ABSTRACT
Water quality concerns have been very crucial because good quality water supplies in abundance and readily available for production and productivity is of great importance. Therefore, the study was conducted to assess irrigation water quality of the lowlands of Delo Menna and Berbere districts. The samples were collected from the upstream and downstream of the canals along the farms of the districts during the medium and low peaks of the rivers’ flow. About 1 L of water sample was collected from each site. Likewise, the sampling date was on the 15th of designated months. Accordingly, the results reveal that the surface irrigation water has less salinity, sodium hazard and residual sodium carbonate hazard in the irrigated canals and canals along the farms. The electrical conductivity ranges from 0.17 to 0.49 ds/m at lower peak and from 0.06 to 0.24 ds/m at medium peak in Delo Menna district. Moreover, the residual sodium carbonate ranges from 1.34 to 2.57 at lower peak flow and 0.79 to 1.30 at medium peak flows; sodium concentration ranges from 0.36 to 0.46 meq/L at lower peak of the river flow and very small concentrations at the medium peak flow period in Berbere district. The salinity indicator parameters such as all cations and anions, total dissolved solid, adjusted sodium ratio, residual sodium carbonates in the irrigated rivers were low in Delo Menna and Berbere. Therefore, the rivers in both districts are normal for agricultural production of all types of crops which are grown in the low lands of Bale.
Key words: Cation, anion, assessment, conductivity, irrigation, water quality.
Water quality issues have often been neglected because there are quite a number of good quality water supplies (Islam et al., 2004). Hydrochemical study reveals the quality of water that is suitable for drinking, agriculture and industrial purposes. The chemical parameters of groundwater play a significant role in assessing water quality which is suitable for irrigation (Sadashaiah et al., 2008). Most of Ethiopian irrigable lands are affected by soil salinity problem. As indicated by Massoud (1997) in Ethiopia, saline soils cover about 11,608,000 ha, and sodic soils, about 425,000 ha, and these are found in arid and semi-arid climate where most irrigable lands are found. Irrigation with poor quality waters may bring undesirable elements to the soil in excessive quantities, affecting its fertility. The quality of groundwater has definite command over yield of crops through its effect on soil environment which is the soul of infinite life (Latha et al., 2002).
In irrigation, poor water quality with excess salts affects plants in many ways, but the most common problems are caused by salts which influence the osmotic relationship between roots and soil moisture (Malash et al., 2005). Irrigation water must have appropriate salt concentrations and be free of chemical and biological pollutants. High salts in irrigation water reduce plant growth and affect the structure, aeration, permeability and texture of soil (Singh et al., 2010; Ackah et al., 2011). The aridity of the study area could raise irrigation water impact on soil through the concentration of minerals in soil during evaporation (Al-Rashdi and Sulaiman, 2015). Salt water increases the osmotic pressure in soil solution and accordingly restricts water uptake by plants (Singh et al., 2010; Embaby and El-Barbary, 2011).
The main sources of salts in these regions are rainfall, mineral weathering, “fossil” salts, and various surface and ground waters which redistribute accumulated salts, often the result of anthropogenic activities (Bresler et al., 1982). For instance, soil saturated with high sodium, especially heavy textured, and high swelling clay soils cause increased hydration, swelling, dispersion and peptization of the soil colloids, structural destruction, and aggregate failure. Therefore, this study was undertaken with the objectives of assessing irrigation water quality.
Study area
The study area is located in the Southeastern part of Oromia Regional State in Bale Zone which lies at latitude 060° 24ʺ N and longitude 390° 50ʺE in Delo Menna District, with 1278 m altitudes and an average rainfall of 986.2 mm and at latitude 06° 49ʺ N and longitude 040° 11ʺE in Bebere District, with 1234 m altitudes and an average rainfall of 725 mm. The districts were purposively selected where irrigation has frequently been used.
Procedure for water sample collection
Water samples were collected from four rivers per district from which the canals were constructed for irrigation purpose, and the three water samples were taken from each river at Delo Menna and Berbere in 2011 during the medium and low peaks of river flow. Water samples were collected in one-liter plastic bottle and were drawn from midstream at 0 to 15 cm depth below the surface of the canal water and along the canal. The collected water samples were tightly sealed as early as possible to avoid exposure to air and immediately analyzed for sensitive parameters like pH, electrical conductivity and total dissolved solids (TDS).
Assessment activities were carried out along the rivers from upstream and downstream of the canals along the farms in the districts. About one litre of water sample was collected from each site. The sampling date was on the 15th of designated months. Water sampling techniques were followed as outlined by Hunt and Wilson (1986) and APHA (1989). The chemical analyses were conducted at the Sinana Agricultural Research Center (SARC) and JIJE Laboglass PVT Limited Company.
