ABSTRACT
This study determined the quality of surface and underground water in Itagunmodi in Nigeria. Physicochemical analysis of water samples collected within gold mining area and the water consumed in the town was carried out to determine their hygienic conditions. The major objective of this study was assessment degree of groundwater pollution around the mining sites and the available water consumed in the town. Four major sources of water were analyzed. Several physical and chemical parameters were tested in groundwater, these include pH, total solids (TS), total dissolved solids (TDS), conductivity, total alkalinity, total hardness (TH), chloride (Cl-1), sulphur (S), heavy metals (Pb, Zn, Cu), major cation (Mg). The results show that the parameters are within the standard acceptable levels which are required for drinking water adapted from World Health Organization. The pH of the samples was suitable for drinking according to the World Health Organization standard. For a safe drinking water, the pH should be between 6.5 and 8.5); it can be said that the water quality of the samples are fairly good based on the monitored elements and physicochemical characteristics.
Key words: Groundwater, conductivity, heavy metals, gold mining site, pollution.
Groundwater is water located beneath the ground surface in soil pore space and in the fractures of lithologic. It makes up about 20% of the world’s fresh water supply which is about 0.61% of the entire world’s water including oceans and permanent ice. Groundwater is naturally replenished by surface water from precipitation, streams and rivers when this recharge reaches the water table. Rain water dissolves soluble salts from vegetations, topsoil, river bed, lake bed into water bodies, hence most ions (Ca2+, Mg2+, Na+, K+ and NH4+) in rain water are also found in surface and groundwater.
Groundwater is an important water resource in both the urban and rural areas of Nigeria but in the cities, pipe borne water is also available. Rural dwellers rely basically on hand-dug wells for potable water supply as the streams usually dry up in dry season. These resources are under threat from pollution either from human life style manifested by the low level of hygiene practiced in the developing nations (Punmia and Jain, 1998; Akujieze et al., 2003). Environmental health involves all the factors, circumstances and conditions in the environment or surroundings of humans that can influence health and well being. The neglect of rural areas in most developing countries in terms of basic infrastructures such as pipe-borne water and sanitation facilities, expose the villagers to a variety of health related problems such as water - borne diseases (Sridha, 2000).
Recent research conducted by Yisa and Jimoh (2010) showed that there was an increase in the demand for freshwater due to rapid growth of population as well as the accelerated pace of industrialization in the last few decades. Groundwater has long been considered as one of the purest forms of water available in nature and meets the overall demand for rural and semi-rural people (Tyagi et al., 2002). This was considered as the major source of water for human activities (consumption inclusive) especially in the rural area (Fasunwon et al., 2008).
Inadequate amounts of water for basic hygiene can contribute to poor hygiene practices, which in turn can lead to skin and eye diseases, and act as a key factor in the transmission of many diarrhoeal diseases (Ramakrishnaiah et al., 2009) reported that once ground water is contaminated, its quality cannot be restored. It therefore becomes imperative to protect it in other to prevent water borne diseases such as typhoid, cholera, diarrhoea and dysentery which are potentially communicable (Okeke and Igboanua, 2003; Suleiman, 2006).
Water from various sources contains varying forms of dissolved gases, minerals, organic and inorganic substances, chemical compounds and suspended matter such as organisms and dirt (Erah et al., 2002). Pure water is never found in nature, but contains impurities which vary from dissolved gases, chemical compounds to suspended matter such as organism and dirt especially in this part of the world (Sandhu et al., 1976). The most important physical characteristics are turbidity, colour, odour, taste, temperature and solids, while the chemical characteristics are acidity, alkalinity, hardness and corrosiveness (Ademoroti, 1996a). Water of good quality is necessary if it is to be acceptable by the people.
Palatability of water describes the characteristics of being pleasing to sense of taste. However, palatable water in real sense is not necessarily safe to drink or potable (Packham, 1996). Trace metals of different types can be found in water. Very low amount of trace metals are required by living things, but in excess concentration, metals can be harmful (Rand and Petriocelli, 1985). Potassium and sodium are very soluble salts and most of them leached from rocks, soils and plants tend to remain in solution. High concentration of potassium and sodium cause foaming in boiling water and have toxic effect on fish (Vermani, 1989).
