ABSTRACT
In Nigeria, lack of good potable water has always been a major problem; hence many homes have wells as a source of water for household uses. The objectives of this study are to determine the predominant sources (human or livestock) of fecal pollution in wells and concentrations of fecal bacteria and pathogens in well water. Thirty Five samples of well water in different places in Igboora community were analyzed for Total Aerobic Bacteria Counts (TABC) and Total Coliform Counts (TCC). The location and distances of wells from latrines were determined using the Global Positioning System (GPS) device and a tape rule respectively. All the wells sampled had high mean total of TABC (5.79±0.51 log CFU/mL) and TCC (2.77±0.42 log CFU/mL) which ranged for TABC (1.40±0.16 to 9.20±0.49 log CFU/mL) and TCC (1.02±0.13 to 4.43±0.77 log CFU/mL) counts which exceeded the international standard of 0 per 100 mL of potable water. TABC increased with a decrease in distance between the wells and latrines though not significant (p<0.05). The average distance (9.21±1.77m) of wells from the latrines was below the limit (15.24 m or 50 ft) set by United State Environmental Protection Agency (USEPA). According to bacteroidales measurements, fecal contaminations in the 32 well samples were from anthropogenic source.
Keywords: Water borne diseases, fecal source, E.coli, Latrine effluent, Igboora.
In Nigeria, inadequate supply of pipe borne water has been a major problem of many houses hence many homes have wells sited around the house at a distance from their latrine. 52% of Nigerians do not have access to improved drinking water supply (Orebiyi et al., 2010). The scarcity of pipe borne water has made communities to find alternative sources of water, in which well water is included. Wells are a common ground water source readily explored to meet community water requirement or make up the short fall (Adekunle, 2008; Olukanni and Ugwu, 2013; Ogwueleka, 2014; Olukanni et al., 2014a). These wells serve as major source of water for household uses (drinking, cooking, washing etc.). Commonest cause of pollution is attributed to close proximity of latrine to wells and unhygienic usage of the wells (Olukanni et al., 2014b). For instance, some wells have no cover/lids, they are dirty and unkempt thus, making the water unfit for use, resulting in water borne diseases.
The Igboora community suffers serious water supply problems, cases of dry taps are virtually common in every part of the city where you see children and women searching for water including during the dry season. Meanwhile, rainfall is highly seasonal and more serious is the fact that the beginning and end of the rainy season fluctuate very much from year to year though the area is within a rainfall zone of 1000 – 1200 mm per annum. The government at different levels has been interested in the problem.
In the 1970s, a dam was constructed in Eruwa by the state government for the purpose of providing pipe-borne water to the major towns in the area (Areola and Akintola, 1979). Rural water supply is provided through the state water and sanitation project (WATSAN) which was established in 1992 with the support of UNICEF, In which the boreholes and deep-wells were sunk by WATSAN in rural areas across the state including Ibarapa.
Potable water is one that does not contain chemical substances or microorganisms in amount that can cause hazards to health (Alonge, 2005). Water must be substantially free of dissolved salts, plant, animal wastes and bacterial contamination in order to be suitable for human consumption (Bourne, 2001). Poorly designed latrines or some houses that do not have latrine and inadequately maintained latrine systems have contaminated ground water with nitrates, bacteria and toxic cleaning agents. This can serve as a vehicle for spreading illnesses caused by such organisms as: Vibrio cholera, Yersinia enterocolitica, Escherichia coli, Cryptosporidium spp. and vector borne diseases such as guinea worm, schistosomiasis, lymphatic filariosis, onchocerciasis, parasitic and viral infections (Swerdlow et al., 1992; Mackenzie et al., 1995).
Microbial fecal contamination indicators are Echerichia coli, Clostridia spp., Streptococci (Binnie et al., 2002; Simpson et al., 2002; Scott et al., 2002) and other bacteria that could be of human or non-human origin. It is noteworthy that Individual houses in Igboora are closely built and in orderly fashion with high number of inhabitants. Refuse dumps, pit latrines and open sewers are common. Environmental sanitation is almost nil. All these suggest possible chances of pollutants and contaminants entering these wells.
