ABSTRACT
Irrigation is a common agricultural practice for farmers in the periphery of flowing rivers in Ethiopia. In the south west Ethiopia, fruits and vegetables are majorly produced under irrigation where the mode of irrigation is by pumping water from flowing rivers. Irrigated agriculture has only been linked to the availability of water in physical terms, while the quality of water is significantly important for sustainable agricultural production. A study was conducted to assess the water quality parameter of the Kulfo River in South West part of Ethiopia. Accordingly, the concentration of calcium cation (21 g/L) is the highest concentrated followed by sodium (8.3 g/L), magnesium (5 g/L) and potassium (1.8 g/L) is the least concentrated in the Kulfo River. The concentration level of the chloride ion has significantly (25 mg/L) increased from what it was reported (3 to 12 mg/L) in 2004. More importantly, both the electrical conductivity and the sodium adsorption ration are relatively low, implicating the water is non saline and can safely be used for irrigation.
Key words: Electrical conductivity, irrigation water, pumping, sodium absorption ratio, river.
Irrigation practices have played an important role in increasing agricultural production and productivity so as to meet the ever increasing food, fibre, etc. demands of the growing population. Water resources like rivers and streams are serving as source of irrigation water, while crossing different territories. Irrigated agriculture, however, has only been linked to the availability of water in physical terms while the quality of water is significantly important for sustainable agricultural production. However, the latter has often been neglected, until the situation when most of irrigated agriculture is heavily dependent on lower water quality and less desirable sources elsewhere in the world (FAO, 1985; Islam et al., 2004). Water quality concerns have often been neglected because good quality water supplies have been plentiful and readily available (Islam et al., 2004; Causapé et al., 2004; Rao and Devadas, 2005; Raju, 2006; Singh et al., 2008; Dhirendra et al., 2009; Megersa et al., 2009).
Salinity is a common problem facing farmers who irrigate in arid climates. This is because all irrigation waters contain soluble salts. Whether derived from springs, diverted from streams, or pumped from wells, the
waters contain appreciable quantities of chemical substances in solution, dissolved from the geological strata through and over which the waters have flowed. Waters with a high salt content may have moved from a saline water table. In areas with intensive agriculture, fertilization is a major cause of aquifer salinization. The composition of salts in water varies according to the source and properties of the constituent chemical compounds (Guy, unpublished).
These salts include substances such as gypsum (calcium sulphate, CaSO4.2H2O), table salt (sodium chloride NaCl) and baking soda (sodium bicarbonate NaHCO3). When dissolved in water, salts separate into ions; e.g. sodium chloride breaks down into sodium and chloride ions. Thus, it is customary to refer to ions rather than salts under low quality water, the performance of irrigated agriculture is expected to be below normal, in addition to its environmental impact on soil system. The application of irrigation water to the soil introduces salts into the root zone. Plant roots take in water but absorb very little salt from the soil solution. Similarly, water evaporates from the soil surface but salts remain behind. Both processes result in the gradual accumulation of salts in the root zone, even with low salinity water. This situation may affect the plants in two ways: (a) by creating salinity hazards and water deficiency; and (b) causing toxicity and other problems (Singh et al., 2008; Sukhdev, 2012).
The build-up of salinity in the root zone increases the osmotic pressure of the soil solution and causes a reduction in both the rate of water absorption by the plants and the soil water availability. Thus, a continuous water deficiency may exist even though the field is heavily irrigated. Plant wilting symptoms may not become apparent, but growth and yield are depressed. Under such circumstances, it is not possible to maintain good crop growth and development conditions and obtain high yields. Instead, plant growth is delayed and there will be a considerable reduction in yield. Seed germination is also affected by the presence of salts. It is usually delayed and in some cases does not occur. The level of salinity build-up depends on both the concentration and the composition of salts in the water. Chloride is highly soluble and remains in the soil solution, while sulphate and bicarbonate combine with calcium and magnesium, where present, to form calcium sulphate and calcium carbonate, which are sparingly soluble compounds (Quddus and Zaman, 1996).
The use of such saline water with excessive salt content may also cause secondary salinization of cultivated soils, and bring a series of serious consequences to the agricultural environment and the ecosystem (Kumar et al., 2015). The use of saline irrigation directly affects the soil profile and the dissolved salts may cause changes in the salt balance of the soil system. Zhai et al. (2015) reported that the salt concertation in the soil profile under tomato production was increased by 47% for the use of saline water more than 5 dS/m.