Some water bodies may contain pathogens that are harmful in the body. The water sources in the area were either from bore-hole, streams or other sources, in which one may not be sure if analysis were carried out or not. Access to potable water is still a major concern in all the developing nation of the world as millions of people died of water-borne diseases annually.
In this study, the levels of some physical, chemical, water quality parameters in hand-dug wells, streams, borehole, deep well located in the residential areas and in the vicinities of Itagunmodi, a rural settlement in southwest Nigeria was carried out. In this work water from gold mining area was included in the analysis. Since gold is extremely resistant to weathering, when freed from enclosing rocks, is carried downstream as metallic particles consisting of "dust," flakes, grains, or nuggets.
Geology of the study area
Itagunmodi is located on latitude (DMS) 7031’60’’ longitude (DMS) 4039’0’’ and altitude (meters) 347 the time zone is east (est). The approximate population for 7 km radius from this point is 12655. Itagunmodi is a town very close to towns such as: Ile-Ife, Ilesa in Osun State, Nigeria. The sediment-hosted disseminated gold deposits (SHDGD) in Itagunmodi, Osun State, Nigeria is located in the clayey soil types derived from variably migmatised gneiss, biotite-and-biotite hornblende-gneiss and weathered amphibolites respectively (Figure 1).
Sample collection
The analyses were carried out between the months of February to July, 2013, and their average values were recorded in Table 1. The water sources include: (i) Deep well; (ii) Shallow well; (iii) stream, and (iv) Borehole. Samples were taken to the laboratory within two weeks. It was found out in the course of the work that most of the inhabitants get most of their domestic water from the well and drink directly without purification. The mean concentrations of the samples were calculated and used for the study.
Parameters analyzed
Physical and chemical parameters were analyzed in groundwater samples. The physical parameters include pH and electrical conductivity (EC). The chemical parameters include: Nitrite (NO3), chloride (Cl), sulphate (SO4), lead (Pb), iron (Fe), zinc (Zn), copper (Cu), biological oxygen demand (BOD), and groundwater, these include pH, total solids (TS), total dissolved solids (TDS), conductivity, total alkalinity, total hardness (TH), chloride (Cl-1), sulphur (S), heavy metals (Pb, Zn, Cu), major cation (Mg).
Theory
Where M = Molarity of EDTA used and V = volume of EDTA used.
Fe and Cr were measured with S series atomic absorption spectrophotometer (AAS)
The pH values of the samples were shown in Table 1. The highest average value of 7.83 was measured in sample 2, and the lowest value of 6.5 was measured in sample 5, this was shown in Figure 1. The results obtained were within the standard range of 6.5 to 8.5 required for drinking water according to W.H.O and the Nigeria standard. This occurs in most natural waters. The lower the pH, the more corrosive the water will be. The pH of sample collected from the gold mining site is acidic while the other samples are alkaline. Those samples within the town of Itagunmodi are also regarded as safe drinking water; the average pH values lies between 6.5 and 7.4, these values lie between the permitted ranges in drinking water quality standard used in Nigeria.
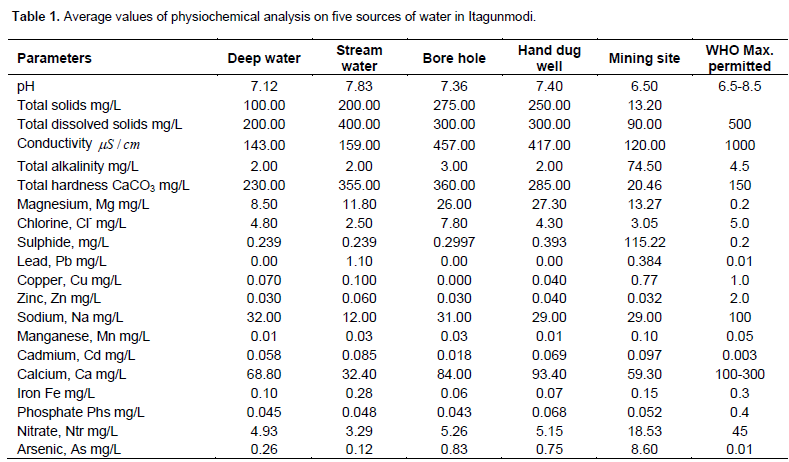
Low values are most often caused by lack of carbonate minerals, such as calcium and magnesium found in limestone. Water leaking from a landfill may also lower pH. Other analysis such as conductivity, total dissolved solids, chlorine, copper and zinc, falls within the maximum permitted level with reference to Nigeria drinking water standard which are: 1000.00

, 500.00 mg/L, 250.00 mg/L, 0.01 mg/L, 1.00 mg/L and 3.00 mg/L.