Methods used for chemical analyses
The pH and electrical conductivity (EC) values were determined electrometrically using digital pH and digital conductivity meter (Ghosh et al., 1983). Residual sodium carbonate (RSC) and adjusted residuals sodium (Adj.Ran.) were calculated using standard equation procedures. Total dissolved solids (TDSs) were estimated by weighing the solid residue obtained by the evaporation of a measured volume of water samples to dryness (Chopra and Kanwar, 1980). Potassium and sodium were determined by flame emission spectrophotometer (Golterman, 1971).
Calcium and magnesium were analyzed directly by atomic absorption spectrophotometer (APHA, 1989). Carbonate and bicarbonate were determined by acidimetric titration (Chopra and Kanwar, 1980) while chloride was determined by argentometric titration (APHA, 1989); whereas sulphate was analyzed turbidimetrically (Wolf, 1982) as was analyzed directly by atomic absorption spectrophotometer with hydride generator (APHA, 1989). Accordingly, the generated data were presented in tabulated form.
Salinity hazard of irrigated rivers
Water quality is determined according to the purpose for which it was used. Regarding irrigation water, for instance, the usual criteria including salinity, sodicity, and ion toxicities has been indicated for quality of water. Likewise, the concentration of soluble salts in irrigation water can be classified in terms of electrical conductivity (EC), total dissolved solids (TDS) and pH. Therefore, the analyzed results show quality of irrigation water in Delo Menna district. Thus, the pH ranges in both peaks were found the same which is near neutral from 6.77 to 7.79 at lower peak (Table 1) and from 7.50 to 7.95 at medium peak (Table 2). Likewise, electrical conductivity at lower peak was higher than the medium peaks flows of rivers, thus indicating non saline which ranges from 0.17 to 0.49 ds/m at lower peak and from 0.06 to 0.24 ds/m at medium peak in Yadot and Gomgoma Rivers, respectively. Similarly, total dissolved solids at lower peak was higher than the medium peak flow which range from 113.00 mg/L at Yadot to 352.00 mg/L at Gomgoma River at lower peak and 133 mg/L at Dayu to 164 mg/L at Gomgoma River at medium peak of the river flow (Table 2).
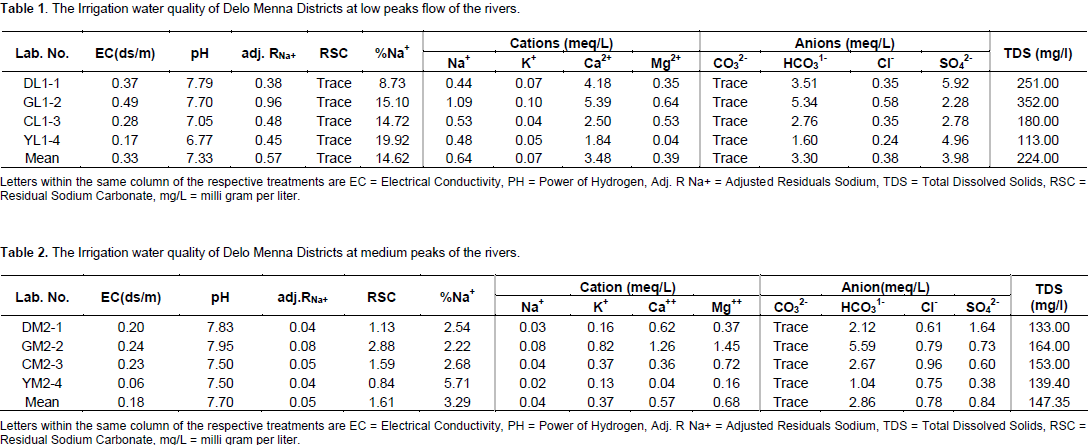
Sodium hazard of irrigated rivers
Among the soluble constituents of irrigation water like sodium, magnesium and calcium is considered most hazardous. Therefore, sodium soils are relatively impermeable to air and water; making both soils and plants adversely affected by high sodium irrigation water. Accordingly, the results reveal that the sodium percent varies from time to time with high percentage at lower peak flows that ranges from 8.73 to 19.92% at lower peak and 2.22 to 5.71% at medium peak (Table 2). Likewise, residual sodium carbonate was found in trace amount at lower peak flow and 0.84 to 2.88 meq/L at medium peak flow and that of sodium concentration ranges from 0.44 to 1.09 meq/L at lower peak flow and 0.02 to 0.08 meq/L at medium peak of the river flow (Table 2).