The chemical constituents of irrigation water can affect plant growth directly through toxicity or deficiency, or indirectly by altering availability of nutrients (Ayers and Westcot, 1985). For instance, in the study conducted in China, it was confirmed that irrigation water more than 5 Ds/m significantly affect the fruit yield of tomato (Zhai et al., 2015). The associated effect of salt in irrigation is attributed to decreased turgor pressure, a lowered speed of cell expansion and damage to chloroplasts, thus reducing the growth rate and photosynthesis. These changes ultimately influence the dry matter accumulation and yield of crops. Ragab (2004) also substantiated that there was a progressive increase in the soil salinity as saline water is continuously used for irrigation. The source of water is the most important phenomenon, whether it is a canal or drainage water. Drainage water is usually affecting the soil system than the water from the canal, in terms of salinity. The complicated nature of the interaction of soils systems and water is governed by the process the exchange of chemical between the water and the soil (Asante et al., 2005).
The other most important problem of the effect of the poor quality water is toxicity of some ions in irrigation water. Many fruit trees and other cultivations are susceptible to injury from salt toxicity. Chloride, sodium and boron are absorbed by the roots and transported to the leaves where they accumulate. In harmful amounts, they result in leaf burn and leaf necrosis. Moreover, direct contact during sprinkling of water drops with a high chloride content may cause leaf burn in high evaporation conditions. To some extent, bicarbonate is also toxic. Other symptoms of toxicity include premature leaf drop, reduced growth and reduced yield. In most cases, plants do not show clear toxicity problems until it is too late to remedy the situation.
Chloride and sodium ions are both present in the solution. Thus, it is difficult to determine whether the damage caused is due to one or to the other. Chloride ions in high concentrations are known to be harmful to citrus and many woody and leafy field crops. A chloride content exceeding 10 meq/litter may cause severe problems to crops. The effect of sodium toxicity is not very clear. However, it has been found that it may cause some direct or indirect damages to many plants. Boron is an essential element to the plants. However, where present in excessive amounts, it is extremely toxic, even at relatively very low concentrations of 0.6 mg/litter. Toxicity occurs with the uptake of boron from the soil solution. The boron tends to accumulate in the leaves until it becomes toxic to the leaf tissue and results in the death of the plant. In arid regions, boron is considered the most harmful element in irrigation water (Guy, unpublished).
In assessing, the quality of irrigation water, one has to identify the relative concentration of the cations, ions and compounds that have a direct effect on plant physiology, growth and also the soil system. More importantly, the acceptable limits of the concentrations should be known. There are a number of laboratory techniques to measure the quality of water for each of the parameters. For instance, the suitability of water for irrigation is usually assessed by measuring the total dissolved salts (TDS) and or the electrical conductivity where a close relationship exists between the two measurements. But the latter is easier and TDS is easily inferred from the electrical conductivity. The acidity or alkalinity of water is assessed by pH reading where the hydrogen ion concentration of water is directly measured in the water solution.
Given that water is available for irrigation practice, proper assessment and analysis of the quality of water is congruently importance. Information on the quality of water will support to develop strategies for appropriate irrigation practices that will reduce the impact of the undesirable chemicals on proposed crop yields and also the soil profile. In Ethiopia, particularly, at Kuflo River where this study has been conducted, data on water quality of rivers is scarcer, and the purpose of this study was therefore to make the information available so that it can assist future irrigation water management options.
The study site is located Arba Minch Zuriya district in the Gamo Gofa zone of the Southern Nations and Nationalities Peoples’ Regional State of Ethiopia. Situated in the Great Rift Valley region, the district is located about 505 km south west of Addis Ababa, the capital of the country. The district has a total of 29 kebeles (the smallest administration unit in the country). Agro ecologically 4 kebeles are highlands, 15 kebeles at mid altitude and the rest 10 are found in the low land zone. The district has a total area of 168,172 hectares and the total population is 74,879 in Arba Minch city (the capital of the Zone and the district) and 164,529 in Arba Minch Zuriya district. The altitude of the district ranges from 1200 - 3300 masl with a rainfall amount of 800 to 1200 mm per annum. The temperature also ranges from 16 - 37°C. There are two cropping seasons, namely, Belg (short rainy season from March to May), Meher (main rainy season of June to September). The district is also surrounded by the two Rift Valley lakes namely Chamo and Abaya that have a great economic as well as ecological value to the area (FDRE-PCC, 2008).
The district has about 8 major rivers of which River Kulfo is being used by farmers and private investors to grow different crops. The smallholder farmers and the private investors grow crops like banana, mango, papaya, maize, cotton, sweet potato, etc. (Zenebe et al., 2015) (Figure 1).