Improving the quality of groundwater resources offers an important economic opportunity for the gradual improvement of the quality of life (Valenzuela et al., 2009). Therefore, evaluation of the microbial quality of water is an important weapon to the achievement of potable water for daily consumption (Narasimha et al., 2014). This necessitated the study to assess the bacteriological quality of ground water at the Igboora community via the use of microbial counts (total aerobic bacteria and total coliform) and the relationship between the distances of the wells to latrines.
Global studies have revealed that substantial decreases in diarrheal infection morbidity by a quarter to over a third (Fewtrell et al., 2005) and improvements in childhood nutritional indexes (Esrey, 1996) have accompanied a gradual switch from open pit latrines to more sanitary latrines consisting of concrete foundation rings to prevent leakage of human feces onto the open ground. Although several diarrheal infection pathogens are transmitted through human feces including, Shigella and rotavirus, livestock feces can carry diarrheal pathogens including Campylobacter, Salmonella and certain types of pathogenic E.coli (Nicholson et al., 2005).
Villagers live very close to their livestock on a daily basis and the livestock are frequently kept near where latrines and tube wells are also located, resulting in opportunities for both types of fecal contamination to impact well water quality. Fecal bacteria in wells of Igboora community are primarily human in origin rather than from domestic livestock; and concentrations of fecal bacteria is related to local population density and to the number of latrines around a well as well as the type of latrine.
This study is to determine the impact of human populations and household latrines on fecal contamination on well water in Igboora community of Ibarapa central local government in Oyo state, South-western Nigeria.
Study area
The study area is Ibarapa Central Local Government Area of Oyo State. Igboora is one of the largest communities in Ibarapa Central Local Government. The community is largely occupied by students and staffs of the Oyo State College of Agriculture and Technology, and Obasanjo Farm International as well as other local occupants. The choice of Ibarapa as one of the study areas is due to the fact that it is a predominantly rural area with over 1,200 communities or localities that are grouped around fourteen large communities that are classified as semi-urban towns. Administratively, the area is organized into three local government areas Ibarapa east, Ibarapa central and Ibarapa north and it forms part of the Oyo South Senatorial District which has nine local governments in all. Oyo state as a whole is organized into three Senatorial districts and thirty three local governments.
Ibarapa Central Local Government which has its headquarter in the town of Igboora and covers about 440 km2 derived from the savannah to the west of Ibadan, the Ogun and Oyan rivers form its eastern and western boundaries respectively, between approximately 7.53° Latitude and 3.08° Longitude, with a population of 102,979 at the 2006 census. The people of Igboora in Ibarapa Central Local Government Area (LGA) are predominantly farmers. Although farming activities in the area is largely subsistence in nature, in the last few years, there has been an upsurge in the number of large commercial farms in the area. This implies that water demand in the area is essentially for agricultural and domestic purposes.
Sample collection
Thirty five ground water samples with a replicate were collected at random from 25 different areas of Igboora community for bacteriological analysis. All the samples were collected aseptically into sterile 10 ml bijou bottles that were fitted with screw caps and properly labeled. Two samples each were collected from each well after which they were transported to the Department of Science Laboratory Technology of Oyo State College of Agriculture and Technology, Igboora on ice in order to maintain the microbial population.
Field methods and wells classi?cation
GPS coordinates were collected for all wells, latrines and households throughout the community for all the selected areas during August 2013 using an eTrex GPS device. GPS data were post-processed using Path?nder Of?ce 3.0. Latrines were classi?ed as sanitary if the wells are covered; clean environment, drawer and metal lock were intact with no visible sign of ef?uent discharging onto the ground. A latrine was classi?ed as unsanitary if the wells are covered or uncovered in dirty environment and uncovered with high or dense population, if visible effluent discharged directly into a well. A population survey was conducted to determine well ownership and the number of persons living in each household and the distances between the wells and latrines were also determined (Livestock were not counted during the community-wide survey).
