ABSTRACT
Fonio (Digitaria spp.) is a highly nutritious, health promoting and cheaply available cereal which is, however, marginalized in many parts of Nigeria, and research attention on the crop is yet to measure up to its usefulness as a dietary staple. In this study therefore, five accessions each of two common species (Digitaria exilis and Digitaria iburua) were assessed for intra and inter- species variation in antioxidant activities. Total phenols were higher (p<0.05) in D. iburua than D. exilis accessions (0.64 to 1.59 and 0.43 to 0.63 mg gallic acid equivalents g-1 specimen, respectively). D. iburua accessions scavenged 50% of stable 2,2-diphenyl-1-picrylhydrazyl (DPPH) radicals with greater potency (EC50 = 0.278 to 0.816 mg/mL) compared to D. exilis accessions (0.916 to 2.325 mg/ml). D. iburua accessions further showed higher (p<0.05) 2,2'-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS) radical scavenging potency (6.939 to 8.677 trolox equivalents) than D. exilis accessions (0.365 to 6.070 trolox equivalents). Total antioxidant capacity of D. iburua accessions ranged from 17.76 to 27.44 ascorbic acid equivalents, and was significantly higher (p<0.05) than those of D. exilis (8.69 to 15.80 ascorbic acid equivalents). Correlation analysis revealed that antioxidant activity of the fonio accessions was significantly associated with their total phenols content, but had little association with extract yields. Taken together, the study results provide evidence that fonio grains contain useful antioxidants, which varied significantly within and across species. Optimum antioxidant benefits in fonio would be derived more from D. iburua than D. exilis. However, environmental influence on antioxidant activity of the crop could also be prevalent, given the observed significant variation among accessions of both species.
Key words: Antioxidant, accessions, fonio, Digitaria iburua, Digitaria exilis, variation
With the rapidly growing global population, the demand placed on world agriculture, to among other things, provide healthy and nutritious food for all, is compelling. It has been argued quite reasonably that agricultural and
food systems will need to make greater use of traditional crop species and varieties that have been hitherto marginalized in large scale commercial food production (Nono-Wondim et al., 2013). Such crops, which include fonio (Digitaria species), according to Padulosi et al. (2013), present tremendous opportunities for fighting poverty, hunger and malnutrition, and making agricultural systems more resilient to climate change. Also called Hungry Rice, Fonio is a cereal crop particularly pertinent to sub-Saharan Africa. It belongs to the Poaceae family and is indigenous to West Africa where it is cultivated for its small edible grains (Ballogou et al., 2013).
In Nigeria, fonio is locally called “acha” and grown mostly in the cool regions of Plateau State, and some other parts of Bauchi, Taraba, Nassarawa, Kaduna and Niger States. Two species, Digitaria exilis and Digitaria iburua, usually physically distinguishable by their colour differences (Chukwu and Abdulkadir, 2008) are common. D. exilis is commonly called “white fonio” because of its light brown seed coat colour while D. iburua, which has darker and sometimes black seed coat, is referred to as “black fonio”. Nutritionally, according to a review conducted by Ballogou et al. (2013), husked grains of fonio contain about 5.1 to 11% proteins, 67.1 to 91% carbohydrates (mainly starch), 1.3 to 5.2% fats and 1 to 6% ash as reported by various authors, including a rich content of both essential and non-essential amino acids. Higher energy value compared to other cereals like rice, sorghum and maize was also reported by Jideani and Akingbala (1993).
Just like other cereals, fonio, and indeed many other underutilized species, represent unique sources of a wide variety of bioactive substances (Nono-Wondim et al., 2013), including antioxidants (Uyoh et al., 2013), which are essential for preventing free-radical induced degenerative disorders like cancers, heart diseases and diabetes. For example, Glew et al. (2013) reported that grains of acha (D. exilis) grown on the Jos Plateau of Nigeria contained total phenolic compounds comparable to that of common cereals, and had substantial amounts of free radical scavenging substances. Natural antioxidants from plant sources, like fonio, have continued to be craved against synthetic antioxidants that have long been implicated in carcinogenesis (Chukwurah et al., 2014).