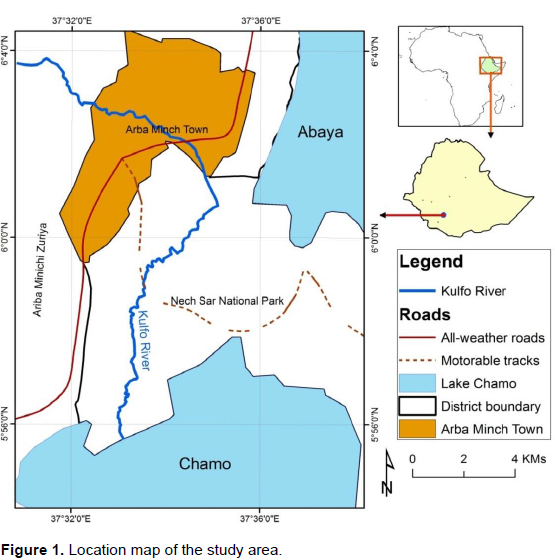
The upper catchment of the Kuflo River where samples were taken is presented in Figure 2. The widely accepted threshold values for classifying the suitability of water for irrigation (FAO, 1989) were used. The analysis for the physio-chemical parameters of the samples was carried out using the standard laboratory procedures. Electrical conductivity (ECw) and pH (H2O) were determined using electrical conductivity meter provided with automatic temperature compensation and potentiometrically with pH-meter equipped with single probe combined glass-calomed electrode (Greenbergs et al., 1992) respectively. Soluble Na+ and K+ were determined by flame-photometer after proper calibration with combined Na-K standard solutions (RTI, 1991), while soluble Ca+2 and Mg+2, were analyzed directly by atomic absorption spectrophotometer (APHA, 1998). Chloride (C1-) and HCO3 ions were measured by the argentometric method, by titrating against silver nitrate standard solution with potassium chromate indicator with a procedure from (Greenbergs et al., 1992). The turbid metrical procedures were solid residues weighed after evaporation for measuring TDS (Chopra and Kanwar, 1980). Similarly, sulfate (SO42-) was analyzed turbidmetrically as described by Rezwanul et al. (2007) and nitrate content (NO3-) was determined with spectrophotometric (AOAC, 1990).

The water quality parameters tested and the procedures and equipment used is described in Table 1.
The concentration of important cations of the irrigation water is presented as in Table 1. Calcium cation (21 g/L) had the highest concentrated followed by sodium (8.3 g/L), magnesium (5) and potassium (1.8) has the least concentrated in the Kuflo River. The relatively higher concentration of the calcium cations can be linked with the existence of the calcium bicarbonate in the river (Ababu and Bern, 2014) which might have originated from basaltic formation of parent rock in the region. The relatively high concentration of calcium cation may limit the hazardous effect of sodium which latter causes the dispersion of the soil aggregates and eventually creating problem water and movement in the soil systems (Mathess, 1982). The low level of potassium concentration in the water may implicate minimum wash away of the fertilizers from the soil into the water from the upstream farming areas. However, this could be relatively higher in the downstream areas where compounds may settle (Table 2).
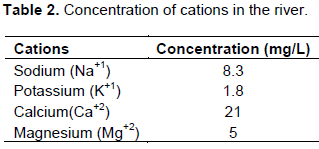
Similarly, the ion concentrations of the important anions for the quality of water are presented in Table 3. The bicarbonate ion is highly concentrated followed by the chlorides and nitrates. The abundant presence of the bicarbonate is again associated with the basaltic formation of the parent material in the vicinity areas of the river, Kulfo. The relatively high nitrates in the form of NO3 could be associated with high use of nitrogen fertilizers and agrochemicals in the upstream areas. Furthermore, the relative high concentration of nitrates in this form might have been linked to the repeated irrigation practices in the area.
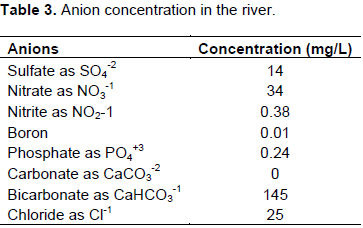
The concentration level of the chloride ion has significantly (25 mg/L) increased from what it was reported (3 to 12 mg/L) in 2004 by Ababu and Bern (2004). The chloride ion can be taken up by plant roots and accumulate in the leaves. The progressive increase of the chloride ion is a warning that there could be some sorts of pollution to the water due to human activity, though the current range is no hazardous. Excessive accumulation of chloride my cause burning of the leaf tops or margins, bronzing and permanent yellowing. Most importantly, fruit trees are sensitive while there are moderate, crops, like vegetables. In general, intents of the nature of the concentration of ions and anions, the water is still safe for irrigating for most of the crops.
