ABSTRACT
The fundamental goal of this research is to provide a method to predict the fatigue life of Magnesium and its alloys in human body fluid environment. Estimation of fatigue life of Magnesium and its alloys in human body fluid environment can help doctors and patients to choose the best biomaterial for implantation surgery. This fact is true due to the reason that based on the fatigue life of these materials it can be easily predicted how long these biomaterials can last in human body. However, this is due to the reason that Magnesium and its alloys may dissolve in human body by the end of their fatigue life. As a result, no second surgery may be required for the patients and doctors. This study aims to present a method to determine the fatigue life of Magnesium and its alloys in human body fluid. The results can help to select the best option of biomaterials.
Key words: Fatigue life, magnesium, magnesium alloys, human body fluid.
It has been years that scientists and doctors are looking for biomaterials with adequate mechanical properties and biocompatibility to use in human body as a replacement for bones, joints, hips, etc (Sedmak and Rakin, 2006). Recently, in order to investigate the characteristics of nanomaterials a few books are published. Among those, “Nanomaterials and polymer membranes: Synthesis, characterization, and applications” is submitted by Saleh and Gupta (2016), and “Nanotechnology in oil and gas industries: Principles and applications” is presented by Saleh (2018). Furthermore, in the field of nanomedicine “Aptamer-drug-conjugate: Targeted delivery of doxorubicin in a HER3 aptamer-functionalized liposomal delivery system reduces cardiotoxicity” is provided by Dou et al. (2018). In order to investigate the environmental effect of human body on biomaterials, many experimental procedures are performed (Dewidar, 2012). In 2017, the role of metallic materials as biomaterials in human body is introduced by Santos (2017). Metallic materials can be used in artificial valves in the heart, stents in blood vessels, replacement implants in shoulder, knees, hips, elbows, ears and ortho-dental structures (Santos, 2017). Recently, Titanium alloys have attracted many researchers due to their excellent biocompatibility (Wang and Xu, 2017; Findik, 2017). Furthermore, numbers of investigations are performed to characterize the corrosion and fatigue resistance of Titanium and Magnesium alloys.
The reason behind this is that corrosion of metallic materials in human body is one of the important issues that need to be studied. This is due to the corrosive environment of human body fluid. In addition, fatigue is due to the cyclic loads in human body as a result of dynamic loads such as walking, running, etc. Among the investigations in this area, “comparative corrosion behavior of Titanium alloys for dental implants” is provided by Lopes et al. (2016). “Resistance of Magnesium alloys to corrosion fatigue for biodegradable implant applications” is submitted by Raman and Harandi (2017). In addition, “fatigue and corrosion fatigue of Ti-6Al-4V implant grade Titanium alloy in ringer solution” is presented by Yazdani et al. (2017). Moreover, many attempts have been done to improve the corrosion and fatigue resistance of biomaterials in human body fluid. One of the methods to increase the corrosion resistance is formation of stable oxide surface layer on biomaterial. Furthermore, it has been discovered that the factors which increase the tensile strength, can also improve the fatigue strength as well (Hosseini, 2012). In addition, in 2013, “effect of microstructure on the fatigue properties of Ti-6Al-4V Titanium alloys” is recommended by Wu et al. (2013). However, currently, further researches are performed to evaluate the fatigue life of implants and stents. Among these studies, “Material fatigue research for Zirconia ceramic dental implant” is provided by Karacali (2015), “Fatigue of metallic stents: From clinical evidence to computational analysis,” is published by Auricchio et al. (2016), and “Fatigue life prediction of Titanium implants with Titanium dioxide surface” is submitted by Major et al. (2017).