Laboratory investigation
All media used in this study were prepared and sterilized according to manufacturer’s instructions. The media used include: MacConkey agar (Biotec, UK) for Total coliform counts and Plate count agar (Biotec, UK) for Total aerobic plate count.
The samples were serially diluted to 108. Using the Standard plate method, 0.1 ml was inoculated using a glass spreader and incubated at 37°C for 24 h. For the Total coliform count a 0.1 dilution level was used and only lactose fermenters (pink colonies) were counted. The bacteria isolates were characterized by studying the following:
1. Colony characteristics such as Gram staining according to Donald et al. (2013)
2. Endospores test, according to Marise and Anne (2013)
3. Biochemical test such as catalase test, oxidase test, coagulase test, sugar fermentation, according to MacFadding (2000).The morphological identification of isolate bacteria was also determined in percentage (Figure 1).
Analysis of data
In view of the high microbial counts, the wells sampled were classified into 7 different categories, in which five well samples were in each group. Wells are grouped according to distance between the wells and latrines; number of persons residing in a household; condition of wells and microbiological analysis of well water was performed. One–way ANOVA was used to compare the seven groups. A correlation analysis was also carried out to establish the degree of relationship between the counts and the distances between the latrines.
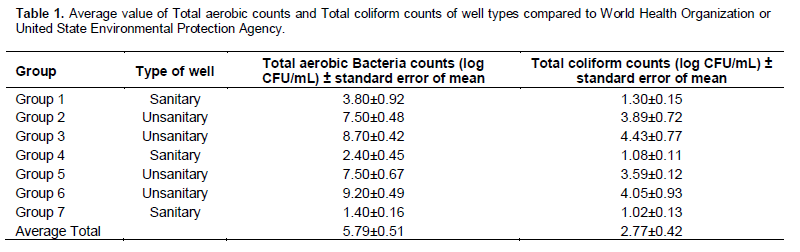
It was observed that all the water samples collected were contaminated with coliforms and other bacteria. The values for microbial counts obtained were ranging from 1.40±0.16 to 9.20±0.49 log CFU/mL for total aerobic bacteria count and from 1.02±0.13 to 4.43±0.77 log CFU/mL for Total coliform count. The mean distance of the wells to the latrines was 9.21±1.77m. In virtually all the wells sampled, the Total aerobic bacteria counts were higher than the Total coliform counts.
In the mean values of Total Aerobic Bacteria count (5.79±0.51) and Total Colifrom counts (2.77±0.42) of different well types, there was a weak negative correlation (r2 = ?0.121) which is not significant at p<0.05 between the distances from latrines and the Total Aerobic Bacteria Counts though a very weak positive correlation (r2 = 0.023) ensued between the distance from latrines and Total coliform counts which was significant at p < 0.05.
Also, a weak negative correlation (r2 = ?0.261) not significant at p<0.05 was seen between the Total coliform and the Total aerobic bacteria counts. However, wells with uncovered and metal locks had the highest Total Aerobic Bacteria counts (9.20±0.49 log CFU/mL), the least (1.40±0.16 log CFU/mL) was observed in covered and metal locks. While highest value was recorded in Total Coliform counts (4.43±0.77 log CFU/mL) of the wells covered and high population, the least value was (1.02±0.13 log CFU/mL) for the wells covered and metal locks, Table 1.
Distance between wells and latrines was highest, in wells (13.93±2.63 m) in which the wells were covered, metal locked and clean environment, while the least was (5.87±0.61m) in uncovered and dense population. All results obtained were below the international standards of 15.24m or 50ft (USEPA Standard). No statistical significant difference at p<0.05 between the average distance of the wells from latrines and the microbial counts (Total aerobic bacteria count and Total coliform counts) was observed for the sanitary and unsanitary wells compared to World Health Organization or United State Environmental Protection Agency, Table 2.
