ABSTRACT
Co-infection with HIV and hepatitis B virus (HBV) has become an important factor of co-morbidity and mortality. The aim of this study was to determine the seroprevalence of HIV/HBV co-infection and its effect on the disease progression in people living with HIV/AIDS identified in Yaoundé Central Hospital. Blood samples from 75 HIV positive patients were collected in Yaoundé Central Hospital from November 2015 to February 2016, for the determination of hepatitis B virus surface antigen (HBsAg) using immunoassays. Cluster of differentiation 4 (CD4) T-cells count and biochemical markers of liver function were also collected and analyzed. The socio-demographic data were also collected. The effect sizes were confirmed using G*Power version 3.1.9.2 software. The data were entered and analyzed using the SPSS Version 22.1 software. The statistical tests performed were x2, and Pearson correlation, with significant difference at the threshold p ≤ 0.05. Hepatitis B virus surface antigen (HBsAg) was identified in 12 patients out of 75 HIV-positive patients, for a HIV/HBV co-infection prevalence of 16%. The co-infection rate was higher in women 9 (12%) than in men 3 (4%). Among HIV infected patients, a negative and significant correlation was observed between CD4 count and ALT activity, and between the concentration of conjugated bilirubin and the activity of alkaline phosphatase (ALP) p≤ 0.05. The prevalence of HIV/HBV co-infection is higher among HIV positive patients in the Yaoundé Central Hospital. HIV associated with HBV plays a role in the disease progression. Consequently, it is important that a national management programme is in place in the country to monitor the incidence and morbidity rates of these affections.
Key words: Co-infection, seroprevalence, hepatitis B virus (HBV), human immunodeficiency virus (HIV), Cluster of differentiation 4 (CD4) T-cells, liver enzymes, disease progression.
Acquired immunodeficiency syndrome (AIDS) due to human immunodeficiency virus (HIV) is a major threat to the development of resources-limited countries. It is a poverty related disease that has destroyed many lives and contributed to maintain poverty. Sub-Saharan Africa with only 13% of the world population is the hardest hit region, home to nearly 70% of people living with HIV/AIDS worldwide. In 2015, there were 36.7 million people living with HIV, with about 2.1 million new infections (UNAIDS, 2016). Western and Central Africa is home to 18% of these infections, right after the Eastern and Southern Africa (UNAIDS, 2016). Cameroon remains in a situation of generalized epidemics for HIV, with a seroprevalence of 4.3% in adults aged 15 to 49 years (National Institute of Statistics, 2011).
Essentially, the effectiveness of highly active antiretroviral therapy (HAART) in improving the quality and lifespan of HIV patients has revolutionized the field of HIV. However, co-infections with viruses like hepatitis B virus (HBV) appear to compromise the benefits of efficient antiretroviral drugs by increasing the morbidity and mortality in HIV-infected populations. HIV and HBV are blood-borne pathogens, and because of their shared modes of transmission, people at risk for HIV infection are also at risk for HBV infection (WHO, 2016). Cameroon is in an endemic area for HBV where HBV infection in the general population accounts for 12%, with high prevalence in the younger population (Noah et al., 2011; Njouom and Tejiokem, 2016). HIV-HBV co-infection would not be without impact on the progression of AIDS, and despite advances on HBV prevention, an affordable and widely accessible mean to eradicate HBV infection worldwide is still needed. Furthermore, HIV infection alters the natural history of HBV and accelerates the progression to chronic hepatitis, resulting in the complication of the patient's condition and leading to progressive deterioration of several vital organs specifically the liver; hence the abnormal level of the liver enzymes like alanine aminotransferase in the blood stream (Dieterich, 2007). Managing extremely such co-infections is therefore compulsory in people affected, and the effectiveness of antiretroviral treatment is contingent.
