ABSTRACT
Human immunodeficiency virus (HIV) has emerged as one of the leading causes of childhood mortality and morbidity in sub Saharan Africa. But, the attention given to HIV-infected children in terms of providing antiretroviral treatment (ART) had so far been ranked second. The study had the objectives of identifying predictors that had significant impacts on the survival status of HIV infected children who received antiretroviral treatment care in the University of Gondar Comprehensive Specialized Hospital, Gondar, Ethiopia. The data used in the study was based on secondary data from hospital records of HIV infected children aged below 15 years who started ART between 2008 and 2013 and who followed through April 2015 in University of Gondar Comprehensive Specialized Hospital, Gondar, Ethiopia. The Multivariable Cox Proportional model was fitted to identify factors affecting the survival of children after initiation of ART. The median survival time frame was found to be 55 months. At the end of the follow up, 46 (17.1%) children died due to the disease, the remaining 223 (82.9%) were alive and lost to follow-up. The multivariate analysis of the Cox Regression model showed that the age of a patients (for age < 1.5 years HR: 3.590 ; 95% CI: 1.439, 8.953; P = 0.006, baseline hemoglobin level (for hemoglobin level < 7g/dl HR: 6.286; 95% CI: 2.328, 16.973; P=0.000, WHO clinical stage (For stage III HR: 0.308 ; 95% CI: 0.150, 0.630; P = 0.001); and baseline CD4 count(HR: 0.180 ; 95% CI: 0.084, 0.388; P = 0.000) are significant factors of survival of HIV infected children during the 92 months of follow up. Therefore, special attention should be given to younger children in ART; patients with low CD4 cell count, patients with advanced WHO clinical staging (stage III and IV); and patients with low hemoglobin level to improve the survival of HIV infected children treated with ART.
Key words: Children, antiretroviral therapy (ART), HIV, survival, Ethiopia.
The occurrence of the acquired immunodeficiency syndrome (AIDS) epidemic is amongst the forefront public health challenges that the world has faced (UNAIDS The Gap Report, 2014). It has had some strong emotional effects on individuals and families with the implications of untimely death along with medical, financial and social burdens for the past three decades. Millions of people have died of the human immunodeficiency virus (HIV) infection. Globally, an estimated 35 million (33.2 million to 37.2 million) people were living with HIV in 2013 (Newell et al., 2004). In sub-Saharan Africa, the number of AIDS-related deaths fell by 39% between 2005 and 2013. The region still accounted for 74% of all the people dying from AIDS-related causes in 2013 (UNAIDS The Gap Report, 2014). There are 2.9 million (2.6 million to 3.2 million) children (aged 0 to 14) living with HIV in sub-Saharan Africa. Of the estimated 1.8 million people living with HIV, 1.5 million were living in this region. There were also 190,000 child deaths of AIDS-related illnesses during 2013, out of 1.5 million people overall (WHO, 2014). Ethiopia is one of the sub-Saharan African countries hardly-hit by HIV/AIDS in all of its manifestations. In 2013, there were an estimated 793,700 (716,300-893,200) people living with HIV including 200,300 (172,400 to 232,400) children according to the EPP/Spectrum modelling (UNAIDS, 2014 EPP/Spectrum).
Tremendous progress has been made over the past few years in diagnosing and treating infants and children with HIV infection. However, much remains to be done to effectively scale up and sustain prevention efforts and treatment services for all in need. Treatment of HIV- infected children with Antiretroviral Therapy (ART) leads to immune reconstitution which results in an increase in CD4 lymphocyte counts, decreased risk of opportunistic infection and improved survival (UNAIDS The Gap Report, 2014; Newell et al., 2004). Moreover, children may die with an undetectable viral load and inadequate CD4 count recovery (Estimation and Project Package for HIV, 2014). However, very little attention has been given to how other factors, not relating to ART drugs, may influence the survival of HIV infected children. Therefore, this research is undertaken to explore the factors that have strong association with the survival experience of HIV-infected children treated with ART in the University of Gondar Comprehensive Specialized Hospital. The study had the objectives to assess the relationship of explanatory variables to survival time, estimate the survival duration and identify predictors that have significant impacts on the survival status of HIV infected children who received Antiretroviral Treatment and care in the University of Gondar Comprehensive Specialized Hospital, Gondar, Ethiopia.
