ABSTRACT
Provision of integrated care for human immunodeficiency virus (HIV) co-infected tuberculosis (TB) patients is challenging. Many persons with TB and HIV co-infection are not yet receiving anti-retroviral therapy (ART) and initiation of ART is not always timely. This study investigated ART uptake among HIV co-infected TB patients and its time of initiation in an urban primary health care facility in Ethiopia. A retrospective cohort study was conducted using routine program data. All adult HIV co-infected TB patients registered in a large TB-HIV clinic in Addis Ababa from September, 2008 to August, 2014 were included. Both descriptive and inferential statistics were used to summarize and analyse findings. A total of 993 TB patients were registered in the study period and included. HIV counselling and testing was offered to 738 (74.5%) and HIV testing was performed for 678 (68.3%) patients. Of those tested, 226 (33.3%) were HIV co-infected of whom 125 (57.6%) were started on ART. The median period from commencement of TB treatment to starting of ART was 41 days. ART initiation was delayed beyond the period advised in the National TB-HIV Guideline for 31 (27%) of HIV co-infected TB patients. For 109 (48.2%) of co-infected TB patients the ART status evaluation could not be done due to missing data. A considerable proportion of HIV co-infected TB patients did either not receive ART or started it later than recommended by national guidelines. For better outcomes to HIV co-infected TB patients, the actual implementation of national recommendations on when to start ART needs to be monitored closely.
Key words: ART-uptake delay, TB-HIV, primary health facility.
Screening of all tuberculosis (TB) patients for human immunodeficiency virus (HIV) co-infection and referral of HIV positive patients for antiretroviral treatment (ART) initiation and chronic care services are essential components of collaborative TB-HIV activities and crucial to reduce mortality and morbidity in TB patients (FMOH, 2007; Sileshi et al. 2013). Medical management of HIV co-infected TB requires standardized anti-TB treatment combined with trimethoprim-sulphamethoxazole (co-trimoxazole) prophylactic therapy (CPT) and ART (WHO, 2010). The recommended timing of ART initiation after TB treatment start has been adapted several times in the last decade based on the available evidence (Lawn et al., 2009). The latest insights are that early initiation of ART started within 2 weeks of commencing TB treatment, in all HIV co-infected TB patients regardless of CD4 count significantly improves survival (Abdool Karim et al., 2010; Nglazi et al., 2015)and is particularly beneficial for patients with CD4 counts <50 cells/μl (Franke et al., 2011; Salim et al., 2011; Naidoo et al., 2013; Stockdale et al., 2013). In 2014, globally, 51% of notified TB patients had a documented HIV test result and 77% of TB patients known to be HIV co-infected started ART. In Ethiopia with a high burden of both TB and HIV, 75% of TB patients had a documented HIV test result while only 39% of HIV co-infected TB patients had started ART (WHO, 2014).
The National Ethiopian TB-HIV guideline was revised in 2007 and 2013 specifically with regards to timing of ART and how to prioritize HIV co-infected TB patients for ART initiation (FMOH, 2007; 2013). The 2007 guideline recommended ART to be started within 8 weeks of commencing TB treatment for patients with a CD4 count <200/μl and at completion of the intensive phase of TB treatment for patients with a CD4 count of 200 to 350/μl while deferring ART commencement for those with a CD4 count >350/μl. The updated Ethiopian TB-HIV 2013 guideline following new global guidance recommends ART initiation as soon as TB treatment is tolerated (usually within 2 to 8 weeks) for those with a CD4 count <200/μl, to start within 8 weeks of starting TB treatment for TB co-infected patients with a CD4 count ranging from 200 to 350/μl, while it is recommended to defer ART and reassess at 24 weeks or at completion of TB treatment for those with a CD4 count >350/μl. Whether or not the National guideline is fully adhered to and TB co-infected patients are started timely on ART, is not known. The objective of this study was to investigate ART uptake among HIV co-infected TB patients and timing of ART initiation in an urban primary health care facility, against the standard of care as described by the Federal Ministry of Health of Ethiopia in the National TB/HIV guidelines.
Study design and setting
A retrospective analysis of routine program data was carried out in a primary health facility in Addis Ababa. Addis Ababa, the capital city of Ethiopia, has an estimated population of 3 million (FDRE Population Census Commission, 2007). Addis Ababa regional health bureau oversees 11 hospitals and 26 health centers which provide comprehensive health services for its urban residents. Diagnostic and treatment services for TB and HIV are provided at both the hospital and health center level. Patients who cannot be managed at the health center level are referred to the hospital. Bole 17 health center is located in Bole sub-city of Addis Ababa. This health center is one of the first primary health care facilities to start HIV care in the city, including ART initiation and follow up. An average of 40 people living with HIV and TB attend this clinic every year In Ethiopia, HIV co-infected TB patients are referred to ART clinics to be registered and assessed for ART initiation according to the prevailing National TB/HIV guideline.
