaluation of the effect of dead slurry volume present in the outlet chamber is important since the gas generated from this portion of slurry is generally not collected. Above all, for efficient performance of the biogas plant, it is necessary to regulate all the above factors suitably. The analysis of the effluent slurry indicates that it is rich in nutrients and can be used as an organic fertilizer.
CONFLICT OF INTERESTS
The authors have not declared any conflict of interests.
ACKNOWLEDGEMENTS
The authors would like to thank the Managements of Tertiary Education Trust Fund (TETFUND) Abuja and Auchi Polytechnic, Auchi for providing the needed funding for this research work. This type of collaboration is crucial for sustainable development at the tertiary education level in Nigeria.
REFERENCES
|
Arthur M (2004). Biogas Production from Stillage (Scientific, Technical and Economic Aspects): University of Luneburg, Germany. |
|
|
Babatola JO (2008). Comparative Study of Biogas Yield Pattern in Some Animal and Household Wastes, African Research Review. An International Multi-Disciplinary Journal 2(4):54-68. |
|
|
Center for Sustaining Agriculture and Natural Resources (CSANR) (2012). Climate Friendly Farming. Center for Sustaining Agriculture and Natural Resources, Research Report 2012. |
|
|
Dahunsi SO, Oranusi US (2013). Co-digestion of Food Waste and Human Excreta for Biogas Production. British Biotechnology Journal 3(4):485-499. |
|
|
de Mes TZD, Stams JH, Reith JH, Zeeman G (2003). Methane production by anaerobic digestion of wastewater and solid wastes. Bio-methane & Bio-hydrogen 58-102. |
|
|
Dobre P, Nicolae F, Matei F (2014). Main factors affecting biogas production - an overview: Romanian Biotechnological Letters 19(3):9283-9296. |
|
|
Ghaly AE (1996). A comparative Study of Anaerobic Digestion of Acid Cheese Whey and Dairy Manure in a Two-stage Reactor. Biosource Technology 58:61-72. |
|
|
Gupta A, Chandra R, Subbarao PMV, Vijay VK (2009). Kinetics of Batch Biomethanation Process of Jatropha and Pongamia Oil Cakes and their Co-digested Subtrates. Journal of Scientific and Industrial Research 68:624-629. |
|
|
Itodo IN, Philips TK (2002). Covering Materials for Anaerobic Digesters Producing Biogas. Nigerian Journal of Renewable Energy 10(1&2):24-52. |
|
|
Leitão RC, van Haandel C, Zeeman G, Lettinga G (2005). The effects of operational and environmental variations on anaerobic wastewater treatment systems: A review Bioresource Technology pp. 76-77. |
|
|
Mahanta P, Dewan A, Saha UK, Kalita P (2004). Influence of Temperature and Total Solid Concentration on the Gas Production Rate of Biogas Digester. Journal of Energy in Southern Africa 15(4):112-117. |
|
|
Mateescu C (2016). Influence of the Hydrostatic Pressure on Biogas Production in Anaerobic Digesters, Romanian Biotechnological Letters 21(5):11941-11948. |
|
|
Mir MA, Hussain A, Verma C (2016). Design considerations and operational performance of anaerobic digester: A review: Cogent Engineering 3(1):1181696 |
|
|
Mitel KM (1996). Biogas Systems-Principle and Application: New Age International Publishers Ltd., New York; 6:9-12. |
|
|
Mshandete AM, Parawira W (2009). Biogas Technology Research in selected Sub-Saharan African Countries - A review. African Journal of Biotechnology 8:116-125. |
|
|
Ocwieja SM (2010). Life Cycle Thinking Assessment Applied to Three Biogas Projects in Central Uganda. An M.SC project thesis submitted to the Department of Environmental Engineering Michigan Technology University. |
|
|
Olaoye JO, Oyeleke IF, Adeniran KA (2014). Design, Fabrication and Testing of a Modified Floating Drum Bio-digester. Proceedings of the International Soil Tillage Research Oganisation (ISTRO) Nigeria Symposium, Akure 2014. November 3 - 6, Akure, Nigeria.149-161. |
|
|
Rabah AB, Baki AS, Hassan LG, Musa M, Ibrahim AD (2010). Production of Biogas Waste at Different Retention Time. Scientific World Journal 5(4). |
|
|
Rajeshwari KV, Balakrishnan M, Kansal A, LataKishore VVN (2000). State-of-the-artof Anaerobic Digestion Technology for Industrial Wastewater Treatment. Renewable and Sustainable Energy Reviews 4:135-156. |
|
|
Report No. ETSU B 1118 (1986). Research into the Development of Prototype Units for the Production of Biogas Methane from Farm Wastes and Energy Crops, Department of Microbiology, University College, Cardiff. |
|
|
Republic of Rwanda (2012). Technical Guidelines for Construction of Domestic Fixed Dome Biogas Plants pp. 1-39. |
|
|
Sibiya NT, Muzenda E, Tesfagiorgis HB (2014).Effect of Temperature and pH on The Anaerobic Digestion of Grass Silage. 6th Int'l Conf. on Green Technology, Renewable Energy and Environmental Engg. (ICGTREEE'2014) Nov. 27-28, 2014 Cape Town (SA) 198-201. |
|
|
Thakur IS (2006). Environmental Technology: Basic Concept and Applications, New Delhi; IK international Pvt Ltd. |
|
|
Ubwa ST, Asemave K, Oshido B, Idoko A (2013). Preparation of Biogas from Plants and Animal Waste, International Journal of Science and Technology 2(6):6-7. |
|
|
Ukpai PA, Agbo PE, Nnabuchi MN (2015). The Effect of Temperature on the Rate of Digestion and Biogas Production using Cow Dung, Cow Pea, Cassava Peeling. International Journal of Scientific and Engineering Research 6(1):1255-1261. |
|
|
Vidal G, Carvalho A, Mendeza R, Lema JM (2000). Influence of the Content in Fats and Proteins on the Anaerobic Biodegradability of Dairy Wastewaters. Bioresource Technology 74(3):231-239. |
|
|
Wise DL (1987). Global Bioconversions. CRC Press, Florida 4:178-189. |
|
Copyright © 2024 Author(s) retain the copyright of this article.
This article is published under the terms of the Creative Commons Attribution License 4.0