Furthermore, recently, numbers of studies to predict the fatigue life and failure of materials are provided by Anvari (2014, 2017a, b, c, d). As mentioned in the previous paragraphs, many efforts have been made to evaluate the fatigue life of biomaterials in human body fluid. However, it seems that the exact consideration of biomaterials such as Magnesium and its alloys based on fatigue life in human body fluid is still needed to be performed to obtain the relations and cycle numbers of fatigue life in different stress values in human body. However, these results can help doctors choose the best biomaterials for the implantation surgery, and as a result, the second surgery for implantation removal might be eliminated. In the presented research, the goal is to provide a method to estimate the fatigue life of Magnesium and its alloys in human body fluid. Equations to predict the fatigue life are achieved and the cycle numbers for the end of fatigue life are obtained based on analytical method. The cycle numbers for the end of fatigue life can be very advantageous for doctors to treat patients professionally with minimum numbers of surgeries and eliminate the surgery for implantation removal. This is of high significance because sometimes patients are weak or too old for second surgery. In addition, in some cases, the surgery is very inconvenient for doctors or patients or both of them.
BIOMATERIALS IN HUMAN BODY AS IMPLANTS
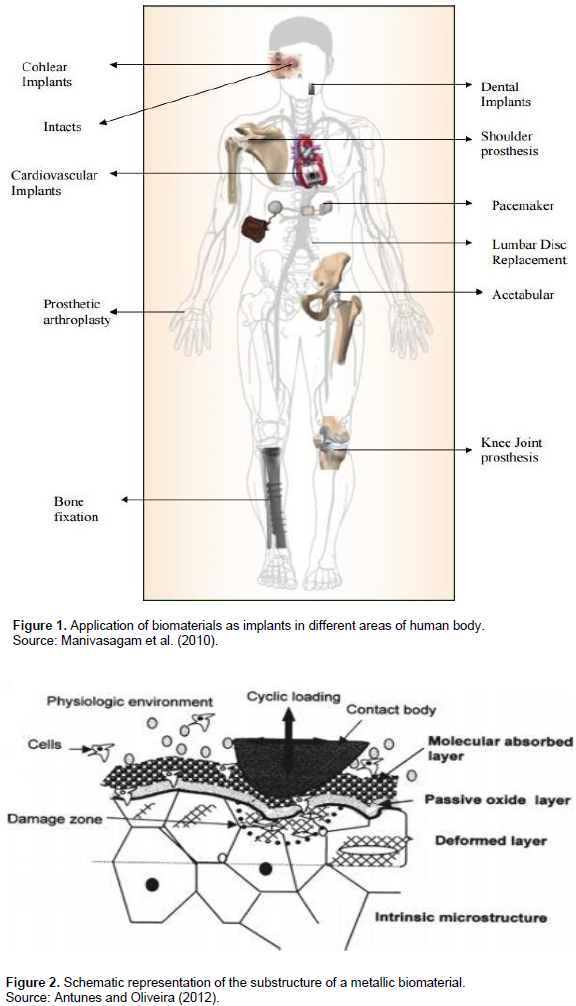
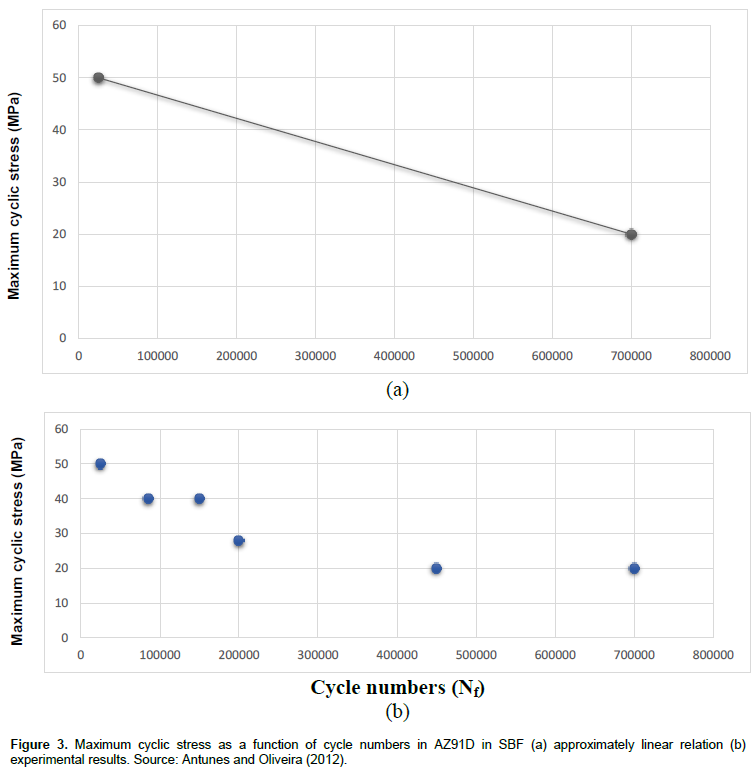
Application of biomaterials in human body as implants is very common nowadays. In Figure 1 (Manivasagam et al., 2010), the locations of implants in different areas of human body are shown. As illustrated, biomaterials can be used in many locations in human body such as shoulders, hips, joints, bones, dental implants, etc. The materials used in the implants are usually from metallic materials such as Steel, Cobalt and its alloys, Titanium and its alloys, Magnesium and its alloys, etc. Once these biomaterials are planted in the human body, they will be subjected to human body fluid environment. The human body fluid is corrosive for metallic biomaterials. As a result, biomaterials degradation occurs ultimately unless they are covered with appropriate coatings that could prevent corrosion. In Figure 2 (Antunes and Oliveira, 2012), a schematic illustrating the implant’s situation in human body is shown. The role of cyclic loading and physiologic environment in biomaterial’s degradation can be perceived in Figure 2.