Study area, design, and period
The data for the study were obtained from patients’ ART follow up records. During the study period, from the HIV cohort database, a The data for the study were obtained from patients’ ART follow up records. During the study period, from the HIV cohort database, a total of 756 children were recorded. However, only 269 patients with a full record of variables who started ART between 2008 and 2013 were included and continued until April 2015 in the University of Gondar Comprehensive Specialized Hospital. The data were collected based on the child’s identification number in HIV cohort database without any direct contact with a child so as to maintain the confidentiality of the child’s record. Demographic data, laboratory and clinical information of all children aged <15 years who started ART were included.
Variables of the study
The study focused only on demographic variables (age, and gender) and clinical/immunological variables (baseline weight, WHO Clinical Stage, Prophylaxis taken, baseline functional status, Baseline TB status, Reason for taking ART, baseline CD4 count, baseline hemoglobin levels, Opportunistic Illness) that can affect the survival time of HIV-infected children. The response variable in the study is the survival time of HIV-infected children measured in months after starting ART. This was measured according to the time a child had follow up from the time the child began to receive treatment until the time of an event (death) or lost to follow up (for those right censored subjects).
Operational definitions
(1) CD4 count is one of the factors used to determine when to start antiretroviral therapy (ART).
(2) Threshold CD4 count: The CD4 cell count of a person who does not have HIV can be anything between 500 and 1500. People living with HIV who have a CD4 count over 500 are usually in pretty good health.
Statistical model
A variety of models and methods have been developed for doing this sort of survival analysis using either parametric or semi-parametric approaches. One of the most popular types of regression models used in survival analysis is the Cox Proportional Hazard Model (Cox, D.R. Regression models and life Tables (with Discussion), 1972). The Cox Regression Model is used to determine which combinations of explanatory variables affect the form of the hazard function. Also the model is used to obtain an estimate of the hazard function for an individual who may be of interest. The Cox Regression Model can be used for data that contains censored observations. The model also takes into account the fact that the probability of experiencing an event differs with duration of exposure to risk. In particular we apply the semi-parametric Cox proportional hazard model because it is the most commonly used model in hazard regression.
A total of 269 participants with full record of variables were included in the study. From this number, 46 (17.1%) children died due to the disease, 223 (82.9%) were alive or loss to follow-up during the time of data collection. Out of the total 269 ART patients, 116 (36.65%) were male and the remaining were females. Among the 269 children, 78 (30%) were at clinical stage IV, 82 (30.5%) were at clinical stage III, 63 (23.4%) were at clinical stage II and the rest which amount to 46 (17.1%) children were at clinical stage I when they started ART (Table 1). From the results of the Multivariate analysis using the significant variables found in Table 2, the covariates age, WHO clinical stages, CD4 counts and hemoglobin level are the four categorical variables that are found to be significantly associated with the survival time of HIV infected children under ATR treatment in the fitted Cox regression model. Let us begin with Baseline CD4 cell count of the patient that is supposed to be significant both clinically and statistically. In this study, a Baseline CD4 cell count has been found to have a significant impact on the survival time of HIV infected children. The estimated hazard ratio for baseline CD4 counts is 0.180 (with a 95% C.I. 0.084 to 0.388). Thus, patients whose CD4 counts are above the threshold levels have an 82% lower risk of death than those whose CD4 counts are below the threshold levels. The confidence interval indicated that the risk of death for those patients that have CD4 counts above the threshold levels could be lower by 38.8% or as low as 8.4% than patients that have CD4 counts below the threshold levels (500 cells/mm3); p < 0.0001.