Composition of chemicals and nutrients in fonio grains however, varies largely, as observed in a review by Ballogou et al. (2013), and was attributed to environmental influences, genetic factors and analytical methods used, among others. This variation may invariably, also affect the antioxidant composition of fonio, as studies on other cereals like wheat (Mpofu et al., 2006) and maize (Nawaz et al., 2013) grains all revealed significant differences in antioxidant composition, and activities among different genotypes and varieties. Mpofu et al. (2006), in their study on wheat antioxidants, further added that the observed genotype variation in the crop indicated possibility of selection for antioxidant properties in breeding programs. Even in non-cereal crops like culinary and medicinal herbs (Wang, 2003), sweet cherry (Prvulovic et al., 2011), tomato (Brezeanu et al., 2013), genetic and environmental factors have all been identified as contributing significantly to variation in antioxidant activity of the crops. In this wise, an assessment of the intra- and inter-species variation in antioxidant composition and activity of fonio (D. exilis and D. iburua) accessions will provide further understanding to the influence of genetic and environmental factors on this parameter.
Collection and extraction of fonio grains
Grains of ten accessions of fonio were sourced from collections maintained at the National Cereals Research Institute, Acha Sub-station, Riyom, Anglo-Jos, Plateau State, Nigeria. Five of the accessions (Churiwe, Gindiri I, Kureep, Ndai and Shen) were D. exilis while the other five accessions (Dampep, Dipiya, Gotip, Nasheleng and Zor) were D.iburua. The grains of each accession were hulled and milled, then sun-dried and ground to powder with the aid of a mortar and pestle, separately. Thereafter, 10 g sample of each accession was extracted three successive times for 24 h at room temperature with 100 ml of methanol. Extracts obtained were filtered using Whatman No. 1 filter paper and supernatants combined. Crude extracts were concentrated under vacuum, and the dry weights determined.
Chemicals
Folin-Ciocalteu reagent, gallic acid, DPPH radical, Iron (III) chloride, ABTS, potassium persulfate and trolox were purchased from Sigma Aldrich Chemicals Ltd., St Louis, USA. Solvents and other chemicals used were of the highest analytical grade, and water was glass distilled.
Determination of Total Phenol Content (TPC)
Total phenol content of the fonio extracts was estimated using the Folin-Ciocalteau colorimetric assay described by Singleton et al. (1999), with slight modifications. Briefly, aliquots of 0.25 ml of extracts (1 mg/ml) were mixed with 1.25 ml of Folin Ciocalteau reagent (0.2N, diluted in methanol). A reagent blank using methanol instead of sample was prepared. After 5 min incubation at room temperature, 1 ml of sodium carbonate solution (7.5%) was added. Finally, the solution was brought to 10 ml by adding distilled water. Samples were incubated for 1 h at room temperature, and absorbance read at 765 nm against the prepared blank. Gallic acid, a standard phenol, in the range of 0 to 50 mg/L was used to prepare a standard reference curve. The total phenol contents (TPC) of the extracts were expressed as mg of gallic acid equivalent (GAE) per gram dry weight of original acha specimen. All measurements were performed in triplicate.
2, 2-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging assay
The ability of the methanol extracts of fonio to neutralize the free radical, 2, 2-diphenyl-2-picrylhydrazyl (DPPH), was determined using the method of Mensor et al. (2001). Different concentrations (50 to 150 µg/ml) of the extracts were prepared. To 2.5 ml solution of each extract concentration, 1 ml of freshly prepared DPPH solution in methanol (0.3mM) was added and allowed to react in the dark at room temperature for 30 min. Absorbance of the resulting solution was measured at 518 nm. Methanol (1 ml) added to 2.5 ml of each extract concentration served as blank, while 1 ml of 0.3 mM DPPH solution added to 2.5 mL of distilled water served as a negative control. Ascorbic acid was used as a reference standard. Measurements were performed in triplicate. Percentage DPPH scavenging activities of the extracts and reference standard were determined using the formula:
% Scavenging activity = 100 – [(As – Ab)/Ac x 100], where As = Absorbance of sample (extracts or reference standard), Ab = Absorbance of blank and Ac = Absorbance of negative control.