Bicarbonate hazard of irrigated rivers
In water having a high concentration of bicarbonate, there is a tendency for calcium and magnesium to precipitate. When this happens, there is a reduction in the concentration of calcium and magnesium and a relative increase in sodium. Accordingly, the results reveal that the bicarbonate concentration at lower peak and medium peak river flow were found in the same and trace amount. Likewise, bicarbonate ranges from 1.60 to 5.34 meq/L at lower peak flow and 1.04 to 5.59 meq/L at medium peak flow in Yadot and Gomgoma Rivers, respectively of Delo Menna District (Table 2).
Chemical properties of irrigated rivers
The water quality during the assessment of eachindicator displayed recommendations for the farmers. Moreover, the analyzed results show that the cations such as potassium, calcium and magnesium were found in small amount which could not affect the growth and developments of all crops grown in the area. Anions such as chlorine were also found in small amount at lower and medium peak, while sulphate was very small at medium flow and 2.28 to 5.92 meq/L at lower peak flow rivers of Delo Menna district.
Salinity hazard of irrigated rivers
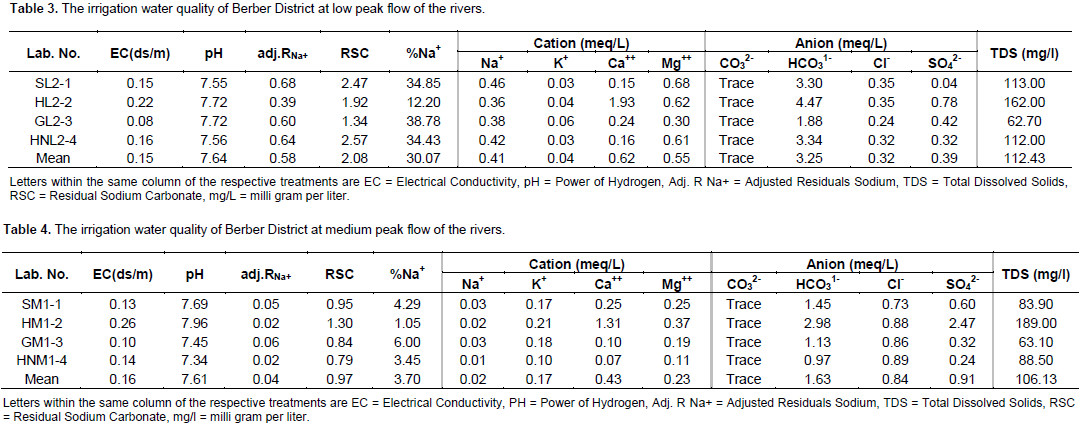
The analyzed results show the quality of irrigation water in Berbere District which were collected from rivers of Gabe, Hambala, Sirima and Hara Nano. Thus, the pH of most of the irrigation water of four rivers ranges from 7.34 to 7.96 at medium stream flow and 7.55 to 7.72 at low stream flows (Table 3), and is within the safe limit for irrigation water quality. Likewise, the electrical conductivity ranges from 0.08 ds/m in Gabe to 0.22 ds/m in Hambala at low stream flow and 0.10 ds/m in Gabe to 0.26 ds/m in Hambala at medium stream flow; total dissolved solids range from 62.70 mg/L in Gabe River to 162.00 mg/L in Hambala River at lower peak and 63.10 mg/L in Gabe River to 189 mg/L in Hambala at medium peak of the river flow (Table 4).
The study is in line with the finding of Raghunath (1987). The amount of total dissolved solids (TDS) ranged from 130 to 359 mgL-1 water containing TDS less than 1000 mgL-1 considered to be of ‘fresh water category. According to Mohammed (2011), irrigation being used had the most influential water quality guideline on crop productivity; the extent of salinity hazard could be measured by the ability of water to conduct an electric current since conductance is a strong function of the electrical conductivity (EC) measurement. In general, the amount of water available to the crop gets lower when the electrical conductivity is higher. The pH of water samples varied from 7.50 to 7.95 at medium stream flow and 6.77 to 7.79 at low stream flows, indicating slightly acidic to slightly alkaline in nature and is within the safe limit recommended for irrigation water quality. The recommended pH limit of irrigation water is 6.0 to 8.5 (Ayers and Westcot, 1985).