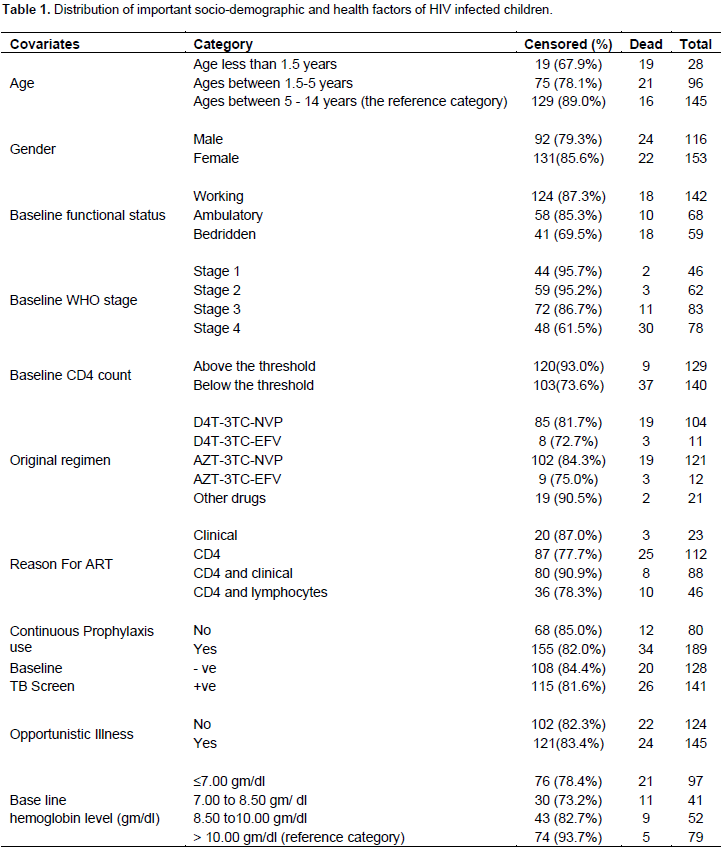
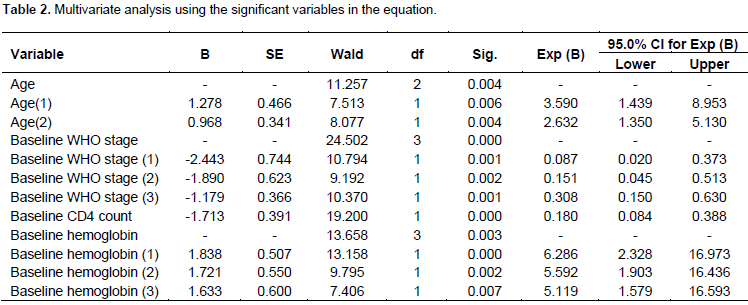
HIV-infected children aged below 1.5 years are 3.59 times more likely to die than children aged between 5 to 14 years. The 95% C.I. confirmed that the hazard of death for this category could be as low as 1.439 and as high as 8.953 compared with children aged between 5 to 14 years. HIV-infected children aged between 1.55 and 5 years are 2.632 times more likely to die than children between the ages of 5 to 14 years. The 95% CI verified that the rate of death could be as small as 1.350 and as large as 5.130. HIV-infected children aged below 1.5 years are 1.36 times more likely to die than HIV-infected children aged between 1.5 and 5 years given that all other factors are constant. The estimated risks of death for a patient with hemoglobin levels less than 7gm/dl as compared to those patients with hemoglobin levels greater than 10gm/dl (reference category) are 6.286 (95% CI: 2.323, 16.973). This means that the hazard rate of death of a child for a hemoglobin level less than 7gm/dl is 6.286 times more likely to die than HIV-infected children with hemoglobin level greater than 10gm/dl. In addition, the estimated relative risk (hazard ratio) of dying patients with hemoglobin levels between 7 to 8.5gm/dl are 5.592 times more likely to die as compared to those patients with hemoglobin level greater than 10gm/dl(reference category).