The identification of co-infected individuals is thus a critical step. Previous studies in sub-Saharan Africa showed that HBV infection prevalence among HIV positive people varies from one region to another: from 17.5% in a hospital setting in Dar Es Salaam, Tanzania (Nagu et al., 2008), 17% in Northern Uganda (Ochola et al., 2013), 12.8% in the North-Eastern Nigeria (Obi et al., 2012), 12.6% in the North-West region of Cameroon (Zoufaly et al., 2012), to 12.2% in The Gambia (Jobarteh et al., 2010). However, in many setting, the dual infection HIV and Hepatitis B virus still goes unnoticed due to the lack of diagnostic means: The spread of this co-infection is rapid while diagnostics means are still deficient. In addition, studies targeting determinants of HIV-HBV co-infection remain unsatisfactory. Given the high prevalence of HIV/HBV co-infection in the different African regions, it might be hypothesize that the infection rate of hepatitis B is higher among people living with HIV/AIDS in Cameroon, the most at risk population. Consequently, more data linking the seroprevalence of co-infection to the disease progression are needed for the management strategy in care hospitals.
In order to contribute to this management process, the purpose of this study was to investigate the seroprevalence of HIV/HCV co-infection and its impact on the disease progression in people living with HIV/AIDS Identified in Yaoundé Central Hospital, a tertiary level teaching hospital in Cameroon. Specifically, it was to determine the seroprevalence of HIV/HBV co-infection, to examine correlations between the biochemical liver markers and CD4-T cell count, and to scrutinize other risk factors associated to the co-infection.
Biological material (serum, plasma) as well as laboratory equipment, reagents and consumable were used in this study.
Study design, period and population
This was a prospective and analytical study carried out in Yaoundé Central Hospital from November 2015 to February 2016. The study population consisted of patients in consultation and observation in the Yaoundé Day Care Central Hospital. A total of 75 HIV-positive patients were enrolled.
Plasma and serum were obtained from the collected blood and directly analyzed, or stored at -20°C for subsequent analyses.
Inclusion and exclusion criteria
Participants in this study were aged 21 to 49 regardless of gender, ethnicity or tribe. The volunteers’ participants who agreed to sign an informed consent form after being informed of the nature, the procedure of the study, the potential benefits and the foreseeable risks, were recruited. Patients with history of jaundice were excluded from this study.
Data collection procedure and laboratory analyses
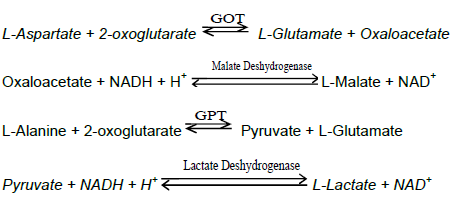
To address confidentiality issue, an identification code without key was assigned to each patient for laboratory analyses, data entry and data analysis. Blood samples were collected under aseptic conditions, in both EDTA and dry tubes. Plasma and serum were separated by a low speed centrifugation at 1500 rpm for 5 min; two aliquots were made for subsequent use, and stored frozen at -20°C until tested. The first aliquot (plasma) was used to test the serology of Hepatitis B virus surface antigen (HBsAg) using the immuno-chromatographic method. The HBsAg serology was confirmed using enzyme linked immunosorbent assay (ELISA). The second aliquot (serum) was used to determine the activity of liver enzymes alanine aminotransferase (ALT), aspartate aminotransferase (AST) and alkaline phosphatase (ALP) using
enzyme kinetic method, as well as the serum concentration of conjugated or direct bilirubin (CB). Kinetic method for the determination of AST and ALT activities was performed according to the recommendations of the Expert Panel of the International Federation of Clinical Chemistry (IFCC), without pyridoxal-phosphate activation.
The principle is based on the following reactions:
ALP activity was determined using kinetic photometric test according to the International Federation of Clinical Chemistry and laboratory Medicine, based on the following reaction:
p-Nitrophenyl-phosphate + H2O ALP p-Nitrophenol + Phosphate
The serum concentration of bilirubin was determined using the colorimetric method. CD4 T-cells count results were also collected and analyzed.
Data preparation and analysis
The effective size for this study was computed using G*Power version 3.1.9.2 software, with post-hoc as type of power analysis. Data obtained were subsequently entered, cleaned and analyzed using the statistical package for social sciences (SPSS) software (version 22.1). Mean, frequencies and percentages were used to summarize descriptive statistics of the data. Chi-square (x2) test was used to assess relationships between selected and/or qualitative variables namely gender, sex, marital status, level of education and occupation. Pearson correlation was used to determine the relationship between the biochemical parameters and CD4 cells count. The significant difference was set at the threshold p ≤ 0.05.