For the study, delay in starting ART was defined as: eligible HIV-co infected TB patients starting ART beyond the period indicated in the prevailing national guideline for TB/HIV collaborative activities at the time of TB treatment start, which is either the 2007 or 2013 edition of the guidelines. As per the guideline, eligibility is identified based on CD4 count, presence of opportunistic infections and WHO clinical stage. Most of the TB patients are diagnosed and start treatment at the health centre although the health centre also receives TB patients diagnosed elsewhere to start or continue TB treatment. All TB patients are offered HIV testing and counselling and HIV co-infected TB patients are referred to the ART clinic in the same health centre to be enrolled into HIV care. One health officer and one nurse are full time employed in the TB clinic with other trained health professionals providing help based on needs.
Sampling technique and study variables
For this study, all adult TB patients (>18 years) registered at Bole 17 Health Center from 1 September, 2008 to 31 August 2014 were included in the study. Patients who were already on ART before TB diagnosis were excluded. Sources of data were unit TB registers and ART registers. Patients registered for TB treatment from September, 2008 to March, 2013 were assessed against the 2007 guideline and those registered for treatment from April, 2013 to August, 2014 against the revised 2013 guideline (FMOH, 2007, 2013). The main outcome, delay in ART initiation was determined by calculating the difference between start date of TB treatment and start date of ART treatment, which is then compared with national TB/HIV management guideline on when to start ART for co-infected patients. Explanatory variables collected were age, sex, site of TB infection, pulmonary TB smear result, TB treatment history, TB treatment start date, HIV test offered, HIV testing done, HIV test result, HIV test date, CPT provision, CPT start date, enrolment to HIV care, date of enrolment to HIV care, WHO clinical stage at enrolment, CD4 cell count, recipient of ART, ART start date, opportunistic infection (OI) diagnosis, outcome of TB treatment, and timing of ART (that is, time between TB treatment start and ART start).
Data management and analysis
The data were double entered and analyzed using SPSS Version 2.0 statistical package (SPSS Inc’). Description of means, frequencies and proportions were used to describe all study variables. Bivariate analysis was performed to test for associations between each explanatory variable and the outcomes of interest. Explanatory variables that were found to be significant in bivariate analysis were included in a multivariate logistic regression model. This estimated the relative effect of the explanatory variables in predicting the outcomes of interest. A P-value ≤ 0.05 was considered as a statistically significant association and the adjusted odds ratio with 95% CI was calculated.
Ethical consideration
Ethical approval was obtained from the Ethical Review Committee of the Addis Ababa Regional Health Bureau and permission was obtained from the Tuberculosis Research Advisory Committee (TRAC) and the Bole 17 health center.
Patient characteristics
A total of 993 TB patients were registered over the six year period. Their median age was 29 years (inter-quartile range (IQR) 23 to 39) and 510 (51%) were male. The majority 947 (95%) patients had a first episode of TB (so called new patients) and 606 (61%) had pulmonary disease. Of the 606 pulmonary TB patients, 340 (56%) were sputum smear- positive for acid-fast bacilli (Table 1). The majority of TB patients 904 (91%) were evaluated against the 2007 guideline and the rest 88 (9%) were evaluated against the revised 2013 guideline.