MAGNESIUM AGAINST NATURAL BONE
In Table 1 (Peron et al., 2017), mechanical properties of natural bone and Magnesium are indicated. With a quick review of the mechanical properties of natural bone and Magnesium, it can be concluded that the values of the material properties of natural bone and Magnesium are close to each other. With the further assessment of Table 1, it is easy to find out that the value of density in natural bone (1.7 to 2.0 g/cm3) is approximately in the same range as Magnesium density (1.74 to 2.0 g/cm3). It is one of the reasons that Magnesium is one of the best biomaterials for the application of implantations in human body as is a perfect match with natural bone based on density.
Materials and specimen preparation
The experimental materials were high purity magnesium (99.99 wt. %, denoted as HP-Mg), Mg–1Ca and Mg–2Zn–0.2Ca all in extruded rods with an extrusion ratio of 11. The raw ingot of HP-Mg was provided by Hebi Wuhua Magnesium Processing Co. Ltd., China. Plate tensile specimens with a gage length of 25 mm and smooth-faced fatigue specimens (surface roughness: 0.3 lm) with a circular cross-section 5 mm in diameter and a gage length of 10 mm were machined parallel to the extrusion direction according to ASTME8-04 and ASTM-E466-96, respectively. Specimens for microstructural characterization were cut perpendicular to the extrusion direction into 2 mm in thickness and 10 mm in diameter disks (Bian et al., 2016).
Microstructural characterization
Disk specimens were mechanically polished with SiC abrasive papers from 400 grit to 2000 grit gradually, then chemically polished into mirror-like surface with the mixture of 2 ml chlorhydric acid, 3 ml nitric acid, 5 ml acetic acid and 20 ml glycerol. Phase constituents were characterized by X-ray diffraction analysis (XRD, Rigaku DMAX 2400) using Cu Ka radiation at a scan rate of 4°/min operated at 40 kV and 100 mA. After etched in 4% nitric acid alcohol solution, the samples were observed under a scanning electron microscope (SEM, HITACHI S-4800) (Bian et al., 2016).