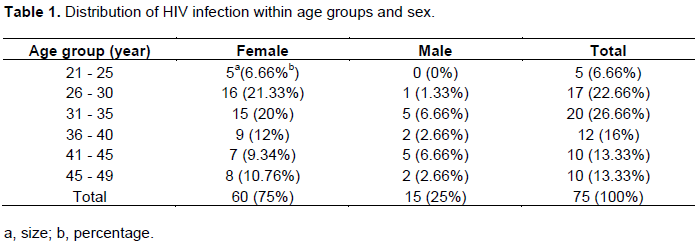
The effect size for this study was computed using G*Power version 3.1.9.2 software (Faul et al., 2007, 2009), with post-hoc as type of power analysis. The sample size (N=75) was in conformity with the effect size, 0.3 with x2 test, and 0.6 with the Pearson correlation. In this study, 75 HIV positive patients among other 15 (20%) men and 60 (80%) women were recruited. The average age was 36 years. The sex ratio male: female was 1:4. 60 (78. 65%) patients were aged between 26 and 45 (Table 1).
Seroprevalence of HIV/HBV co-infection and socio-demographic characteristics
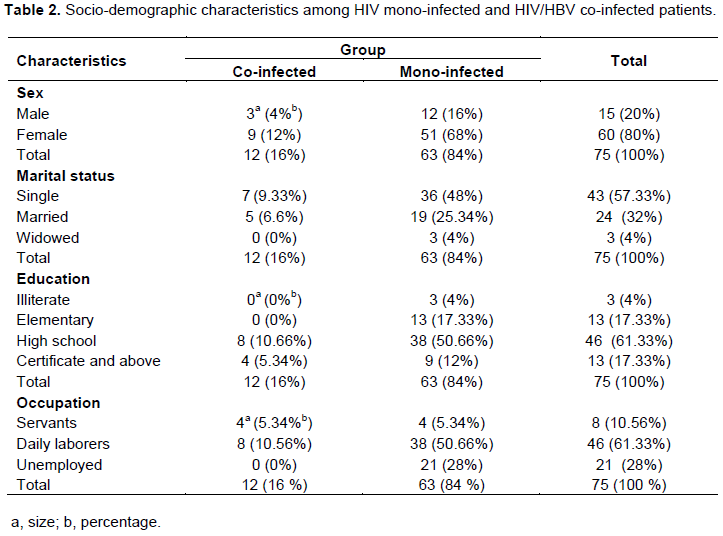
The seroprevalence of HIV/HBV co-infection was 16%, the rate of co-infection was higher in female (12%) compared to male (4%), p≤ 0.05. The average age was 34 years. The co-infection rate was higher in single (9.33%), compared to married and widowed populations. The co-infection rate was higher in patients with higher level of education (10.66%). In addition, this co-infection rate was higher in daily labor workers (10.56%), compared to servants and patients who were jobless (Table 2).
CD4 count analysis
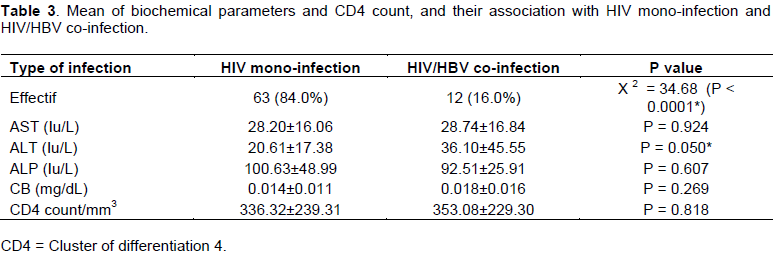
The mean CD4 count in HIV mono-infected patients were 336.32±239.31 cells/mm3 (with a minimum and a maximum CD4 count of 12 and 1355 cells/mm3 respectively), whereas the mean CD4 count in HIV/HBV co-infected were 353.08±229.30 cells/mm3 (with a minimum and maximum CD4 count of 73 and 753 cells/mm3 respectively). However, the difference was not statically significant (Table 3).