In the 35 wells sampled, E.coli spp were detected in 34 wells, Staphylococcus spp in 32 wells, streptococcus spp in 30 wells, Bacillus spp in 25 wells, pseudomonas spp in 22 wells, Enterococcus spp in 17 wells, Enterobacter spp in 11 wells and Human and bovine Bacteroidales were detected in 32 and 33 wells respectively.
Most Probable Numbers of E. coli in the 35 wells ranged from 9.67x102 (100 MPN/100 ml) to 6.02×108 MPN/100 ml, Table 3. The higher percentage for morphological identification of bacteria isolate was recorded by E coli 90% and least percentage was Enterobacter spp 17%, Figure 1.
Tables 1 and 2 shows that the total bacteria load in the groundwater wells would decrease with distance. Several other factors apart from the distances of the wells to latrines or septic tanks promoted the bacterial contamination of the wells. The covered wells in dirty environment, uncovered wells in a dense populated house, uncovered wells in high populated house and uncovered wells in dirty environment that demonstrated high Total aerobic bacteria counts, might have possibly originated from majorly environmental factors.
Covering a well without maintaining a good level of environmental hygiene will lead to contamination of groundwater. Also, if the number of people accessing a particular well is high there is a high degree of water contamination. Effect of distance from pollution sources was more pronounced on fecal and total coliform counts, which decreased with increasing distance from waste dumps and defecation sites (Adekunle et al., 2007). Chiroma et al. (2007) also recorded high bacteria counts in wells at 3 m from drainage effluent and wells in a residential house at 2 m from pit latrine and 2 m from refuse dump.
These results were consistent with the findings of Shimizu et al. (1980) whose studies showed that more microbial activities were found in wells close to organic waste sites in Japan. The dense population of the Igboora community could be a major factor responsible for the high counts observed. Shimizu et al. (1980) have shown that bacteria contaminate well water, depending on location.
In this study, latrines were categorized as sanitary or unsanitary based on previously identi?ed associations between water borne disease incidence and the number of unsanitary latrines surrounding a dwelling (Emch et al., 2008). The number of unsanitary latrines within each well was correlated to fecal bacteria concentrations (p<0.05). The number of unsanitary latrines within a well was more predictive of fecal indicator bacteria concentrations than spatial-buffer counts of any latrine type (sanitary and unsanitary) within each well, Table 1. The fact that unsanitary latrine counts within wells were more predictive likely could be due to the result of topography surrounding each well that determines the direction of latrine ef?uent and surface runoff ?ow. Contamination of wells may occur associated with contamination from latrines (human waste) but also from animals which graze in close proximity to the wells. There are existing minimum recommended distance for latrines citing relative to groundwater, drinking water supplies and also recommendations for the maintenance of wells (covers and concrete rings) to prevent ingress of surface run off. The results also suggest that topographical characteristics are not solely distance from latrine to well, which is a determinant in fecal contamination of well water, hence, these should be considered in citing of wells.
The presence of human and bovine bacteriodales in 32 and 33 wells respectively, also highlight that animal grazing around that drink well water should be restricted. However, concentrations of microbiological contamination indicator organisms observed in groundwater are a function of the contamination sources active at that moment (Solo-Gabriele et al., 2000). These further explain why no significant difference was observed between the covered and uncovered wells. The distances of the wells from latrines recorded in this study averages 9.21±1.77m.