Fatigue test in simulated body fluid (SBF)
As for the corrosion fatigue, a self-designed dynamic circulation system was utilized to monitor the corrosion fatigue environment. This system consisted of three main parts: an acrylic chamber that 352 D. used to keep the specimen (only gage length part) immersing in SBF solution, a temperature controlling apparatus that used to keep the temperature of SBF at 37 ± 1°C, and a pump to generate the flow (65 ml/s). During the corrosion fatigue test, the SBF solution was replaced every 24 h and the dissolution of magnesium and alloying elements into the retrieved SBF was measured until sample failure by inductively coupled plasma atomic emission spectrometry (Leeman, Profile ICP-AES) (Bian et al. 2016). As it is clear, the purpose of this experiment is to measure the fatigue life (cycle numbers to failure) for magnesium and its alloys in the presence of the SBF. In order to make the simulation of the human body environment, dynamic loads (cyclic loads) are applied to the specimen (Magnesium and its alloys) in SBF. As mentioned previously, cyclic loads in human body can be due to walking, running, exercising, etc.
CYCLE NUMBERS TO FAILURE IN SBF
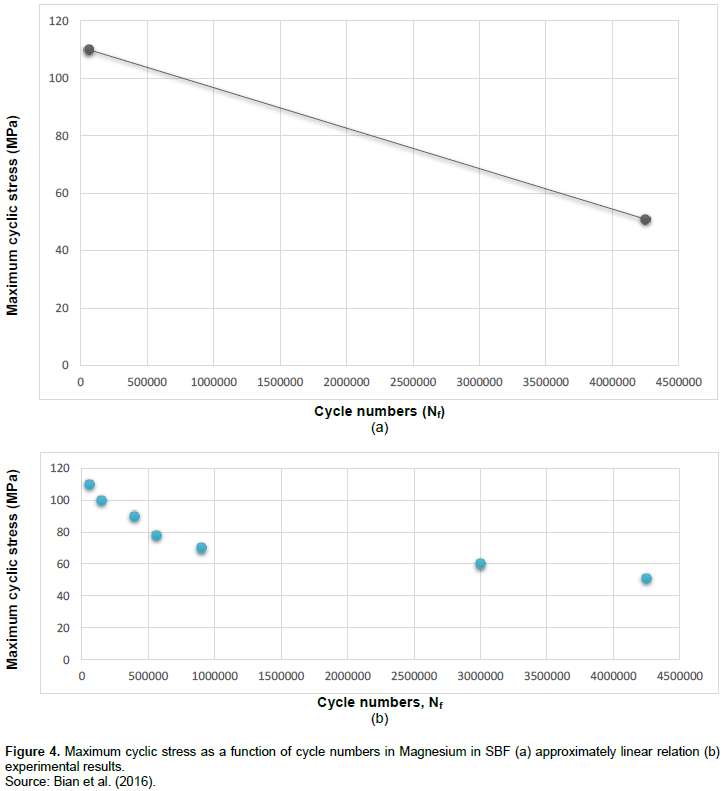
In Figure 3 (Antunes and Oliveira, 2012), maximum cyclic stress as a function of cycle numbers for Magnesium based biomaterial (AZ91D) in human body fluid is illustrated. However, it seems that Cycle-Stress relation in Figure 3 is approximately linear. In the present study, in order to achieve a function that represents the relation between the maximum cycle stress and cycle numbers to failure, two boundary points from Figure 3 is used. By applying point 1; σ = 50 MPa, Nf = 2.5e+4 cycles, and point 2; σ = 20 MPa, Nf = 7e+5 cycles, two equations may be developed. Following above procedure, for point 1,
50 = a (2.5e+4) + b, (1)
And for point 2, there is
20 = a (7e+5) + b. (2)
In above relations a and b are constants. By solving these two dependent relations, a and b can be obtained and equation representing the maximum cyclic stress as a function of cycle numbers is achieved.