Correlation between different parameters and disease progression
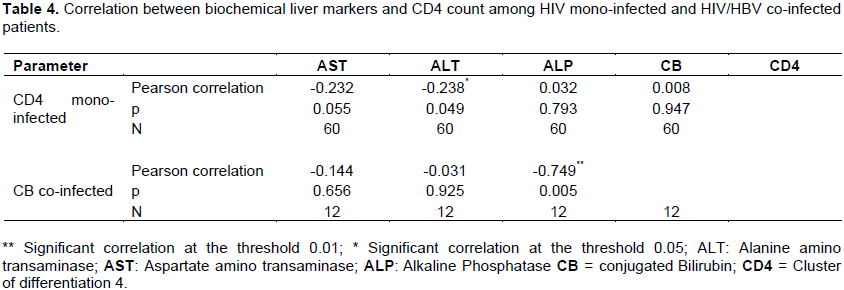
The (bivariate) correlation was investigated between parameters. In HIV mono- infected patients, Pearson correlation showed a negative and significant correlation between ALT activity and CD4 count (r = -0.238; p =0.049) at the threshold 0.05. In HIV-HBV co-infected patients, negative and significant correlation was observed between conjugated bilirubin and alkaline phosphatase APL (r = -0.749; p = 0.005) at the threshold 0.01 (Table 4).
This study investigated the seroprevalence of Human immunodeficiency virus and hepatitis B virus (HIV/HBV) co-infection and effect on the disease progression in people living with HIV/AIDS identified in Yaoundé Central Hospital, Cameroon. In this study, HIV-HBV co-infection rate was 16%, which is higher compared to results obtained by previous authors, 12.5 and 12.6% respectively among HIV-1 infected Cameroonian adults initiating antiretroviral therapy in Cameroon (Zoufaly et al., 2012; Laurent et al., 2010). Also, this seroprevalence is higher compared to results in the general population. In Cameroon, HBV infection in the general population accounts for about 12%. However, the prevalence of HBV is lower in the elder populations. HBV prevalence is about 13% in people under 45 years of age, and between 8 to 9% in people above 45 years (Noah et al., 2011; Njouom ad Tejiokam, 2016). The prevalence in the present study is comparable to 16.8% obtained in studies from Senegal (Diop-Ndiaye), and from Uganda
(Ochola et al., 2013). It is however lower, compared to 19% reported in Northwest Ethiopia (Yoannes et al., 2014) and 20.4% reported in Malawi (Nyirenda et al., 2008). In the present study, the prevalence of co-infection was higher in women than in men (12 vs 4%) and the difference was statistically significant (P = 0.05). This finding is similar to studies from Uganda. This trend can be explained on the basis of higher rate of sexual promiscuity as well as the anatomy of the female genital organs that are most vulnerable. The mucous membrane surface during sexual act is bigger than that of man. In addition, virus concentration in sperm is higher compared to the vaginal secretions. Actually, a multitude of factors increase women’s vulnerability to HIV acquirement, including biological, behavioral, socio-economic, cultural and structural risks (Mabala, 2006; Gita and Brodie, 2013). In the present study, the sex ratio male: female was 1:4. This result is in accordance with the UNAIDS epidemics update, 2016. In fact, women represent more than half of all adults with HIV worldwide, and HIV is the leading cause of death among women of reproductive age. Gender inequalities, differential access to service, and sexual violence are all hallmarks of women’s vulnerability to HIV (UNAIDS, 2016).