The Most Probable Numbers of E. coli which ranged from 9.67x102 to 6.02x108 for all the samples that exceeded the (USEPA) recreational water quality limit up to 10,000 times (USEPA, 1986) and were in fact similar in concentration to fecal coliforms typically detected in untreated wastewater (Sinton et al., 1999; Passerat et al., 2011) and the bacterial detected in all the 35 samples analyzed where E. coli species of 90% were detected in 34 well samples, Staphlococcus species of 89% were detected in 32 well samples, Streptococcus species of 72% were detected in 30 well samples, Bacillus species of 56% were detected in 25 well samples, Pseudomonas species of 38% were detected in 22 well samples, Enterococcus species of 23% were detected in 17 well samples, Enterobacter species of 17% were detected in 11 well samples from all the samples.
A global epidemiological meta-analysis determined that for every 1 log10 increase in E.coli the odds of acquiring water borne disease from recreational contact approximately doubles (Wade et al., 2003). The river that flows through the Igboora community is a potential source of contamination for the wells and some houses do not have toilets or latrines and thereby dispose of defecated materials in the river, thus increasing the level of wells contamination. There is an urgent need to better understand the role of the environment in the transmission of water borne illness in Igboora where an estimated 11% of all deaths are attributable to diarrheal infections. Figure 1 showed the Percentage Morphological Identification of Bacteria Isolates where E. coli species were 90%, Staphlococcus species were 89%, Streptococcus species were 72%, Bacillus species were 56%, Pseudomonas species were 38%, Enterococcus species were 23%, Enterobacter species were 17% were determined from all the samples.
The results of bacteriological analysis of well water from the Igboora community in table 1 showed that all the wells were contaminated with coliforms and other bacteria. Mean total aerobic bacteria counts and Total coliform counts were high with the total aerobic bacteria counts being higher. It confirmed that wells classify as sanitary had lower total coliform count compared with those that were classified as unsanitary. These high counts might have risen due to the poor level of hygiene observed in this community. Wells in this area are constantly exposed to contamination from human activities. Also, the layout of the houses is not well planned such that the distances between wells and latrines is not enough and even refuse dumps are very minimal.
Adekunle (2008), Fasunwon et al. (2008), Oparaocha et al. (2008) and Adelekan (2010) had also reported higher heterotrophic bacterial counts too. In some studies conducted in the country, Ifabiyi (2008) and Akinbile and Yusoff (2011), recorded high values for total coliform counts in various groundwater wells. The presence of coliform in water is an indication of fecal contamination and has been associated with waterborne epidemic (Mackenzie et al., 1995). Any water source used for drinking or cleaning purposes should not contain any organism of fecal origin (Akeredolu, 1991). The analysis of the relationship between bacterial indicator levels and environmental characteristics presents several statistical challenges (Bai and Lung, 2006).
Also other factors such as the environment where the well was cited and the level of hygiene of the well, in terms of the use of drawers and the population of people the well is serving and its surroundings could be considered as other possible sources of contamination aside the latrines. The mean values for total Coliform counts for all the sanitary were relatively low (1.13±0.13 log CFU/mL) compared to unsanitary (5.32±0.85 log CFU/mL) ones.
Wells in dirty environments and in houses with dense population had very high total aerobic plate counts. A cumulative increase use of the wells over and above the latrines might lead to failure of the latrines system. This can increase the risk of contaminants entering drinking water sources (Katz et al., 2011). Dirty environments easily breed microorganisms. This is in agreement with the fact that coliform bacteria are widely found in nature (Binnie et al., 2002; Griffiths et al., 2003).
Poor town planning, dilapidated infrastructure and indiscriminate sitting of wells and boreholes contributed to the low bacteriological quality of domestic water supplies, while rainfall accentuated the impact (Egwari and Aboaba, 2002). Though covered wells with metal locks had low value total aerobic bacteria counts (1.40±0.16 log CFU/mL). This could possibly be as a result of the protection in form of the locks provided for the wells and it equally signified that there is level of restricted access to such wells. Valenzuela et al. (2009) suggested that the most important factors affecting well vulnerability to bacterial contamination were those related to the well itself: construction and site management while its usage and maintenance are also crucial.