Sodium hazard of irrigated rivers
The results reveal that the sodium percent ranges from 12.20% in Hambala to 38.78% in Gabe River at lower peak and 1.05% in Hambala to 6% in Gabe at medium peak of the river flow (Table 4). Likewise, residual sodium carbonate ranges from 1.34 in Gabe to 2.57 in Hara Nano at lower peak flow and 0.79 meq/L in Hara Nano to 1.30 meq/L Hambala at medium peak flow (Table 4); sodium concentration ranges from 0.36 meq/L in Hambala to 0.46 meq/L in Sirima Rivers at lower peak of the river flow (Table 3) and very small concentrations in Sirima, Hambala, Gabe and Hara Nano at the medium peak flow. The study is in line with the finding of Vasanthavigar et al. (2009) higher sodium concentrations observed in (May 2010) because increase river water level that leads to dissolution of minerals from lithological composition.
Bicarbonate hazard of irrigated rivers
The results reveal that the carbonate at lower and medium peak was found in a trace amount in all rivers in Berbere District. Likewise, bicarbonate ranges from 1.88 meq/L in Gabe to 4.47 meq/L in Hambala at lower peak river flow and that of Hara Nano 0.97 meq/L to 2.98 meq/L in Hambala at medium peak flows of the river. As a result, the relative proportion of sodium in the water is increased in the form of sodium bicarbonate (Sadashaiah et al., 2008). Continuous use of waters having residual sodium carbonate of more than 2.5 meq/L leads to salt build up which may hinder air and water movement by clogging the soil pores. This leads to the degradation of the physical condition of soil (Latha et al., 2002).
Chemical properties of irrigated rivers
The analyzed results show that the cations such as potassium, calcium and magnesium were found in small amount which could not affect the growth and developments of all crops. Anions such as chlorine were also found in small amount at lower and medium peak. Sulphate was very small at medium and lower peak flow in all the rivers in Berbere District. Accordingly, the study, in line with some of the soluble constituents, Ca2+, Mg2+, Na-, Cl-, SO42-, HCO31- and B are of prime importance in judging the water quality for irrigation (Michael, 1978). Likewise, some of these ions are beneficial and few ions in excess amounts are more or less detrimental to plant growth and soil properties (Quddus and Zaman, 1996).
Similarly, the relatively high concentration of calcium cations may limit the hazardous effect of sodium which later causes the dispersion of the soil aggregates eventually creating water problem and movement in the soil systems (Mathess, 1982). Generally, the irrigation water qualities in both districts were identified as a good condition for production and productivity. Moreover, Delo Menna is relatively more saline than the Berbere district. Accordingly, salinity, sodium, bicarbonate and chemical properties of the districts were found to be in normal concentration which does not need reclamation for the crops grown in area.
Irrigation is an important practice for farmers to increase their production and productivity, especially where moisture stress is found in lowlands of Oromia. In the area, fruits and vegetables are majorly produced under irrigation where the mode of irrigation is canal from flowing rivers. Accordingly, assessments of irrigation water quality parameters reveal that the percentages of sodium at the low stream flow were higher than those at the medium stream flow of irrigated rivers and that of the carbonate ions in all the kebeles were in trace amounts.
Analysis of the results showed that the relative concentrations of all cations and anions in both districts were very low at medium and lower stream flow of all rivers. Generally, the irrigation water qualities in both districts were identified as a good condition for production and productivity. Moreover, Delo Menna is relatively more saline than the Berbere district. Accordingly salinity, sodium, bicarbonate and chemical properties of the districts were found to be in normal concentration which does not need reclamation for the crops grown in the area. Therefore, the rivers in both Woreda are normal for the agricultural production of all types of crops which are grown in the low lands.
The authors have not declared any conflict of interests.
The authors appreciate all staffs of Soil Fertility Improvement and Soil and Water Conservation team of Sinana Agricultural Research Center, for their contribution towards implementation of the study, and Sinana Agricultural Research Centre for providing necessary logistic support in the course of the study. The generous financial assistance from Oromia Agricultural Research Institute is also gratefully acknowledged.
REFERENCES
|
Ackah M, Agyemang O, Anim AK, Osei J, Bentil NO, Kpattah L, Gyamfi ET, Hanson JEK (2011). Assessment of groundwater quality for drinking and irrigation: the case study of Teiman-Oyarifa Community, Ga East Municipality, Ghana [2011]. Proc. Int. Acad. Ecol. Environ. Sci. 1(3-4):186-194.
|
|
|
|
Al-Rashdi TT, Sulaiman H (2015). Assessment of physiochemical properties of farm soils and irrigation water around a major industrial area in Oman. Proc. Environ. Sci. 28:265-270.