In this study, there was no statistically significant difference between mean CD4 count in HIV mono-infected and HIV-HBV co-infected study participants. However, HIV-HBV co-infected participants in this study had a mean CD4 count (353.08±229.30 cells/mm3) that differs for mean CD4 count of 141.6 cells/mm3 and 121 cells/mm3 in South African and Nigerian studies respectively (Odenyo et al., 2000; Olufemi et al., 2009). The minimum and maximum CD4 count were 73 and 753 cells/mm3, respectively. These different results might be due to the differences in the immune status of the individuals and/or to the fact participants in the present study were newly identified with current history of HIV infection. ALT activity was significantly higher among HIV/HBV co-infected participants compared to HIV mono-infected ones. This is in agreement with findings resulting from other investigations in Cameroon and worldwide in which high level of ALT was reported (Zoufaly et al., 2012; Zhou et al., 2007). The ALT is found in serum and in various bodily tissues, but high level in the serum is most commonly associated with the liver damage (e.g. cytolysis). It has already been demonstrated that high ALT serum level activity principally reflects direct hepatocellular damage or liver dysfunction (Pratt and Kaplan, 2000). Consequently, both HIV and HBV create pressure on liver, leading to elevation of liver transaminase, alanine amino-transferase.
In the present study, no significant correlation was observed between different parameters among co-infected patients; though a significant and negative correlation at the threshold 0.05 was observed between CD4 T-cells and ALT in HIV mono-infected patients. Some HIV mono-infected patients had a rate of CD4 ≤200 cells/mm3 (34%), and the minimum and maximum CD4 count values were 12 and 1355 cells/mm3, respectively. In these patients, CD4 T-cells decrease while ALT activity increases.
Overall, based on the present findings, there is a critical need for management of HIV-HBV co-infections in Cameroon, in people living with HIV as well as in people from hepato-gastroenterology clinics. In 2013, it clearly appeared in the WHO Global policy report on the prevention and control of viral hepatitis that there is no written national strategy or plan that focuses on the prevention and control of viral hepatitis in Cameroon, as in other sub-Saharan African countries (WHO Global policy report, 2013). Four years after the remarks, these written strategies is an urgent need following the new policies currently launched in the Country.
This study has pointed out that the prevalence of hepatitis B virus surface antigen (HBsAg) is significant amongst HIV positive patients identified in the study’s site, 16%. The co-infection rate is higher among women (12%) compared to men (4%). No significant increase in liver parameters was observed in HIV mono-infected patients. A negative and significant correlation was observed between CD4 and alanine amino-transferase (ALT) activity, as well as between conjugated bilirubin and alkaline phosphatase (ALP) activity at the threshold 0.05. These results are without doubt useful in the management of hepatitis B virus in people with HIV/AIDS. Future investigation of hepatitis chronic carriers is required in the follow-up of patients.
Based on the research findings, a national management and active surveillance program for HIV and hepatitis co-infections is essential in the country, as a critical step to reduce the incidence and morbidity rates of these affections. The new policies shall integrate and consider viral hepatitis as serious as HIV infection.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study received an ethical clearance from the Cameroon National Research Ethics Committee for Human Health N° 2015/11/665/CNERSH/SP. In addition, informed consent of participants was obtained prior to their enrollment.
The authors declare that they have no conflict of interests with regard to this work.
REFERENCES
|
Dieterich DT (2007).Special considerations and treatment of patients with HBV-HIV coinfection. Antivir. Ther. 12:H43-H51.
|
|
|
|
Faul F, Erdfelder E, Buchner A, Lang AG (2009). Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behav. Res. Methods 41:1149-1160.
Crossref
|
|
|
|
|
Faul F, Erdfelder E, Lang, AG, Buchner A (2007). G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 39:175-191.
Crossref
|
|
|
|
|
Gita R, Brodie D (2013).Women and HIV in Sub-Saharan Africa. AIDS Res. Ther. 10:30.
Crossref
|
|
|
|
|
Jobarteh M, Marine M, Ingrid P, Adam J, Ramu SN, Abraham A, Kevin P, Matt C, Andrew A, Sarah R-J, Hilton W, Richard T, Assan J, Maïmouna M (2010). Seroprevalence of hepatitis B and C virus in HIV-1 and HIV-2 infected Gambians. Virol J. 7:230.
Crossref
|
|
|
|
|
Laurent C, Bourgeois A, Mpoudi-N E, Kouanfack C, Ciaffi L, Nkoue N (2010). High rates of active hepatitis B and C co-infections in HIV-1 infected Cameroonian adults initiating antiretroviral therapy. British HIV Ass. HIV Med. 11:85-89.