HIV status and CPT and ART uptake
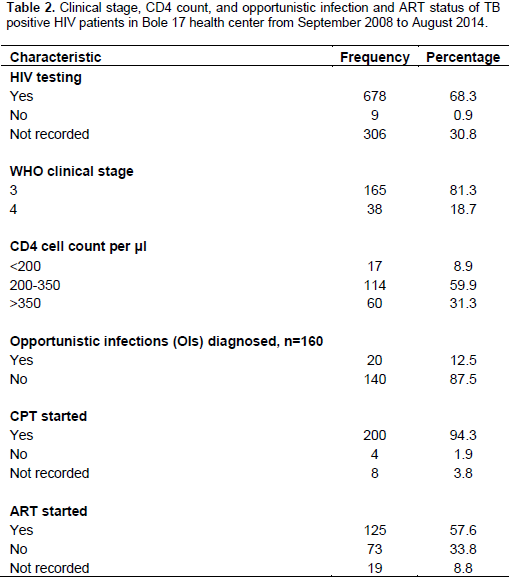
HIV counselling and testing was offered to 738 (74.5%) of the 993 TB patients and HIV-test was performed for 678 (68.3%) of whom, 226 (24.4%) tested HIV positive (Figure 1). Of the 226 HIV co-infected patients, 189 (83.6%) were enrolled in HIV care and 125 (57.6%) had started ART, while 200 (94%) were started on CPT. For 203 (89.8%) HIV co-infected TB patients, staging information was available with 165 (81.2%) being WHO stage 3 and 38 (18.7%) being WHO stage 4 (Table 2). Data on CD4 count was available for 191 HIV positive patients (86.7%): 17 (8.9%) had a CD4 count of <200/μl, while 113 (59.2%) had CD4 between 200 and 350, with remaining 61 >350/μl.

Timing of ART initiation
Of the 226 HIV co-infected TB patients, only 121 (53.4%) had information on the start dates of both TB and ART treatment. Timing of ART initiation after start of TB treatment was computed for these 121 patients. The median time period from TB treatment start to ART initiation was 41 days (IQR: 25 to 84 days). Timeliness of ART initiation was analyzed against the 2007 and 2013 national guideline as outlined in the methods. Of the 117 patients assessed against the 2007 guideline, for 31 (26.5%), ART initiation was delayed (Figure 1). The four patients assessed against the 2013 guideline all had started ART within the required timeframe.
Determinants of timing of ART initiation
Of the factors (age, sex, type of TB by site, smear result, treatment history, year that TB treatment started, CD4 cell count, WHO clinical stage and presence or absence of opportunistic infections) evaluated for association with timing of ART initiation, only CD4 cell count was found to be statistically significantly associated in multivariate analysis. Patients with CD4 counts less than 50/μl were three times more likely to start ART in a timely manner when compared to patients with CD4 count greater than 200/μl (p<0.005) (Table 3).

TB treatment outcomes among HIV co-infected TB patients
Of the 226 HIV co-infected TB patients, 119 (54.1%) completed TB treatment and an additional 38 (17.3%) had documented cure, resulting in a successful TB treatment outcome for 71.4% of HIV co-infected patients. A total of 61 (27.4%) of the patients had undesirable outcomes (died (9.5%), transferred out (9.5%), defaulted (7.0%) and treatment failure (1.4%)). For 3.2%, no treatment outcome was recorded (Table 4). In HIV-negative TB patients, a treatment success rate of 82.7% was observed (Table 4). TB treatment success rate was significantly associated with HIV status (P value < 0.001) with HIV negative TB patients being 2 times more likely to have a successful treatment outcome.

Timing of ART initiation and TB treatment outcome
Of the 226 HIV co-infected TB patients, 21 (9.5%) died of whom 7 (36.5%) were started on ART, with one starting ART late. ART initiation showed a significant association with TB treatment success (P value=0.01) with those not started on ART being 2 times more likely to have an undesirable treatment outcome. ART initiation, ART delay and CD4 count showed no significant association with patient mortality (Table 5). Looking at TB treatment outcome in relation to timing of ART initiation indicated no statistical difference in treatment outcome for those starting ART as per guideline compared to those who started ART late in this study.
WHO recommends time of initiation of ART uptake in HIV co-infected TB patients in relation to starting of TB treatment as a core indicator for programmatic evaluation of collaborative TB/HIV activities, as indicated by TB/HIV indicators for monitoring and evaluation B8 and B9 (World Health Organization, PEPFAR, UNAIDS, 2015). In the current study the time between TB treatment initiation and ART initiating was a median of 41 days (IQR: 25 to 84 days). Less than two thirds of HIV co-infected TB patients (57.6%) were started on ART of whom 27% started ART later than recommended per the National Ethiopian, as well as the global guidelines. This is a cause for great concern as the latest global guidelines of WHO and UNAIDS of 2015 advise that all HIV co-infected TB patients should be started on ART within 2 months after start of TB treatment, and those with a CD4 counts of less than 50 be started as soon as possible within 2 weeks (World Health Organization, 2015). Other countries in the region have reported much better results in ART initiation. An observational cohort study carried out among HIV positive TB patients in South Africa (Lawn et al., 2011)reported that 87% started on ART while in a study in Kenya this was 70% (Tayler-Smith et al., 2011).