σ = -4.444e-5 (Nf) + 51.108. (3)
For estimating the fatigue life of AZ91D which is a Magnesium-based biomaterial in SBF, it is enough to solve the Equation 3 in a state that σ = 0. The result can indicate that due to the fatigue in human body in the presence of corrosion, AZ91D biomaterial can last about 1.15+e6 cycles. As mentioned before, cyclic load in human body could be due to walking, running, and other body movements. This result can help doctors to answer the question, “How long AZ91D can last in human body?” Thus, doctors would be able to estimate the time of second surgery, if required. Magnesium alloys could totally dissolve in human body, hence, the second surgery may not be required at all. In the following Figures 4 and 5, fatigue lives of Magnesium and its alloy in SBF are indicated. As perceived in these two figures, maximum cyclic stress as a function of cycle numbers for Magnesium and its alloy is shown, respectively. It appears that the relation between the maximum cyclic stress and cycle numbers is approximately linear in both Magnesium and its alloy (Mg-2Zn-0.2Ca). In the case of Magnesium, there are two points that can be used to obtain the linear equation similar to Equation 3. These two points would be: σ = 110 MPa, Nf = 6e+4 cycles, and, σ = 51 MPa, Nf = 4.25e+6 cycles. In addition, in the case of Magnesium alloy, two points: σ = 115 MPa, Nf = 104 cycles, and σ = 70 MPa, Nf = 3.8e+6 cycles can be applied to obtain the linear equation. With the application of the same method used to obtain Equation 3, two linear relations for predicting the fatigue life of Magnesium and its alloy in SBF are achieved and shown below, respectively.
σ = -1.408e-5(Nf) + 110.84487, (4)
σ = -1.1873e-5(Nf) + 115.11873. (5)
In order to estimate the cycle numbers to failure in SBF, it is enough to solve these two equations while they are equal to zero. For Equation 4 equal to zero, the fatigue life of Magnesium in SBF is obtained about 7.87e+6 cycles. Furthermore, for Equation 5 equal to zero, the fatigue life of Mg-2Zn-0.2Ca in SBF is achieved about 9.69e+6 cycle numbers. These cycle numbers represent the fatigue life in SBF in a state which the biomaterial is not capable to tolerate any stress. Thus, these cycle numbers may be named as cycle numbers to total failure. However, it appears that at about or after these cycle numbers, biomaterials are in a state that they are dissolving, or already partially or even totally dissolved in human body fluid environment.
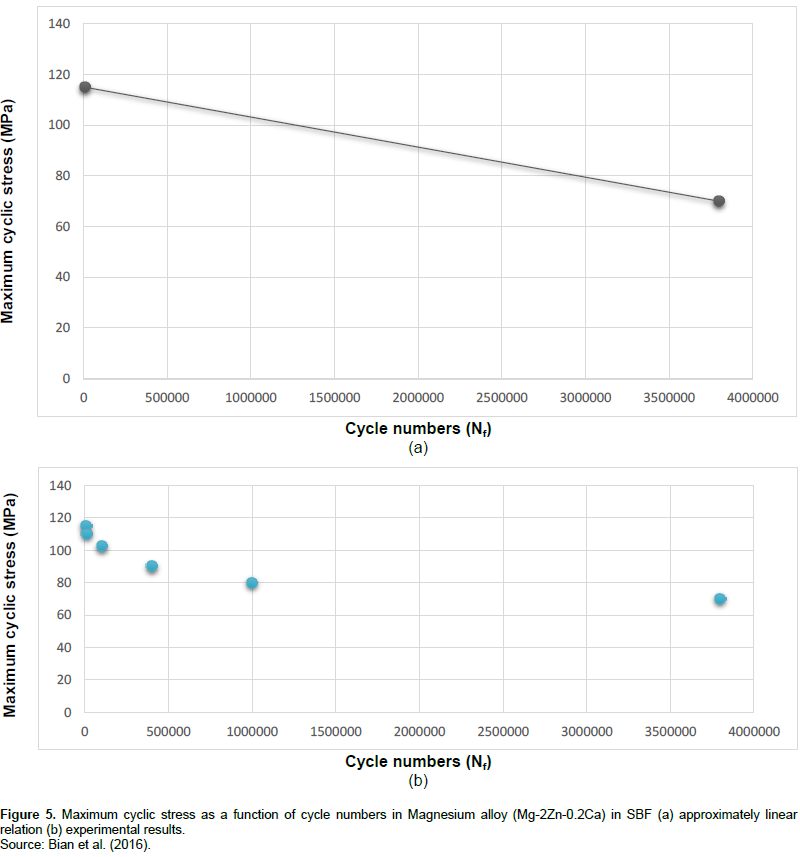
In Table 2, all the equations to estimate the cycle numbers to failure of Magnesium and its alloys are indicated. Based on the results obtained by this method, Mg-2Zn-0.2Ca offers the maximum fatigue life and AZ91D offers the minimum cycle numbers to failure in SBF. These results may be very advantageous for doctors. With these results, they can predict how long each of these materials can last in human body. Hence, they would be able to select the best biomaterial according to their needs. Furthermore, they can choose the biomaterial that guarantee that the second surgery is not required and it will be dissolved in human body fluid before the implantation removal surgery is required. However, in order to apply the results of this research a few calculations are needed to be