Results were expressed as EC50 (effective concentration of extract necessary to neutralize 50% of DPPH radicals), and interpolated from a linear regression curve of concentration (mg/mL) versus % scavenging.
2, 2-azino-bis 3-ethyl benthiazoline-6-acid (ABTS) radical scavenging activity
ABTS radical scavenging activity of the fonio extracts was measured by the ABTS cation decolorization assay as described by Re et al. (1999) and Pellegrini et al. (1999) with some modifications. The ABTS radical cation (ABTS•+) was produced by reaction of 7 mM stock solution of ABTS with 2.45 mM potassium persulfate and allowing the mixture to stand in dark at room temperature for 16 h before use. The ABTS•+ working solution was prepared by diluting the previous stock solution in ethanol to obtain an absorbance of about 0.700 at 734 nm. Fonio extracts (500 µg/ml, 1 mL) were then mixed with 2 ml of the ABTS•+ solution and allowed to react for 1 min after which the absorbance was measured at 734 nm. ABTS scavenging activity was evaluated thus:
ABTS radical scavenging (%) = [(A0-A1)/A0] × 100; where A0 is the absorbance of the control (without extracts) and A1 is absorbance of sample (fonio extracts)
A standard curve was obtained using trolox standard solution at various concentrations (0 to 10 μg/mL) in 95% ethanol. Scavenging activities of the fonio extracts towards ABTS radical were expressed as TEAC (trolox equivalent antioxidant capacity). All tests were performed in triplicate.
Total antioxidant capacity (TAC)
The total antioxidant capacity (TAC) of the fonio extracts were determined by the phosphomolybdate method according to Jayaprakasha et al. (2002). Aliquots (300 μl of 1 mg/mL extract concentration) were mixed with 3 ml of the reagent solution (0.6 M sulphuric acid, 28 mM sodium phosphate, 4 mM ammonium molybdate) taken in test tubes. The tubes were capped with aluminium foil, and incubated in a boiling water bath at 95°C for 90 min. The reaction mixture was allowed to cool to room temperature, and the absorbance of the solutions were measured at 695 nm against a blank containing 3 ml of reagent solution and the appropriate volume of solvent used to dissolve the extracts. The blank was incubated under the same conditions as the test samples.
A standard curve was obtained using ascorbic acid at various concentrations (2.5 to 70 μg/ml). Total antioxidant capacity of the fonio extracts was expressed as ascorbic acid equivalents (AAE).
All tests were performed in triplicate. The experiment was laid out in a Completely Randomized Design (CRD), with the fonio accessions representing ten treatments. Data obtained for all assays were subjected to one way analysis of variance (ANOVA) using the Genstat Discovery Edition 4 Software package. Correlation analysis was also carried out using the same software. The Duncan’s Multiple Range Test (DMRT) was used to separate significantly different treatment means.
Extract yield from D. exilis accessions ranged from 4.14 to 6.84% of original fonio specimen, with a mean of 5.25%, while in D. iburua accessions, a range of 5.17 to 5.48% with a mean yield of 5.30% was recorded (Table 1). Total phenol content of the fonio accessions was interpolated from a linear regression curve (y = 0.0018+0.0076x, r2 = 0.996) of gallic acid, a standard phenol. Accessions of D. exilis contained total phenols in the range of 0.43 to 0.63 mg Gallic Acid Equivalents/g fonio specimen, while those of D. iburua ranged from 0.64 to 1.59 mg Gallic Acid Equivalents/g fonio specimen. Mean total phenol content of D. iburua accessions was significantly higher (p<0.05) than in D. exilis accessions although this also differed among accessions of both fonio species (Table 1).
Whole grain cereals have been identified to constitute good sources of phenolic compounds (Dykes and Rooney, 2007), which have antioxidant properties, and can thus protect against degenerative diseases in which reactive oxygen species are implicated (Naczk and Shahidi, 2004). In fonio particularly, the flavones – apigenin and luteolin have been detected (Sartelet et al., 1996). The study results however show that all accessions of D. iburua contained more phenolic compounds than the D. exilis accessions, in the same amount of sample. This may be caused by inherent genetic differences. Indeed, Dykes and Rooney (2007) reported that different species of grains have a great deal of exploitable diversity in their germplasm resources. Such variation between both fonio species was also subtly established by Chukwu and Abdul-Kadir (2008) in his study on proximate chemical composition of the grains.