The total aerobic bacteria counts and total coliform counts averages 5.79±0.51 log CFU/mL and 2.77±0.42 log CFU/mL which contradicts the USEPA/WHO standards. The minimum standard distance set by the USEPA/US-HUD/FHA, Canadian, and UK governments is 15.24 m (50 ft) (Inspect A Pedia, 2010). Presence of latrine systems and well depth were found to be related to detection of coliforms in groundwater, although these relationships were not statistically significant (Francy et al., 2000). Wells in dirty environments and in houses with dense population had very high total aerobic plate counts.
As the importance of safe guarding quality of water supply has become widely recognized, standards regarding septic tank placement should be reviewed if there be any at all. This can be achieved by imposing minimum lot size requirements larger than those which have been found to be associated with ground water contamination. Another is to evaluate each potential site individually taking into consideration the geological characteristics of the site and methods of waste disposal being practiced in the area.
Results of this study are significant because, drinking water supplies in the area of survey (Igboora) fluctuate seasonally, often with water shortage during dry season. Piped surface water supplies are not available in Igboora with a reliance on ground water (boreholes or deep wells) and a large population of people in the area of survey relies on latrines and wells. Therefore residents may be at risk of suffering from water borne illnesses after consumption of water without treatment. In addition, the study indicates that present distances between wells and latrines coupled with the level of hygiene of these wells are insufficient to prevent ground water contamination. It is recommended that when siting, topographical conditions and surface water flow during flooding or wet season should be considered, minimum buffer zone as well as ensuring that wells are covered with rings (intact). Microbiological water quality monitoring (E.coli) should be used as a tool to evaluate the effectiveness of resiting latrines, introduction of minimal set back distances for cattle (and other animal grazing) and well maintenance. The problem of ground water contamination via latrine is not an isolated problem of the area surveyed but rather a nationwide problem. Hence precautions should be taken by setting standards for citing of wells from latrines and treatment of well water before use.
The authors have not declared any conflict of interest.
The authors wish to acknowledge the staff and students of the Department of Science Laboratory Technology, Oyo State College of Agriculture and Technology, Igboora, for their co-operation and use of department’s laboratory facilities.
REFERENCES
Adekunle IM, Adetunji MT, Gbadebo AM, Banjoko OB (2007). Assessment of groundwater quality in a typical rural settlement in Southwest Nigeria. Int. J. Environ. Res. Publ. Health 4(4):307-318.
Crossref |
|
|
|
Adekunle AS (2008). Impacts of industrial effluent on quality of well water within Asa Dam Industrial Estate, Ilorin, Nigeria. Nature and Science 6(3):1-5. |
|
|
|
Adelekan BA (2010). Water quality of domestic wells in typical African communities: Case studies from Nigeria. Int. J. Water. Res. Environ. Engr. 2(6):137-147. |
|
|
|
Akeredolu FA (1991). Setting water Quality-water Quality Standards for Nigeria. In: Proceedings of First National Conference On Water Quality Monitoring and Status in Nigeria, Kaduna. Pp. 216-224. |
|
|
Akinbile CO, Yusoff MS (2011) Environmental impact of leachate pollution on groundwater supplies in Akure, Nigeria. Int. J. Environ. Sci. Dev. 2(1):81-86.
Crossref |
|
|
|
Alonge DO (2005). Textbook of meat and milk hygiene. Farmco press, Ibadan. P. 32. |
|
|
|
Areola O, Akintola F (1979). "Domestic Water Consumption in Urban Areas: A Case Study in Ibadan City, Nigeria". Water Supply and Manage. 1:001-009. |
|
|
Bai S, Lung WS (2006). Three-dimensional modeling of fecal coliform in the tidal basin and Washington channel, Washington, DC. Part A, Toxic/ Hazardous Substances and Environmental Engineering. J. Environ. Sci. Health 41:1327-1346.