Both countries have adopted the recent WHO guideline that all TB patients (irrespective of CD4 count) are started on ART within two months of start of TB treatment. The lower observed proportion of TB patients starting ART in Ethiopia could be due to differences in the national guidelines as the National guideline in Ethiopia does not yet recommend start of ART irrespective of CD4 in line with the latest global recommendations. The guidelines are currently being updated to be in line with the latest global guidance. Also, poor recording and reporting could have affected the findings, as for nearly 50% the timeliness of ART could not be determined as both the dates of ART initiation and TB treatment start were not available. This calls for better routine recording and reporting to ensure that program data can be used to asses program performance. At the same time as being a limitation, use of routine data was the main strength of the study as despite the shortcomings, the findings likely reflect the operational reality on ground.
In the current study, CD4 count was found to be a predictor for timing of ART where patients with lower CD4 count were more likely to be put on ART in a timely man-ner. This was also the case in other studies where ART is not considered when the CD4 count is high (Chilton et al., 2008). CPT was well implemented, with nearly 95% of eligible patients started on CPT. This was higher than CPT uptake reported in another study carried out in Addis Ababa which reported that only 43.6% patients benefited from CPT (Denegetu and Dolamo, 2014)while in a referral hospital in North-West Ethiopia, 45.9% of patients eligible for CPT actually received treatment (Alemayehu et al., 2009). Besides, a 10-year review of the scale-up of TB and HIV program collaborative activities in Zambia revealed CPT coverage of 70% in 2010 (Kapata et al., 2012). The possible reasons for a relatively higher CPT uptake in the present study might be attributed to trainings provided to health workers on importance of initiation of CPT.
The median time to start ART after commencement of TB treatment was 41 days and 27% of HIV co-infected TB patients started ART late if evaluated against the prevailing national guideline. Early ART initiation in TB patients is a life saving intervention and through consistent follow-up and training of health workers it should be ensured that all HIV positive TB patients receive ART as per the latest national guidelines. Recording and reporting of patient information should be improved through regular follow-up and mentoring of health care providers to ensure that quality data can be used to guide programs. TB/HIV collaborative activities should be strengthened to ensure timely ART initiation.
The authors have not declared any conflict of interests.
The researchers would like to acknowledge the support of the National Tuberculosis Research Advisory Committee (TRAC) and KNCV Tuberculosis Foundation in Ethiopia for the technical and financial assistance. We would also like to acknowledge the institutions of the participating researchers for providing researchers’ time to carrying out this operational research project. Our acknowledgement also goes to the Addis Ababa Health Bureau and Bole 17 Health Center for their support to conduct this research project.
This study was conducted under the Ethiopia Operational Research capacity building initiative of TRAC/FMOH supported by USAID/TB CARE I project. The Global Health Bureau, Office of Health, Infectious Disease and Nutrition (HIDN), US Agency for International Development, supported this study financially through TB CARE I under the terms of Agreement No. AID-OAA-A-10-00020. This study was made possible by the generous support of the American people through the United States Agency for International Development (USAID). The contents are the responsibility of the authors and do not necessarily reflect the views of USAID or the United States Government. The funding body had no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
REFERENCES
|
Abdool Karim SS, Naidoo K, Grobler A, Padayatchi N, Baxter C, Gray A, Gengiah T, Nair G, Bamber S, Singh A, Khan M (2010). Timing of Initiation of Antiretroviral Drugs during Tuberculosis Therapy. N. Engl. J. Med. 362(8):697-706.
Crossref
|
|
|
|
Alemayehu YK, Bushen OY, Muluneh AT (2009). Evaluation of HIV/AIDS clinical care quality: the case of a referral hospital in North West Ethiopia. Int. J. Q. Health Care 21(5):356-362.
Crossref
|
|
|
|
|
Chilton D, Edwards SG, Pellegrino P, Miller RF (2008). Factors influencing delay in initiating antiretroviral therapy among HIV infected patients coinfected with tuberculosis. Thorax 63(10):935-936.
Crossref
|
|
|
|
|
Denegetu AW, Dolamo BL (2014). HIV screening among TB patients and co-trimoxazole preventive therapy for TB/HIV patients in Addis Ababa: facility based descriptive study. PloS one 9(2):e86614. Dis. 15(1):536.