The total solids analyzed, the highest value was recorded in sample 3, and lowest in sample 5. It was high in sample 4 and the lowest was recorded in sample 5 while in total dissolved solids, the highest was recorded in sample 2 and the lowest was in sample 5, samples 3 and 4 were higher than that in sample 5. The conductivity was higher in sample 3 than in sample 4 while those of samples 1 and 2 were lower and sample 5 has the lowest value. The alkalinity was higher in sample 5, while those of samples1, 2, 3 and 4 were low. The concentration of CaCO3 in samples 2 and 3 were higher than the value obtained sample 1 and 4 with the lowest value in sample 5. All the samples have higher concentration in Mg because they exceed the WHO standard for drinking.
The average concentration of chlorine was higher in sample 3 then sample 1, 5 and 4, with the lowest value in sample 2. Average sulphate concentration was highest in sample 5, high in sample 4 and low in samples: 1, 2 and 3. For Lead, sample1, 3, 4 has zero concentration and sample 2 and 3 concentrations exceed WHO standard. The average concentration of Cu was highest in sample 5 than in other sample. The average concentration of Zn was higher in sample 2 than in other samples, The maximum permitted level for magnesium in Nigeria is 0.20 mg/L, this value depend on consumer acceptability e.t.c., can be used to confirm the water samples as been hygienic for human consumption. This does not pose any significant water quality problem, because these results are within the standard acceptable levels of drinking water used in Nigeria. For Arsenic, all the samples are polluted with Arsenic because the arsenic concentrations exceed WHO standard. Arsenic can cause damage to the nervous system. Examples of diseases caused by arsenic are: Cancer of the lung, skin, prostate, kidney and liver, diabetes mellitus and hypertension. The WHO’s guideline value for arsenic in drinking water is 0.05 mg/L (given a provisional guideline value of 0.01 mg/L in 2006).
Phosphate in all the samples exceeds the WHO standard which means that water is polluted with phosphate in all the samples. In the sample Iron in sample 2 has the highest concentration, samples 3, 4, and 5 have higher concentration and sample 1 has the lowest concentration. Iron in all the samples is within WHO standard for drinking water. Although, iron is an important dietary requirement in humans needed by haemoglobin and good for several other functions, when high concentrations of iron are absorbed, iron is stored in the pancreas, the liver, the spleen and the heart. This may damage those vital organs. The presence of excess iron in water imparts the taste and it also promotes growth of iron bacteria that hasten rusting process of all the ferrous metals that come in contact with the water (Chukwu et al., 2008).
A FORTRAN program was also used to determine the present state of water sources in the town, the data for each set of parameters were insert into program at various stage and run. The results show that the samples were within the intake of the people.
Water is the essence of basic survival. Without it, life on Earth would cease to exist. In order to ensure that human life continues to exist, we must work together and do our part to improve the quality of drinking water. Researchers must do their part in the laboratory to come up with treatment methods to improve the quality of drinking water. When the quality of drinking water is good, human health is also good. The non detection of pollution or small concentration of these metals implies that the groundwater in the study area were good. Pollution has not taken place and could be a result of the distant from the pollutants. It could also be due to the direction of groundwater flow, that is the pattern and movement, it could be due to the quality of recharge water as well as the complicated reaction between the water and minerals contained in the rocks. Therefore water sources from the study area were suitable for domestic use and are not likely to pose a major health risk to consumers. This research may serve as a reference for future studies on the assessment of water quality in the study area and its surrounding.