The study results also showed that geographical differences affected the total phenolic compounds in fonio, as evidenced by intra-species variation among the accessions. Such location effect on total phenolics has also been found in other cereals like wheat (Mpofu et al., 2006) and non-cereals like Catharanthus roseus (Kumar et al., 2013). Comparatively, however, the study values for total phenol content (0.43 to 1.59 mg/g dry weight) were lower than a previously reported mean value of about 2.0 mg/g dry weight (Glew et al., 2013), although it was not specified which fonio species were used. It has however been established that numerous factors, including sample difference due to species, cultivation, harvest and storage conditions, can affect content of phenols in crop specimens.
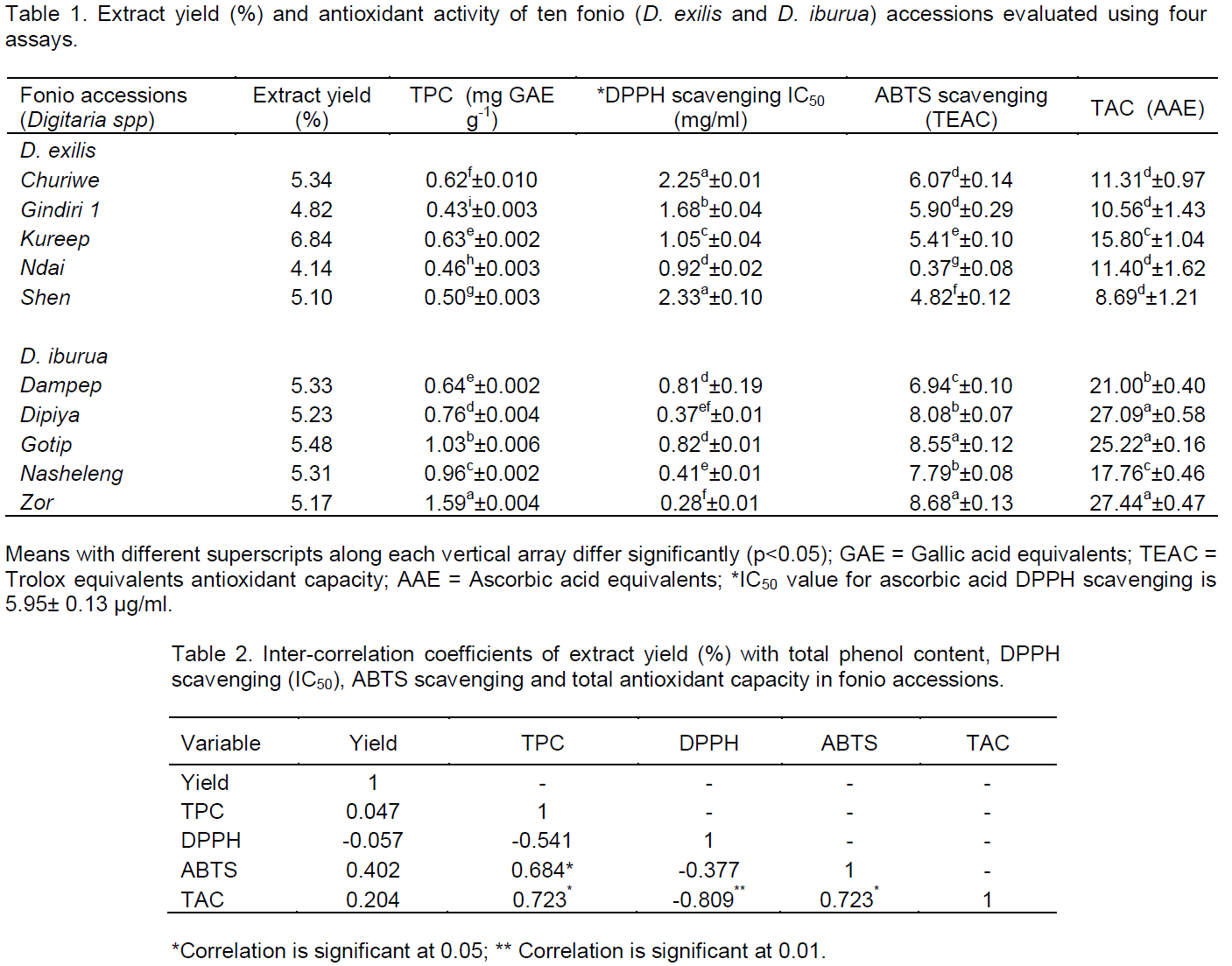
The DPPH radical scavenging potency of the fonio extracts, expressed in terms of effective concentration of extract required to scavenge 50% (EC50) of stable DPPH radicals, are presented in Table 1. Accessions of D. exilis scavenged 50% of the DPPH radicals in the concentration range of 0.916 to 2.325 mg/mL while D. iburua accessions scavenged in a lower range of 0.278 to 0.816 mg/ml. 60% of the D. iburua accessions were significantly more potent (p<0.05) than all D. exilis accessions, while the least potent D. iburua accessions (Dampep and Gotip) equalled (p>0.05) the most potent D. exilis accession (Ndai) in this activity. The fonio extracts however were incomparable with standard ascorbic acid with EC50 of 0.006 mg/mL. Antioxidant activity using the DPPH assay is based on measurement of reducing ability of the antioxidants in a sample towards DPPH radical, usually by discoloration.
The study results indicate that all fonio extracts studied decolorized the radical, thus confirming the presence of radical scavenging substances and thus corroborating the report of Glew et al. (2013). However, this study results further showed greater potency of D. iburua extracts in this activity than D. exilis, evidenced by lesser concentrations of the former needed to neutralize 50% of the radicals compared to the latter. This finding may not be unconnected, at least in part, with