Crossref |
|
|
|
Binnie C, Kimber M, Smethrust G (2002). Basic water treatment. Royal Society of Chemistry, Cambridge, UK. |
|
|
|
Bourne AC (2001). Assessing the contamination risk of private well water supplies in Virginia. A MSc (Biological Systems Engineering) Thesis submitted to the faculty of the Virginia Polytechnic Institute and State University, USA. |
|
|
|
Donald Breakwell, Bryan Macdonald, Kyle Smith, Richard Robison, Christopher Woolverton (2013). Colony Morphology Protocol. American Society for Microbiology, USA. |
|
|
|
Chiroma TM., Ugheoke BI, Patrick DO (2007). Environmental impact on the quality of water from hand-dug wells in Yola Environs. Academic Direct 10:67-76. |
|
|
Egwari L, Aboaba OO (2002). Environmental impact on the bacteriological quality of domestic water supplies in Lagos, Nigeria. Revista de Saúde Pública 36(4):513-520.
Crossref |
|
|
Emch M, Ali M, Yunus M (2008). Risk areas and neighborhood-level risk factors for Shigella dysenteriae 1 and Shigella fexneri. Health Place. 14:96-115.
Crossref |
|
|
Esrey SA (1996). Water waste and well-being: a multicountry study. Am. J. Epidemiol. 143:18-23.
Crossref |
|
|
|
Fasunwon O, Olowofela J, Akinyemi O,Fasunwon B, Akintokun O (2008). Contaminants evaluation as water quality indicator in Ago-Iwoye, South-western, Nigeria. African Physical Review 2:0012. |
|
|
Fewtrell L, Kaufmann RB, Kay D, Enanoria W, Haller L, Colford Jr JM. (2005). Water sanitation and hygiene interventions to reduce diarrhea in less developed countries: a systematic review and meta-analysis. Lancet Infect. Disease 5:42–52.
Crossref |
|
|
Francy DS, Helsel DR, Nally RA (2000). Occurrence and distribution of microbiological indicators in ground water and stream water. Water Environ. Res. 72: 152-161.
Crossref |
|
|
|
Griffiths JF, Welsberg BS, McGee DC (2003). Evaluation of microbial source tracking methods using mixed feacal sources in aqueous test samples. J. Water Health 1:141-151. |
|
|
|
Ifabiyi IP (2008). Depth of hand dug wells and water chemistry: Example from Ibadan Northeast Local Government Area (L.G.A.), Oyo-state, Nigeria. J. Soc. Sci. 17(3):261-266. |
|
|
|
Inspect A Pedia (2010). Online table of required well clearances: Distances between drinking water wells and septic systems, treated soils, farm buildings & other site features.
View
|
|
|
Katz BG, Eberts SM, Kauffman LJ (2011). Using Cl/Br ratios and other indicators to assess potential impacts on groundwater quality from septic systems: A review and examples from principal aquifers in the United States. J. Hydrol. 397:151-166.
Crossref |
|
|
|
MacFadding JF (2000). Biochemical tests for identification of medical bacterial, 3rd edition, Lippincott William & Wilkins, Philadephia, PA. 1: 912. |
|
|
Mackenzie WR, Schell WL, Blair KA, Addiss DG (1995). Massive outbreak of waterborne cryptosporidium infection in Milwaukee, Wiscousin: recurrence of illness and risk of secondary transmission. Clin. Infect. Dis. 1: 57-62.
Crossref |
|
|
|
Marise AH, Anne Z (2013). Endospore Stain Protocol, American Society for Microbiology, USA. |
|
|
|
Narasimha RC, Dorairaju SV, Bujagenda RM, Calapathi PV (2014). Statistical analysis of drinking water quality and its impact on human health in Chandragiri, near Tirupati India.
View
|
|
|
Nicholson FA, Groves SJ, Chambers B (2005). Pathogen survival during livestock manure storage and following land application. Bioresour. Technol. 96:135–43.
Crossref |
|
|
Ogwueleka TC (2014). Assessment of water quality and identification of pollution sources of Kaduna River in Niger State (Nigeria) using exploratory data analysis. WEJ. 28(1):31-37.