Crossref
|
|
|
|
|
FDRE Population Census Commission (2007). Summary and Statistical Report of the 2007 Population and Housing Census, Population Size by Age and Sex. Available at:
View
|
|
|
|
|
Federal Ministry of Health (FMOH) (2007). Implementation Guideline for TB/HIV Collaborative Activities in Ethiopia. Available at:
View
|
|
|
|
|
Federal Ministry of Health (FMOH) (2013). Guidelines for Clinical and Programmatic Management of Tb, Tb/hiv and Leprosy in Ethiopia Fifth Edition. Available at:
View
|
|
|
|
|
Franke MF, Robins JM, Mugabo J, Kaigamba F, Cain LE, Fleming JG, Murray MB (2011). Effectiveness of Early Antiretroviral Therapy Initiation to Improve Survival among HIV-Infected Adults with Tuberculosis: A Retrospective Cohort Study. PLoS Med. 8(5):e1001029.
Crossref
|
|
|
|
|
Kapata N, Chandaâ€Kapata P, Grobusch MP, O'Grady J, Schwank S, Bates M, Jansenn S, Mwinga A, Cobelens F, Mwaba P, Zumla A (2012). Scale-up of TB and HIV Programme Collaborative Activities in Zambia - a 10-Year Review. Trop. Med. Int. Health 17:760-766.
Crossref
|
|
|
|
|
Lawn SD, Campbell L, Kaplan R, Little F, Morrow C, Wood R, Africa IS (2011). Delays in Starting Antiretroviral Therapy in Patients with HIV-Associated Tuberculosis Accessing Non-Integrated Clinical Services in a South African Township. BMC Infect. Dis. 11(1):258.
Crossref
|
|
|
|
|
Lawn SD, Kranzer K, Wood R (2009). Antiretroviral therapy for control of the HIV-associated tuberculosis epidemic in resource-limited settings. Clin. Chest Med. 30(4):685-699.
Crossref
|
|
|
|
|
Naidoo K, Baxter C, Karim SSA (2013). When to start antiretroviral therapy during tuberculosis treatment. Curr. Opin. Infect. Dis. 26(1):35-42.
Crossref
|
|
|
|
|
Nglazi MD, Bekker LG, Wood R, Kaplan R (2015). The impact of HIV status and antiretroviral treatment on TB treatment outcomes of new tuberculosis patients attending co-located TB and ART services in South Africa: a retrospective cohort study. BMC Infect.
Crossref
|
|
|
|
|
Salim S. Abdool K, Naidoo K, Grobler A, Padayatchi N, Baxter C, Gray AL, Gengiah T, Gengiah S, Naidoo A, Jithoo N, Nair G, El-Sadr WM, Friedland G, Karim QA (2011). Optimal Timing of Antiretroviral Therapy during Tuberculosis Treatment : The SAPiT trial, 17th Conference of Retroviruses and Opportunistic Infections Boston, 28 February 2011. Available at:
View
|
|
|
|
|
Sileshi B, Deyessa N, Girma B, Melese M, Suarez P (2013). Predictors of mortality among TB-HIV Co-infected patients being treated for tuberculosis in Northwest Ethiopia: a retrospective cohort study. BMC Infect. Dis. 13(1):297.
Crossref
|
|
|
|
|
Stockdale AJ, Nkuranga J, Török ME, Faragher B, Lalloo DG (2013). Initiation of antiretroviral therapy in HIVâ€infected tuberculosis patients in rural Kenya: An observational study. Trop. Med. Int. Health 18(7):907-914.
Crossref
|
|
|
|
|
Taylerâ€Smith K, Zachariah R, Manzi M, Kizito W, Vandenbulcke A, Sitienei J, Chakaya J, Harries AD (2011). Antiretroviral Treatment Uptake and Attrition among HIV-Positive Patients with Tuberculosis in Kibera, Kenya. Trop. Med. Int. Health 16(11):1380-1383.
Crossref
|
|
|
|
|
World Health Organization (2015). Guideline on when to start antiretroviral therapy and on pre-exposure prophylaxis for HIV. In Guideline on when to start antiretroviral therapy and on pre-exposure prophylaxis for HIV.
|
|
|
|
|
World Health Organization (WHO) (2010). Antiretroviral Therapy for HIV Infection in Adults and Adolescents Recommendations for a Public Health Approach. Available at:
View
|
|
|
|
|
World Health Organization (WHO) (2014). Zhurnal Eksperimental'noi i Teoreticheskoi Fiziki Global TB Report. Available at:
View
|
|
|
|
|
World Health Organization PEPFAR, UNAIDS (2015). Guide to Monitoring and Evaluation for Collaborative TB/HIV Activities--2015 Update. World Health Organization.
|
|