the total phenol content of the accessions. Indeed, the study correlation results (Table 2) showed an inverse relationship between both, thus confirming that species or accessions with higher amounts of phenols neutralized 50% of DPPH radicals at lesser extract concentrations. Similar trend has been reported earlier in wheat (Li et al., 2005; Mpofu et al., 2006). It can therefore be taken that total content of phenolic compounds in fonio is a good indicator of its antioxidant activity, especially in free radical scavenging.
ABTS radical scavenging activity of the fonio extracts, which was interpolated from a linear regression curve (y = 46.107+4.906x, r2 = 0.970) of Trolox, a standard ABTS scavenger, differed significantly (p<0.05) among accessions of both fonio species. Accessions of D. iburua, although significantly different from one another in this activity, however generally showed higher ABTS scavenging activity (6.94 to 8.68 Trolox Equivalents Antioxidant Capacity) compared to accessions of D. exilis (0.37 to 6.07 Trolox Equivalents Antioxidant Capacity) (Table 1). The ABTS assay, which is based on the antioxidant ability (in terms of radical-scavenging capacity) to react with ABTS+ generated in the system, is employed widely to evaluate antioxidant activity in foods, and biological systems and high TEAC values indicate high levels of antioxidant activity (Fan et al, 2010).
The study results in this assay showed that D. iburua extracts scavenged ABTS cation more potently than D. exilis extracts. This was evidenced by higher TEAC values for the former compared to the latter, at the same tested concentration. Higher TEAC values for D. iburua extracts correlated inversely with their IC50 values for DPPH scavenging (Table 2), confirming superior potency of D. iburua extracts to those of D. exilis in radical scavenging. These findings may be attributed to inherent genetic differences in both fonio species as was also evident in their differential content of phenolic compounds. Differences in ABTS scavenging activity among accessions of both species collected from different locations suggest that environmental factors may play crucial roles in antioxidant activity of fonio.