Crossref
|
|
|
|
|
American Public Health Association (APHA) (1989). Standard Methods for the Examination of Water and Waste Water'. 17th ed. Washington, D.C. 200005. 1-30:40-175.
|
|
|
|
|
Ayers RS, Westcot DW (1985). Water Quality for Agriculture. FAO Irrig. Drainage Pap. Rev. 29 (I):8-96.
|
|
|
|
|
Bresler E, McNeal BL, Carter DL (1982). Salin and soils. Principles-dynamics –modeling. Springer- verlga. NY. Adv. Series Agric. Sci. No. 10.
|
|
|
|
|
Chopra SL, Kanwar JS (1980). Analytical Agricultural Chemistry. Kalyan Publishers Ludhiana and New Delhi. pp. 168-307.
|
|
|
|
|
Embaby AAA, El-Barbary SMAJ (2011). Evaluation of Quaternary aquifer for agricultural purposes in northwest Sinai, Egypt. J. Am. Sci. 7(3):344-361.
|
|
|
|
|
Ghosh AB, Bajaj JC, Hasan R, Singh D (1983). Soil and Water Testing Methods. A Laboratory Manual, Division of Soil Science and Agricultural Chemistry. IARI, New Delhi 110012:36-45.
|
|
|
|
|
Golterman HL (1971). Methods for chemical Analysis for Fresh Waters. Blockwell Scientific Pub. (ed), Oxford. pp. 41-42.
|
|
|
|
|
Hunt DTE, Wilson AL (1986). 'The Chemical Analysis of Water - General Principles and Techniques', 2nd Ed. R. Soc. Chem. Cambridge, pp. 29-43.
|
|
|
|
|
Islam MR, Jahiruddin M, Islam S (2004). Assessment of Arsenic in the water-soil-plant systems in Gangetic Floodplains of Bangladesh. Asian J. Plant Sci. 3(4):489-493.
Crossref
|
|
|
|
|
Latha MR, Indirani R, Sheeba S, Francis HJ (2002). Groundwater quality of Coimbatore district Tamil Nadu. J. Ecobiol. 14(3):217-221.
|
|
|
|
|
Malash N, Flowers TJ, Ragab R (2005). The Effect of irrigation systems and water management practices using saline and non-saline water on tomato production. Agric. Water Manage, Department of Agriculture Engineering, University of Cape Coast, Ghana. 78:25-38.
|
|
|
|
|
Massoud F1 (1997). Basic principles for prognosis and monitoring of salinity and sodicity. In: proc. International Conference on Managing saline water for irrigation. Texas Technological University, Lubbock Texas. 16-22, August, 1976. pp. 432-454.
|
|
|
|
|
Mathess G (1982). The Properties of Groundwater. John Willey and Sons, New York. P 406.
|
|
|
|
|
Michael AM (1978). Irrigation: Theory and Practice. Vikas Pub. House Pvt. Ltd. pp. 448-452.
|
|
|
|
|
Mohammed MN (2011). Quality Assessment of Tigris River by using Water Quality Index for Irrigation Purpose. Eur. J. Sci. Res. 57(1):15-28
|
|
|
|
|
Quddus KG, Zaman MW (1996). Irrigation water quality in some selected villages of Meherpur in Bangladesh. Bangladesh J. Agril. Sci. 23(2):51-57.
|
|
|
|
|
Raghunath HM (1987). Ground Water',2nd edition. Wiley Eastern Limited. New Delhi, India. P 353.
|
|
|
|
|
Sadashaiah C, Ramakrishnaiah CR, Ranganna G (2008). Hydro chemical analysis and Evaluation of ground water Quality in Tumkur Taluk, Karnataka State, India. Int. J. Environ. Res. Public health 5(3):158-164.
Crossref
|
|
|
|
|
Singh AK, Mahato MK, Neogi B, Singh KK (2010). Quality assessment of mine water in the Raniganj coalfield area, India Mine Water Environ. 29:248-262.
Crossref
|
|
|
|
|
Vasanthavigar M, Srinivasamoorthy K, Vijayaragavan K, Ganthi RR, Chidambaram S, Anandhan P, Manivannan R, Vasudevan S (2009). Application of water quality index for groundwater quality assessment: Thirumanimu-ttar sub-basin, Tamilnadu, India. J. Environ. Monitor. Assess. DOI 10.1007/s10661-009-1302-1.
|
|
|
|
|
Wolf B (1982). A comprehensive system of leaf analysis and its use for diagnostic crop nutrient statuses. Comm. Soil Sci. Pl. Anal. 13(12):1044-1045.
Crossref
|
|