performed. In the first step, doctors need to calculate the stress imposed to the implant and the cycle numbers associated to the movement in human body. In the next step, by using Figures 3, 4, and 5, can calculate the cycle numbers to failure for that stress imposed to implants. As an instance, an athlete might repeat one kind of body movement with its associated stress in certain cycle numbers each year. Thus, a doctor can estimate the applied stress and its associated cycle numbers each year. Following this procedure, if the doctor can calculate the stress for the movement, then it would be able to find out the cycle numbers to failure for that stress from Figures 3, 4, and 5. As a result, by dividing the cycle numbers to failure to cycle numbers repeated each year, doctor would be able to predict the fatigue life of the implant.

The target of this research is to provide a method to establish relations to predict the cycle numbers to failure of Magnesium and its alloys in human body fluid. Analytical method with linear relations are recommended to model the stress-fatigue behavior of Magnesium and its alloys. The recommended equations are solved and cycle numbers to failure for Magnesium and its alloys are obtained. The results have shown that the Mg-2Zn-0.2Ca offers the maximum cycle numbers to failure and AZ91D offers the minimum cycle numbers to failure in human body fluid. However, it is interesting to mention that the cycle numbers to failure for Magnesium is between the cycle numbers to failure for these two Magnesium alloys. These results can help doctors to select the best biomaterial for the implantation surgery because they would be able to predict how long these biomaterials will last in human body fluid.
The authors have not declared any conflict of interests.
The authors appreciate the opportunity of performing this research in University of Missouri-Columbia. All the funding related to this research is provided by the presented author.
REFERENCES
|
Antunes RA, Oliveira MCL (2012). Corrosion fatigue of biomedical metallic alloys: Mechanisms and mitigation. Acta Biomaterialia 8:937-962.
Crossref
|
|
|
|
Anvari A (2014). Fatigue life prediction of unidirectional carbon fiber/epoxy composite in Earth orbit. Int. J. Appl. Math. Mech. 10(5):58-85.
|
|
|
|
|
Anvari A (2017a). Crack growth as a function of temperature variation in carbon fiber/epoxy. J. Chem. Eng. Mater. Sci. 8(3):17-30.
Crossref
|
|
|
|
|
Anvari A (2017b). Failure of Nickel-based super alloy (ME3) in aerospace gas turbine engines. J. Chem. Eng. Mat. Sci. 8(6):46-65.
Crossref
|
|
|
|
|
Anvari A (2017c). Thermal fatigue life of carbon nanotube wire and unidirectional carbon fiber/epoxy composite (UCFEC) in earth orbit. J. Chem. Eng. Mater. Sci. 8(8):101-111.
Crossref
|
|
|
|
|
Anvari A (2017d). Fatigue life prediction of unidirectional carbon fiber/epoxy composite on Mars. J. Chem. Eng. Mater. Sci. 8(8):74-100.
Crossref
|
|
|
|
|
Auricchio F, Constantinescu A, Conti M, Scalet G (2016). Fatigue of Metallic Stents: From Clinical Evidence to Computational Analysis. Ann. Biomed. Eng. 44(2):287-301.
Crossref
|
|
|
|
|
Bian D, Zhou W, Liu Y, Li N, Zheng Y, Sun Z (2016). Fatigue behaviors of HP-Mg, Mg-Ca and Mg-Zn-Ca biodegradable metals in air and simulated body fluid. Acta Biomaterialia 41:351-360.