Total antioxidant capacity of the fonio extracts was evaluated by interpolation from a standard regression curve (y = 0.0495+0.0075x, r2 = 0.972) of ascorbic acid and a standard antioxidant compound are presented in Table 1. Accessions of D. exilis showed total antioxidant capacity in the range of 8.69 to 15.80 Ascorbic Acid Equivalents while D. iburua accessions showed this capacity in the range of 17.76 to 27.44 Ascorbic Acid Equivalents. D. iburua accessions thus showed significantly higher (p<0.05) total antioxidant capacity than all D. exilis accessions, with significant differences occurring, however, among accessions of both species. Reduction of metal ions is an important mechanism of antioxidant action, as a good antioxidant often acts as a good reductant (Niki, 2010).
Results of the total antioxidant capacity (TAC) assay indicate that extracts of both fonio species contained reductants which could serve as antioxidants but that D. iburua extracts were more potent in reducing Mo (VI) ions to Mo (V) than D. exilis, at the same tested concentration. This finding, which confirms higher reducing potential of D. iburua species of fonio, may also be connected with its higher content of phenolic compounds as indeed both showed positive relationship (Table 2). Intra-species variation in total antioxidant capacity among accessions of both fonio species, on the other hand, is a further confirmation of the influence of location and underscores how environment affects fonio antioxidants.
The results from this study, put together, are a confirmation that fonio contains useful antioxidants and radical scavenging substances, with greater proportion found in D. iburua than D. exilis grains. Optimal derivation of fonio antioxidants, however, may not be without regard to environmental factors like location, which showed significant influence in this study. It may therefore be necessary in future to optimize antioxidant production by standardizing a range of environmental conditions.
The authors have not declared any conflict of interests.
The authors gratefully acknowledge Wadi, S. Mamza of the National Cereals Research Institute, Acha sub-station, Riyom, Jos, Plateau state of Nigeria, for assistance with the fonio accessions used in this study.
REFERENCES
|
Ballogou VY, Soumanou MM., Toukourou F, Hounhouigan, JD (2013). Structure and Nutritional Composition of Fonio (Digitaria exilis) Grains: Rev. Int. Res. J. Biol. Sci. 2(1):73-79.
|
|
|
|
Brezeanu PM, Munteanu N, Brezeanu C, Ambarus S, Draghici E, Calin M, Cristea TO (2013). Antioxidant activity in selected tomato genotypes cultivated in conventional and organic culture systems. Afr. J. Biotechnol. 12(20):2884-2899.
|
|
|
|
|
Chukwu O, Abdulkadir AJ (2008). Proximate chemical composition of Acha (Digitaria exilis and Digitaria iburua) grains. J. Food Technol. 6(5):214-216.
|
|
|
|
|
Chukwurah PN, Brisibe EA, Osuagwu AN, Okoko T (2014). Protective capacity of Artemisia annua as a potent antioxidant remedy against free radical damage. Asian Pac. J. Trop. Biomed. 4 (1):S92-S98.
Crossref
|
|
|
|
|
Dykes L, Rooney LW (2007). Phenolic Compounds in Cereal Grains and Their Health Benefits. Cereal Foods World 52(3):105-111.
Crossref
|
|
|
|
|
Fan H, Yang G, Zheng T, Mei Z, Liu X, Chen Y, Chen S (2010). Chemical Constituents with Free Radical Scavenging Activities from the Stem of Microcos paniculata. Molecules 15: 5547-5560.
Crossref
|
|
|
|
|
Glew CL, Laabes EP, Presely MJ, Schulze J, Andrews R, Wang Y, Chang Y, (2013). Fatty acid, amino acid, mineral and antioxidant contents of Acha. Int. J. Nutr. Metab. 5(1):1-8.
|
|
|
|
|
Jayaprakasha GK, Jena BS, Negi PS, Sakariah KK (2002). Evaluation of antioxidant activities and antimutagenicity of turmeric oil: a byproduct from curcumin production. Z. Naturforsch. C 57(9-10):828-835.
Crossref
|
|
|
|
|
Jideani IA, Akingbala JO (1993). Some physicochemical properties of acha (Digitaria exilis) and iburu (Digitaria iburua). J. Sci. Food Agric. 63:369-374.