Crossref |
|
|
|
Olukanni DO, Ugwu NC (2013). Mud Rotary Drilling in Southern Nigeria: Potential adverse effects of its by-products on the environment. Int. J. Water. Res. Environ. Engr. 5(5):262-271. |
|
|
|
Olukanni DO, Ajetomobi MO, Tebowei SO, Ologun OO, Kayode OM (2014a).Water supply and Sanitation challenges in an urban setting: A case study. IJEAS. 1(33):34-38. |
|
|
|
Olukanni DO, Ebuetse MA, Anake WU (2014b). Drinking water quality and sanitation issues: A survey of a semi-urban setting in Nigeria. IJRES. 2(11):58-65. |
|
|
|
Oparaocha ET, Iroegbu OC, Obi RK (2008). Assessment of quality of drinking water sources in the Federal University of Technology, Owerri, Imo State, Nigeria. J. Appl. Biosci. 32:1964- 1976. |
|
|
Orebiyi EO, Awomeso JA, Idowu OA, Martins O, Oguntoke O, Taiwo AM (2010). Assessment of pollution hazards of shallow well water in Abeokuta and Environs, Southwest, Nigeria. Am. J. Environ. Sci. 6(1):50-56.
Crossref |
|
|
Passerat J, Ouattara NK, Mouchel J, Rocher V, Servais P (2011). Impact of an intense combined sewer overflow event on the microbiological quality of the Seine River. Water Res., 45: 893-903.
Crossref |
|
|
Scott TM, Rose JB, Jenkins TM, Farrah SR, Lukasik J (2002). Microbial source tracking: Current methodology and future directions. Appl. Environ. Microbiol. 68:5796-5803.
Crossref |
|
|
|
Shimizu TM, Kigotake TM, Agatomo HN (1980). Bacteria contamination of drinking water from wells in Miyazaki, Japan. In: Bulletin for Agriculture. Miyakazi University. pp. 21-28. |
|
|
Simpson JM, Sarto Domingo JW, Reasoner DJ (2002). Microbial source tracking: state of science. Environ. Sci. Technol. 36: 527-528.
Crossref |
|
|
|
Sinton LW, Finlay RK, Lynch PA (1999). Sunlight inactivation of fecal bacteriophages and bacteria in sewage-polluted seawater. Appl. Environ. Microbiol. 65:3605–3613. |
|
|
Solo-Gabriele HM, Wolfert MA, Desmarais T R, Palmer CJ (2000). Sources of Escherichia coli in a coastal subtropical environment. Appl. Environ. Microbiol. 66:230-237.
Crossref |
|
|
Swerdlow DL, Woodruff BA, Brady RC (1992). A waterborne outbreak in Missouri of Escherichia coli 0157:H7 associated with bloody diarrhea and death. Annals of Internal Medicine 117(10):812-819.
Crossref |
|
|
|
United Nations Children's Fund and World Health Organization (UNICEF) (2009). Diarrhoea: why children are still dying and what can be done.
View
|
|
|
|
United States Environmental Protection Agency (USEPA) (1986). Bacteriological Water Quality Criteria for Marine and Fresh Recreational Waters, Cincinnati, OH: U.S Environmental Protection Agency, Office of Water Regulations and Standards. EPA-440/5-84-002. |
|
|
Valenzuela M, Lagos B, Claret M, Mondaca MA, Pérez C, Parra O (2009). Fecal contamination of groundwater in a small rural dryland watershed in central Chile. Chilean. J. Agric. Res. 69(2):235-243.
Crossref |
|
|
Wade TJ, Pai N, Eisenberg JNS, Colford JM (2003). Do US Environmental Protection Agency water quality guidelines for recreational waters prevent gastrointestinal illness? A systematic review and meta-analysis. Environ. Health Persp. 111:1102-9.
Crossref |