Human activities such as the use of chemicals in agriculture, effluent from homes, and sewage disposal, industrial waste discharged are all factors that contaminate water. After running the initial set of tests, well users should continue to test for bacteria once in a year. It’s also a good idea to test for nitrate annually for several years. However, a nitrate test should always be conducted if an infant is drinking the water. Hence, it was recommended that physicochemical analysis should be carried out from time to time. At least once every three years. And on site drinking water systems shall be checked at least every 3 years (Nigerian Industrial Standard NIS 554: 2007).
Water is susceptible to various toxic pathogens and chemicals. Therefore, it is of extreme importance that the quality of water is tested through frequent monitoring. Water sources must be protected from potential source of contamination.
For on-site drinking water system, a minimum distance of 15 m shall be kept between the water system and potential source of contamination. Communities shall keep clean the protected area surrounding on-site drinking water systems.
The Federal Ministry of Environment in consultation with the States should declare special protection zones for Chemical Elements Sensitive Areas (such as Nitrates, heavy metals), wetlands based on such local peculiarities. For mechanized centralized drinking water, systems (high yield), broader protection zones shall be established and enforced by the Ministry of Environment.
There should be a long term sustained programme of monitoring the quality of groundwater in the community, to have a complete understanding of the ground water resources. Awareness should be created among the people on the possible danger of polluted water consumption and the diseases associated with it if not treated.
The authors have not declared any conflict of interests.
The authors appreciate the contributions made by Geophysics Group at the University of Ibadan, Nigeria.
REFERENCES
|
Ademoroti CMA (1996a). Environmental Chemistry and Toxicology, Foludex press Ltd. Ibadan, Nigeria pp. 215-218. |
|
|
Akujieze CN, Coker S, Oteze GE (2003). Groundwater in Nigeria – A millennium experience: distribution, practice, problems and solutions. Hydrogeol. J. 1:259-274.
Crossref |
|
|
|
Chukwu O (2008). Analysis of Groundwater pollution from abattoir waste in Minna, Nigeria. Res. J. Dairy Sci. 2(4):74-77. |
|
|
|
Erah PO, Akujieze, CN, Oteze GE (2002). Quality of groundwater in Benin City: A baseline study on inorganic chemicals and microbial contaminants of health importance in boreholes and open wells. Trop. J. Pharm. Res. 1:75-82. |
|
|
|
Fasunwon O, Olowofola J, Akinyemi O, Fasunwon B, Akintoku O (2008). Contaminants Evaluation as Water Quality Indicator in Ago-Iwoye, South-western, Nigeria. Afr. Phys. Res. 2:12. |
|
|
|
Nigerian Industrial Standard (NIS) (2007). 554:1-30. |
|
|
|
Okeke CO, Igboanua AH (2003). Characteristics and quality assessment of surface water and groundwater recourses of Awka Town, Southeast, Nigeria. J. Nig. Assoc. Hydrol. Geol. 71-77. |
|
|
|
Packham RF (1996). Drinking water quality and health: In pollution causes, Effects and control. Third Edition. (Harrison RM Ed.) Royal Society of Chemical, U.K pp. 52-65. |
|
|
|
Punmia BC, Jain AK (1998). Wastewater Engineering. Laxmi Publications (P) Ltd, New Delhi. |
|
|
|
Ramakrishnaiah CR, Sadashivaiah C, Ranganna G (2009). Assessment of water quality index for the groundwater in Tumkur Taluk, Karnataka State India 6(2):523-530. |
|
|
|
Rand GN, Petrocelli SR (1985). Fundamentals of aquatic toxicity Hemisphere Publishing Corporation, pp. 1-46. |
|
|
Sandhu SS, Waren WJ, Nelson SP (1976). Traces of inorganic in rural potable water and their correlation to possible sources. J. Water Res. 12:257-261.