Crossref
|
|
|
|
|
Dewidar M (2012). Influence of processing parameters and sintering atmosphere on the mechanical properties and microstructure of porous 316L stainless steel for possible hard-tissue applications. Int. J. Mech. Mechatron. Eng. 12(1):10-24.
|
|
|
|
|
Dou XQ, Wang H, Zhang J, Wang F, Xu GL, Xu CC, Xu HH, Xiang SS, Fu J, Song HF (2018). Aptamer-drug conjugate: targeted delivery of doxorubicin in a HER3 aptamer-functionalized liposomal delivery system reduces cardiotoxicity. Int. J. Nanomed. 13:733-762.
Crossref
|
|
|
|
|
Findik F (2017). Titanium Based Biomaterials. Eng. Biosci. 7(3):1-3.
Crossref
|
|
|
|
|
Hosseini S (2012). Fatigue of Ti-6Al-4V. Biomedical Engineering-Technical Applications in Medicine, Chapter 3:75-92.
Crossref
|
|
|
|
|
Karacali O (2015). Material fatigue research for Zirconia ceramic dental implant: a comparative laboratory and simulation study in dentistry. Acta Physica Polonica A 127(4):1195-1198.
Crossref
|
|
|
|
|
Lopes CSD, Donato MT, Ramgi P (2016). Comparative Corrosion Behavior of Titanium Alloys (Ti-15Mo and Ti-6Al-4V) for Dental Implants Applications: A review. Corros. Prot. Mater. 35(2):5-14.
|
|
|
|
|
Major S, Kocour V, Bryscejn J (2017). Fatigue life prediction of Titanium implants with Titanium Dioxide Surface. Int. J. Mechanics 11:288-298.
|
|
|
|
|
Manivasagam G, Dhinasekaran D, Rajamanickam A (2010). Biomedical Implants: Corrosion and its Prevention-A Review. Recent Patents on Corrosion Science 2:40-54.
Crossref
|
|
|
|
|
Peron M, Torgersen J, Berto F (2017). Mg and its alloys for biomedical applications: exploring corrosion and its interplay with mechanical failure. Metals 7:1-41.
Crossref
|
|
|
|
|
Raman RKS, Harandi SE (2017). Resistance of Magnesium Alloys to Corrosion Fatigue for Biodegradable Implant Applications: Current Status and Challenges. Materials 10:1-11.
Crossref
|
|
|
|
|
Saleh TA (2018). Nanotechnology in oil and gas industries: principles and applications (Topics in mining, metallurgy and materials engineering). 1st Edition.
Crossref
|
|
|
|
|
Saleh TA, Gupta VK (2016). Nanomaterials and polymer membranes: synthesis, characterization, and applications. 1st Edition.
|
|
|
|
|
Santos GAD (2017). The importance of metallic materials as biomaterials. Adv. Tissue Eng. Regenerative Med. 3(1):1-3.
Crossref
|
|
|
|
|
Sedmak A, Rakin M (2006). Biomaterials-Joints and Problems of Contact Interfaces. FME Transactions 34(2):81-86.
|
|
|
|
|
Wang SP, Xu J (2017). TiZrNbTaMo high-entropy alloy designed for orthopedic implants: As-cast microstructure and mechanical properties. Materials Science and Engineering C. 73:80-89.
Crossref
|
|
|
|
|
Wu GQ, Shi CL, Sha W, Sha AX, Jiang HR (2013). Effect of microstructure on the fatigue properties of Ti-6Al-4V Titanium alloys. Materials and Design. Doi: http://dx.doi.org/10.1016/j.matdes.2012.10.059.
Crossref
|
|
|
|
|
Yazdani SY, Hajisafari M, Bidaki AZ (2017). Fatigue and corrosion fatigue of Ti-6Al-4V implant grade Titanium alloy in ringer solution. J. Adv. Mater. Processing 5(3):12-22.
|
|