The 95% C.I. suggests that the rate of death could be as low as 1.903 and as high as 16.436. Moreover, the estimated hazard ratio of hemoglobin levels between 8.5 to 10gm/dl compared to the reference hemoglobin level is 5.119 (95% CI: 1.579, 16.593). This implies that the risk of dying for patients with hemoglobin levels between 8.5 to 10gm/dl is 5.119 times more likely to die than those patients with hemoglobin levels greater than 10gm/dl (reference category). Children with hemoglobin levels less than 7gm/dl are 1.124 times more likely to die than children with hemoglobin levels between 7gm/dl to 8.5gm/dl. Moreover, children with hemoglobin levels greater than 10gm/dl are 84% less likely to die than children with hemoglobin value less than 7gm/dl provided that all other factors are held constant. The reference category for the design variables of WHO clinical stage is patients who are in the specified WHO clinical stage IV. The estimated hazard ratio for clinical stage III is 0.308 (with a 95% C.I. 0.15-0.630). Thus, the hazard of death for clinical stage III is 70% less likely than those of clinical stage IV. On the other hand, the estimated hazard ratio of stage III compared to stage II was 2.04 =
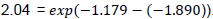
. Since the confidence interval does not contain 1, an individual in clinical stage III has a significantly higher hazard rate than patients in clinical stage II. Thus, patients in stage III are 2.04 times more likely to die than patients in stage II.
Assessing the goodness of fit of the model
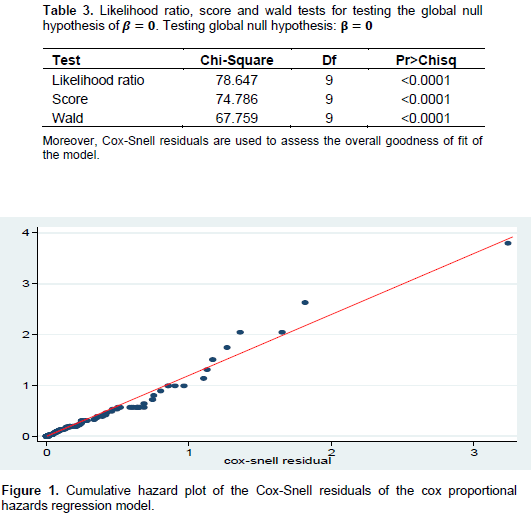
The goodness of fit of the proportional hazard model was checked based on the empirical data. Therefore, for the model fitted in this study, the Likelihood Ratio, Score and Wald tests were used to compare (at 5% significance level) the goodness of fit of the model. The SAS output in Table 3 revealed that the log partial likelihood function (-2LL) without covariate was 489.281 while the function with significant covariates was 410.634. This result showed that the model is appropriate with a chi-square of 78.647 with 9 degrees of freedom and a p-value of <0.0001. The plot in Figure 1 of the cumulative hazard function of the Cox-Snell residual against the Cox-Snell residuals are fairly close to the 45° straight line through the origin. This suggested that the model fit to the data is satisfactory. The 450straight line through the origin is drawn for reference.
This study identified variables/factors that are significantly associated with survival time of HIV-infected children under ART treatment. The results of this study showed that the risk of death among HIV infected children among the age groups of less than 1.5 years and 1.5 to 5 years are higher than those ages between 5 to 14 years. A study by Gebremedhin et al. (2013) identified independent predictors of children’s mortality on ART. The result suggested that the mortality of children on ART was low and factors that affect mortality of children on ART were among those children who were less than 18 months of age. A similar study by Munyagwa et al. (2012) also found that mortality among HIV-infected children was highest among those aged less than 2 years. Thus, mortality among these high risk groups contributed to the higher rate of mortality. CD4 cell count is the most important marker of HIV disease progression and a strong predictor of the survival of HIV-infected children, similar to the plasma viral load. That may be due to the fact that HIV attacks CD4 cells, and as time elapses people with HIV often notice their CD4 cell counts drop. Hence, the lower the CD4 cell count the greater the chances of acquiring a number of very serious diseases. The significant impact of CD4 cell counts on patients’ survival rate has been acknowledged by many studies. A study by Brady et al. (2010), Lumbiganon et al. (2011) and Phongsamart et al. (2013) reported that HIV-infected children with low baseline CD4 cell counts had a strong likelihood of early mortality. The results in this current study are also consistent with the findings in the above studies. But, a similar study conducted by (Habtamu and Eshetu, 2012) in Bahir-Dar found that low baseline CD4 cell counts was not a predictor of survival time of HIV infected children. His finding contradicts our findings that a low baseline CD4 cell counts were a strong risk factor for survival time of HIV infected children.