The most important water quality parameters are presented in Table 3. Accordingly, both the electrical conductivity and the sodium adsorption ration are relatively low and it implicates that the water is non saline and can safely be used for irrigation. The electrical conductivity of water is an important quality parameter that categorizes the nature of water for irrigation use. Irrigation water with more than 0.7 dS/m and TDS greater than 450 mg/l are labelled as saline water and caution need to be taken in using the water. The high electrical conductivity of water affects the water availability to plants once it enters the soil profile and eventually reduces the growth and yield of crops. For instance, under repeated irrigation, salinity levels of a saline water with greater than 3 dS/m would reduce the total yield of lemon and keeping the salinity of irrigation water is critical for areas that are irrigated continuously (Blaine et al., 2006).
In the due courses of climate change that causes more evaporative demand and repeated irrigation practices, the development of soil salinity is expected. Due to the complicated interaction of the soil system and water bodies, the rise in the salt concentration of water is axiomatic. Kulfo River is located in the semi-arid areas of Ethiopia, the contribution of climate change and interest in the expansion of irrigation would impact the chemical composition of the water. Hence, proper use of irrigation water demands protection of the river from polluting substances from the upper catchment that may be engaged in irrigated agriculture (Table 4).
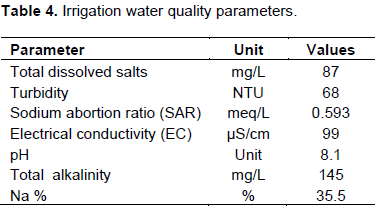
As expected, the alkalinity (145 mg/l) of the water is relatively high and corresponds to the high concentration of the bicarbonates in the water. The turbidity of water is high and might have resulted from anthropogenic activities in the vicinity of the river. Among which, construction and agricultural production trend might have disturbed land surfaces, potentially contributing to soil particles as well as nutrients to aquatic systems. Landscape fertilization and chemicals for weed control activities tend to be leached into the river.
Irrigation is a common practice for farmers in the periphery of flowing rivers in Ethiopia. In the south west Ethiopia, fruits and vegetables are majorly produced under irrigation where the mode of irrigation is by pumping water from flowing rivers. Irrigated agriculture has only been linked to the availability of water in physical terms while the quality of water is significantly important for sustainable agricultural production. A study was conducted to assess the water quality parameter of the Kuflo River in South West part of Ethiopia. Accordingly, the analysis of the results showed that calcium cations is the highest concentration followed by sodium, magnesium and potassium is the least concentrated in the Kuflo River. The relatively higher concentration of the calcium cations can be linked to the existence of the calcium bicarbonate in the river which might have originated from basaltic formation of parent rock in the region. Similarly, the bicarbonate ion is the highset concentrated followed by the chlorides, and nitrates. The abundant presence of the bicarbonate is again associated with the basaltic formation of the parent in the vicinity areas of the river, Kuflo. The relatively high nitrates in the form NO3 could be associated with high use of nitrogen fertilizers and agrochemicals in the upstream areas. Furthermore, the relative high concentration of nitrates in this form might have been linked to the repeated irrigation practice in the area. More importantly, both electrical conductivity and sodium adsorption ration are relatively low and it implicates that water is non-saline and can safely be used for irrigation.
The authors have not declared any conflict of interests.
REFERENCES
|
Ababu T, Bern E (2004). Water Quality of Monitoring the Abaya-Chamo Drainage Basin. FWU VOL. 4, Lake Abaya Research Symposium 2004 proceedings.
|
|
|
|
AOAC (1990). Official Methods of Analysis of the Association Analytical chemists. 15th ed. Virginia, USA.
|
|
|
|
|
APHA (American Public Health Association) (1998). Standard Methods for the Examination of Water and Waste Water'.17th ed. Washington, D.C. 200005. pp. 1-30 to 40-175.
|
|
|
|
|
Asante K, Quarcoopome T, Amevenku F (2005). Water Quality of the Weija Reservoir after 28 years of Impoundment. West Afr. J. Appl. Ecol. 13:171-180.
|
|
|
|
|
Ayers RS, Westcot DW (1985). Water Quality for Agriculture, Irrigation and Drainage Paper 29, rev. 1, FAO, United Nations, Rome.
|
|
|
|
|
Blaine RH, Stephen RG, Allan F (2006). Agricultural Salinity and Drainage. Division of Agriculture and Natural Resources Publication 3375, University of California Irrigation Program, University of California, Davis.
|
|
|
|
|
Causapé J, Quílez D, Aragües R (2004). Assessment of irrigation and environmental quality at the hydrological basin level irrigation quality. Agric. Water Manage. 70:195-209.