Crossref |
|
|
|
Sridha MKC (2000). Ground water in Nigerian urban centers: problems and options. Schriftenr VerWasser Boden Luftyg. (105):393-397. |
|
|
|
Suleiman FB (2006). Analysis of Some Sachet Water Samples in Katsina, Nigeria. Chem. Class J. Chem. Soc. Nig. Zaria Chapter 3:42-44. |
|
|
|
Tyagi PD, Buddhi R, Chaudhary KC, Sawhney RL (2002). Degradation of ground water quality in industrial area in India. Ind. J. Environ. Prot. 20:174-181. |
|
|
|
Vermani OPN (1989). Applied Chemistry, Theory and Practice by Inily Eastern Ltd, New Delhi, pp. 34-60. |
|
|
Yisa J, Jimoh T (2010). Analytical Studies on Water Quality Index of River Landzu. Am. J. Appl. Sci. 7(4):453-458.
Crossref |
APPENDIX
This program is designed in the Department of Physics University of Ibadan to interprete geochemical data from leachates-polluted groundwater with main focus on 19 physio-chemical priority parameters (mainly inorganic) in accordance with WHO (2006) Standard and the United States Geological Survey and the United State Environmental Protection Agency Standard (2002) on secondary drinking water regulation.
TMP: Temprature
CLR: Colour
CDTY:Conductivity
ODR: Odour
TDS: TOTAL DISSO
LVED SOLIDS
PH: HYDROGEN ION CONCENTRATION
NA: SODIUM
CA: CALCIUM
MG: MAGNESIUM
FE: IRON
MN: MANGANESE
AL: ALUMINIUM
CU: COPPER
ZN: ZINC
AG: SILVER
CHL: CHLORIDE
SUL: SULPHATE
PHS: PHOSPHATE
FL: FLUORIDE
NTR: NITRATE
AS: ARSENIC
CD: CADMIUM
PB: LEAD
Z: SUMMATION OF THE DATA OF THE SEVEN MAJOR CATIONS: CA,MG,FE,MN,AL,AS,CD,PB FOR THE HARDNESS TEST
A: SUMMATION OF ANIONS
C: SUMMATION OF CATIONS
7 FORMAT(T1,A25,F9.3,A20)
10 FORMAT(T1,A160)
17 FORMAT(T1,A35,T1/T1,32('='),1X)
REAL:: TMP,CLR,CDTY,ODR,TDS,PH,NA,CA,MG,FE,MN,AL,CU,ZN,AG,CHL,PHS,FL,NTR,AS,CD,PB,Z
OPEN(UNIT=1111,FILE='RESULTS')
PRINT*, 'THE INTERPRETATION OF RESULT'
PRINT*,''
WRITE(1,7)'DATE: 15/05/2013'
WRITE(1,10)
WRITE(1,7)'SAMPLE ID: MINING SITE'
WRITE(1,10)
WRITE(1,7)'SITE/LOCATION: ITAGUNMODI'
WRITE(1,10)
PRINT*,'INSERT THE DATA OF THE PHYSICAL PARAMETERS:TMP,CLR,CDTY,ODR,TDS'
READ(*,*) TMP,CLR,CDTY,ODR,TDS
PRINT*,''
WRITE(1,17)'INTERPRETATION AND TEST RESULT'
WRITE(1,10)
WRITE(1,17)'PHYSICAL PARAMETERS'
WRITE(1,10)
WRITE(1,7)'TEMPERATURE =',TMP,'C'
IF(TMP.GE.12.AND.TMP.LE.25)THEN
WRITE(1,10)'TEMPERATURE WITHIN STANDARD'
ELSEIF(TMP.LT.12)THEN
WRITE(1,10)'TEMPERATURE BELOW STANDARD,VISCOCITY AND IONIZATION MAY INCREASE'
ELSE
WRITE(1,10)'TEMPERATURE EXCEEDS STANDARD,POSSIBLITIES OF INCREASE IN VAPOUR,PRESSURE AND SOLUBILITY'
ENDIF
WRITE(1,10)
WRITE(1,7)'COLOUR =',CLR,'UNIT'
IF(CLR.GT.15)THEN
WRITE(1,10)'COLOUR EXCEEDS STANDARD'
ELSEIF(CLR.LT.15)THEN
WRITE(1,10)'COLOUR WITHIN STANDARD'
ELSE
WRITE(1,10)'COLOUR EQUALS STANDARD'
ENDIF