The finding of this study observed that a higher risk of mortality was found among HIV-infected children with lower hemoglobin levels (anemic groups) compared to hemoglobin levels greater than 10gm/dl. This study was consistent with other studies conducted elsewhere. According to Ebissa et al. (2015), it was found that hemoglobin levels less than 7 gm/dl were significant independent predictors of death after controlling for other factors. For instance, another study also identified that the determinants of mortality in Bahir Dar showed that the risk of death is higher among HIV-infected children with a lower hemoglobin level [13]. Therefore, the above studies confirm the same conclusion as ours. Like CD4 cell counts, the WHO Clinical Staging system has been shown to be a practical and accurate way to manage HIV-infected patients. In this study, we found that the advanced WHO clinical stages III and IV were independent markers of mortality for patients on ART. The possible justification for the finding is that the advanced clinical stage of the disease is the cause for HIV-associated complications. A study in Lumbiganon et al. (2011) found that those children with WHO clinical stage IV had an increased risk of death. Similar to our findings, studies by Atnafu et al. (2012), Ebissa et al. (2015) and Adem et al. (2014) provided evidence that HIV infected children on ART in advanced clinical stages (III and IV) had a strong association with high mortality. The aforementioned sources showed that the most significant predictors of survival of children were CD4 count, advanced WHO clinical stages, age, weight and to some extent opportunistic diseases like anemia and pneumonia. The findings of the current study identified and focused on advanced WHO clinical stage, age, hemoglobin level and baseline CD4 count as determinant predictors of survival of HIV-infected children who were treated with ART at the University of Gondar Comprehensive Specialized Hospital. Weight did not come out as a strong predictor although it is a clinically meaningful determining variable.
In this study, we tried to identify the factors that are associated with survival time of HIV infected children treated with ART in the University of Gondar Comprehensive Specialized Hospital using the methods of survival analysis. The Kaplan-Meier and log-rank test are used to estimate and compare the survival time of children after initiation of ART treatment. The study has shown that the overall median survival time of HIV infected children under the study was 51.1 months. During the follow-up period, out of the 269 HIV infected children 46 (17.1%) of them experienced the event (that is, death). Moreover, the results of the multivariable proportional hazards Cox regression model showed that CD4 count at the start of ART, age, advanced WHO clinical stages and low hemoglobin level (less than 7gm/dl and between 7 to 8.5gm/dl) are associated with a higher risk of mortality. And also HIV- infected children at ages less than 1.5 years and ages between 1.5 to 5 years and who were at advanced WHO clinical stage III & IV are also associated with increased rate of mortality in both the univariable and multivariable analysis. Similarly, patients with poor health indicators like low baseline CD4 cell counts and low hemoglobin levels are less likely to survive. Therefore, special attention should be given to younger children in ART; children should start ART treatment at an early age, with CD4 cell counts at the normal level or above the threshold level and when they have higher hemoglobin values and when they are at a lower clinical stage.
The authors have not declared any conflict of interests.
The authors appreciate the support of the University of Gondar Comprehensive Specialized Hospital staff members for dedicating their time in providing the necessary data.