|
|
|
|
|
Chopra SL, Kanwar JS (1980). Analytical Agricultural Chemistry', Kalyan Publishers, New Delhi. pp. 168-307.
|
|
|
|
|
Dhirendra M, Alok K, Namita A (2009). Assessment of the irrigation water quality of river ganga in Haridwar district. RasÄyan J. Chem. 2(2):285-292.
|
|
|
|
|
FAO (Food and Agriculture Organization) (1985). Water quality for agriculture. FAO Irrigation and Drainage Paper No. 29 (Rev. 1). Rome. Italy. pp. 1-140.
|
|
|
|
|
FAO (1989). Water quality for agriculture. FAO, Rome, P 163.
|
|
|
|
|
FDRE-PCC (Federal Democratic Republic of Ethiopia, Population Census Commission) (2008). Summary and Statistical Report of the 2007 Population and Housing Census. Addis Ababa, Ethiopia.
|
|
|
|
|
Guy (undated). Irrigation Water Quality Standards and Salinity Management, the Texax A and M university, access date 24 -11-2016
View
|
|
|
|
|
Greenberg AE, Clesceri LS, Eaton AD (1992). Standard Methods for the Examination of Water and Waste water (18thEd.). American public Health Association, American Water Works Association, Water Pollution Control Federation, Washington DC.
|
|
|
|
|
Islam MR, Jahiruddin M, Islam S (2004). Assessment of Arsenic in the water-soil-plant systems in Gangetic Floodplains of Bangladesh'. Asian J. Plant Sci. 3(4):489-493.
Crossref
|
|
|
|
|
Islam MS, Shamsad SZKM (2009). Assessment of Irrigation Water Quality of Bogra District in Bangladesh. Bangladesh J. Agric. Res. 34(4):597-608.
|
|
|
|
|
Mathess G (1982). The Properties of Groundwater. John Willey and Sons, New York. P 406.
|
|
|
|
|
Megersa O, Willibald L, Josef F (2009). Effect of Lake Basaka expansion on the sustainability of Metahara SE in the Awash River basin, Ethiopia. Water, sanitation and hygiene: sustainable development and multi sectoral approaches. 34th WEDC International Conference, Addis Ababa, Ethiopia.
|
|
|
|
|
Quddus KG, Zaman MW (1996). Irrigation water quality in some selected villages of Meherpur in Bangladesh. Bangladesh J. Agric. Sci. 23(1):51-57.
|
|
|
|
|
Ragab AM (2004). Influence of irrigation with saline drainage waters on some soil physico-chemical properties of the northern west area of Nile delta. Fayom J. Agric. Res. Dev. 18(1):133-142.
|
|
|
|
|
Raju NJ (2006). Hydrogeo chemical parameters for assessment of groundwater quality in the upper Gunjanaeru River basin, Cuddapah District, Andhra Pradesh, India. Environ. Geol. 52(6):1067-1074.
Crossref
|
|
|
|
|
Rao NS, Devadas DJ (2005). Quality criteria for ground water use for development on an area. J. Appl. Geochem. 7(9):145-160.
|
|
|
|
|
Rezwanul M, Naoto I, Ranjit S (2007). Assessment of Irrigation Water Quality by Using Principal Component Analysis in Arsenic Affected Area of Bangladesh. J. Soil Nature 1(2):08-17.
|
|
|
|
|
RTI (Royal Tropical Institute) (1991). Analytical Methods of the Laboratory for Soil, Plant and Water Analysis. Part I: Methods for Soil Analysis. Amsterdam, the Netherlands.
|
|
|
|
|
Singh AK, Mondal GC, Suresh Kumar TB, Singh BK, Tewary A (2008). Major ion chemistry, weathering processes and water quality assessment in upper catchment of Damodar River basin, India. Environ. Geol. 54:745-758.
Crossref
|
|
|
|
|
Sukhdev K (2012). Assessment of Surface Water Quality for Drinking and Irrigation Purposes: A Case Study of Ghaggar River System Surface Waters. Bull. Environ. Pharmacol. Life Sci. 1(2):1-6.
|
|
|
|
|
Zenebe W, Ali M, Derbew B, Zekarias S, Adam B (2015). Assessment of Banana Production and Marketing in Ethiopia. Int. J. Sci. Basic Appl. Res. 24(3):283-307.
|
|
|
|
|
Zhai Y, Yang Q, Hou M (2015). The Effects of Saline Water Drip Irrigation on Tomato Yield, Quality, and Blossom-End Rot Incidence --- A 3a Case Study in the South of China. PLoS ONE 10(11): e0142204.
Crossref
|
|