Crossref
|
|
|
|
|
Mabala R (2006). From HIV prevention to protection: addressing the vulnerability of girls and young women in urban areas. Environ. Urban 18 (2):407-432.
Crossref
|
|
|
|
|
Nagu TJ, Bakari M, Matee M (2008). Hepatitis A, B and C viral co-infections among HIV-infected adults presenting for care and treatment at Muhimbili National Hospital in Dar es Salaam, Tanzania. BMC Public Health 8:416.
Crossref
|
|
|
|
|
National Institute of Statistics (2011). Cameroon Demographic and Health Survey and Multiple Indicators Cluster Survey DHS-MICS. HIV prevalence and associated factors. pp. 265-285.
|
|
|
|
|
Noah ND, Njouom R, Bonny A, Pirsou P, Meli J, Biwole Sida M (2011). HBs antigen prevalence in blood donors and the risk of transfusion of hepatitis B at the Central hospital of Yaoundé, Cameroun. Open J. Gastroenterol. 1(02):23.
Crossref
|
|
|
|
|
Nyirenda M, Beadsworth M, Stephany P, Hart C, Hart I, Munthali C (2008). Prevalence of infection with hepatitis B and C virus and co-infection with HIV in medical inpatients in Malawi. J. Infect. Dis. 57:72-77.
|
|
|
|
|
Obi SO, Baba HA, Baba MM, Amilo GI, Bukar A (2012). The Effect of Co-infection of HIV and Hepatotropic Viruses on Selected Biochemical and Haematological Markers of Patients in Northeastern Nigeria. Int. J. Trop. Dis. Health 4:568-581.
Crossref
|
|
|
|
|
Ochola E, Ocama P, Orach CG (2013). High burden of hepatitis B infection in Northen Uganda: Results of a population-based survey. BMC Public Health 13(1):727.
Crossref
|
|
|
|
|
Odenyo H, Schoub B, Ally R, Kairu S, Segal I (2000). Hepatitis B and C virus infections and liver function in AIDS patients at Chrishanibaragwanath hospital Johannesburg. East Afr. Med. 77(1):13-15.
|
|
|
|
|
Olufemi A, Emmanuel A, Zaccheus A, Ibrahim W, Funmilayo E, Patience A (2009). Hepatitis B and C virus co-infection in Nigerian patients with HIV infection. J. Infect. Dev. Ctries. 3(5):369-375.
|
|
|
|
|
Pratt DS, Kaplan MM (2000). Evaluation of abnormal liver enzyme results in asymptomatic patients. New England J. Med. 342:1266-711.
Crossref
|
|
|
|
|
The Joint United Nations Programme on HIV and AIDS (UNAIDS) (2016). Global AIDS Update 2016.
|
|
|
|
|
The Joint United Nations Programme on HIV and AIDS (UNAIDS) (2016). Fact Sheet 2016.
|
|
|
|
|
WHO (World Health Organization) (2013). Global policy report on the prevention and control of viral hepatitis in WHO Member States 2013: ISBN 978 92 4 156463 2.
|
|
|
|
|
WHO (World Health Organization) (2016). HIV and hepatitis coinfections: Available at:
View
|
|
|
|
|
Yohannes Z, Wondemagegn M, Mulat Yimer, Bayeh A (2014). Seroprevalenceand risk factors of hepatitis B virus and human immunodeficiency virus infection among pregnant women in Bahir Dar city, Nortwest Ethiopia: a cross sectional study. BMC Infect. Dis. 14:118.
Crossref
|
|
|
|
|
Zhou J, Dore GJ, Zhang F, Lim PL, Chen YM (2007). TREAT Asia HIV Observational Database. Hepatitis B and C virus co-infection in The TREAT Asia HIV Observational Database. J. Gastroenterol Hepatol. 22:1510-1518.
Crossref
|
|
|
|
|
Zoufaly A, Onyoh EF, Tih PM, Awasom CN, Feldt T (2012). High prevalence of hepatitis B and syphilis co-infections among HIV patients initiating antiretroviral therapy in the north-west region of Cameroon. Int. J. STD AIDS. 23:435-438.
Crossref
|
|