REFERENCES
|
Adem AK, Alem D, Girmatsion F (2014). Factors affecting survival of HIV positive children taking antiretroviral therapy at Adama Referral Hospital and Medical College, Ethiopia. J. AIDS Clin. Res. 5(3):289.
|
|
|
|
Atnafu H, Wencheko E (2012). Factors affecting the survival of HIV-infected children after ART initiation in Bahir-Dar, Ethiopia. Ethiop. J. Health Dev. 26(3):193-199.
|
|
|
|
|
Brady MT, Oleske JM, Williams PL, Elgie C, Mofenson LM, Dankner WM, Van Dyke RB (2010). Declines in Mortality Rates and Changes in Causes of Death in HIV-1-Infected Children during the HAART Era. J. Acquir. Immune Defic. Syndr. 53(1):86-94.
Crossref
|
|
|
|
|
Cox DR (1972). Regression Models and Life-Tables (with Discussion). J. R. Stat. Soc. Series B (Methodological) 34(2):187-220.
|
|
|
|
|
Ebissa G, Deyessa N, Biadgilign S (2015). Predictors of early mortality in a cohort of HIV-infected children receiving high active antiretroviral treatment in public hospitals in Ethiopia. AIDS Care 27(6):723-730.
Crossref
|
|
|
|
|
Federal Democratic Republic of Ethiopia (2014). Country Progress Report on the HIV response. 2014. Available at:
View
|
|
|
|
|
Gebremedhin A, Gebremariam S, Haile F, Weldearegawi B, Decotelli C (2013). Predictors of mortality among HIV infected children on anti-
|
|
|
|
|
Habtamu A, Eshetu W (2012). Factors affecting the survival of HIV-infected children after ART initiation in Bahir-Dar, Ethiopia. Ethiop. J. Health Dev. 26(3):193-199.
|
|
|
|
|
Lumbiganon P, Kariminia A, Aurpibul L, Hansudewechakul R, Puthanakit T, Kurniati N, Kumarasamy N, Chokephaibulkit K, Nik Yusoff NK, Vonthanak S, Moy FS, Razali KA, Nallusamy R, Sohn AH (2011). Survival of HIV-infected children: a cohort study from the Asia-Pacific region. J. Acquir. Immune Defic. Syndr. 56(4):365-371.
Crossref
|
|
|
|
|
Munyagwa M, Baisley K, Levin J, Brian M, Grosskurth H, Maher D (2012). Mortality of HIV-infected and uninfected children in a longitudinal cohort in rural south-west Uganda. Trop. Med. Int. Health 17(7):836-843.
Crossref
|
|
|
|
|
Newell ML, Coovadia H, Cortina-Borja M, Rollins N, Gaillard P, Dabis F (2004). Mortality of infected and uninfected infants born to HIV-infected mothers in Africa. Lancet 364(9441):1236-1243.
Crossref
|
|
|
|
|
Phongsamart W, Hansudewechakul R, Bunupuradah T, Klinbuayaem V, Teeraananchai S, Prasithsirikul W, Kerr SJ, Akarathum N, Denjunta S, Ananworanich J, Chokephaibulkit K (2013). Long-term outcomes of HIV-infected children in Thailand. Int. J. Infect. Dis. 22:19-24.
Crossref
|
|
|
|
|
United Nations Programme on HIV/AIDS (UNAIDS) (2014). Strengthening HIV Estimates: EPP/Spectrum 2015. Report and recommendations from a meeting of the UNAIDS Reference Group on Estimates, Modelling and Projections, Geneva, Switzerland, 27-29 October 2014. Available at:
View
|
|
|
|
|
United Nations Programme on HIV/AIDS (UNAIDS) Gap Report (2014). The Gap Report, 2014. Available at:
View
|
|
|
|
|
World Health Organization (WHO) (2014). WHO report in partnership with UNICEF and UNAIDS. Global update on the health sector response to HIV, 2014. Available at:
View
|
|
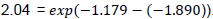 . Since the confidence interval does not contain 1, an individual in clinical stage III has a significantly higher hazard rate than patients in clinical stage II. Thus, patients in stage III are 2.04 times more likely to die than patients in stage II.
. Since the confidence interval does not contain 1, an individual in clinical stage III has a significantly higher hazard rate than patients in clinical stage II. Thus, patients in stage III are 2.04 times more likely to die than patients in stage II.