Crossref
|
|
|
|
|
Kumar A, Singhal KC, Sharma RA, Vyas GK, Kumar V (2013). Total Phenolic and Antioxidant Activity of Catharanthus roseus in Different Geographical Locations of Rajasthan. Asian. J. Exp. Biol. Sci. 4(1):155-158.
|
|
|
|
|
Li W, Shan F, Sun S, Corke H, Beta T (2005). Free radical scavenging properties and phenolic content of Chinese blackgrained wheat. J. Agric. Food Chem. 53:8533-8536.
Crossref
|
|
|
|
|
Mensor LL, Menezes FS, Leitao GG, Reis AS, Dos Santos TC, Coube CS, Leitao SG (2001). Screening of Brazilian Plant Extracts for Antioxidant Activity by the Use of DPPH Free Radical Method. Phytother. Res. 15(2):127-130.
Crossref
|
|
|
|
|
Mpofu M, Sapirstein DH, Beta T (2006). Genotype and environmental variation in phenolic acid composition and antioxidant activity of Hard Spring wheat. J. Agric. Food Chem. 54:1265-1270.
Crossref
|
|
|
|
|
Naczk M, Shahidi F (2004). Extraction and Analysis of Phenolics in Food. J. Chromatogr. A 1054 (1-2):95-111.
Crossref
|
|
|
|
|
Nawaz H, Shad MA, Batool Z (2013). Inter-varietal Variation in Biochemical, Phytochemical and Antioxidant Composition of Maize (Zea mays L.) Grains. Food Sci.Technol. Res. 19(6):1133-140.
Crossref
|
|
|
|
|
Niki E (2010). Assessment of Antioxidant Capacity In Vitro and In Vivo. Free Rad. Biol. Med. 49(4):503-515.
Crossref
|
|
|
|
|
Nono-Wondim R, Achigan-Dako GE, Baudoin W, NeBambi L, Apane J, Noorani A, Gosh K, Hodder A, Pichop GN (2013). Agrobiodiversity of Tropical Africa.Promoting African Indigenous Fruits and Vegetables for Improved Livelihood. FAO, Rome, Italy.
|
|
|
|
|
Padulosi S, Thompson J, Rudebjer P (2013). Fighting poverty, hunger and malnutrition with neglected and underutilized species (NUS): needs, challenges and the way forward. Bioversity int.
|
|
|
|
|
Pellegrini N, Re R, Yang M, Rice-Evans C (1999). Screening of dietary carotenoids and carotenoid-rich fruit extracts for antioxidant activities applying 2, 2'- azinobis (3- ethylbenzothiazolyne-6-sulfonic acid) radical cation decolorization assay. Meth. Enzymol. 299:379-389.
Crossref
|
|
|
|
|
Prvulović D, MalenÄić D, Popović M, Ljubojević M, Ognjanov V (2011). Antioxidant Properties of Sweet Cherries (Prunus avium L.) -Role of Phenolic Compounds. World Acad. Sci. Eng. Technol. 59:1149-1152.
|
|
|
|
|
Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C (1999). Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Rad. Biol. Med. 26:1231-1237.
Crossref
|
|
|
|
|
Sartelet H, Serghat S, Lobstein A, Ingenbleck Y, Anton R, Petitfrere E, Aguie-Aguie G, Martiny L, Haye B (1996). Flavonoids extracted from Fonio Millet (Digitaria exilis) reveal potent anti-thyroid properties. Nutrition 12(2):100-106.
Crossref
|
|
|
|
|
Singleton VI, Orthofer R, Lamuela-Ravantos RM (1999). Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by means of Folin Ciocalteau Reagent. Meth. Enzymol. 299:152-179.
Crossref
|
|
|
|
|
Uyoh EA, Chukwurah PN, Urua IS, Umoffia HM (2013). Upgrading the Medicinal Value Chain of Neglected and Underutilized Eremomastax (Lindau) Species through Antioxidant Health Benefits. Int. J. Med. Aromat. Plants 3(3):334-342.
|
|
|
|
|
Wang SY (2003). Antioxidant Capacity of Berry Crops, Culinary herbs and Medicinal Herbs. Acta Hortic. 620:461-473
Crossref
|
|