Entomopathogenic nematodes (EPNs) are obligate parasites to insects. They are natural enemies of numerous insects, which employ mutually related bacterial symbionts to rapidly kill their insect host. They are among the frequently used beneficial biocontrol agents of numerous insect pests in agriculture, forestry and health. These EPNs are continuing to constitute a great deal of interest for both scientists and industries. This is demonstrated from the breadth of research activities on EPNs in many countries throughout the world. More scientists are becoming trained in working with EPNs and the number of newly discovered EPN species is increasing. In South Africa (S.A.) although various studies have revealed an incredible richness of EPNs fauna with potential use as bio-control agents adapted to some soil texture and environmental conditions and underline the value of conducting more intensive surveys in natural and different parts of the country, few studies have been done in this area. This review gives an overview of the EPNs genera that include the main bio-control agents. The main species of EPNs and their symbiotic bacteria, interaction, associated effects on the insects’ host, as well as their use and main insects’ hosts range in S.A are described. In addition, their production technology is also discussed.
Entomopathogenic nematodes (EPNs) are obligate parasite to insects (Dillman et al., 2012; Lacey and Georgis, 2012; Malan and Ferreira, 2017). They are natural enemies of numerous insects, which: 1) employ mutually related bacterial symbionts rapidly; 2) kill their insect host, usually within 72 h of infection (Dillman and Sternberg, 2012; Dillman et al., 2012; Stubbins et al., 2016; Malan and Ferreira, 2017); and 3) pass on the associated bacteria to future generations (Dillman et al., 2012).
Entomopathogenic nematodes are among the beneficial bio-control agents that are frequently used for pests control in agriculture, forestry and health (Stock, 2005; Stock and Hunt, 2005; Lacey and Georgis, 2012; Kalia et al., 2014; Devi and Nath, 2017; Edmunds et al., 2017; Torrini et al, 2017; Azazy et al., 2018; Saleh et al., 2018). As bio-control agents, EPNs possess the advantages of having a broad host range (Stubbins et al., 2016) and no known negative effect on both environment and non-targeted organisms (Hazir et al., 2003; Lacey and Georgis,
2012). They can search and kill their hosts rapidly, are easily massed produced in vivo and in vitro, are susceptible to genetic selection of desirable traits and, are easily applied using conventional equipment (Hazir et al., 2003). In addition, they can be used with many chemical or biological pesticides or adjuvants (Rezaei et al., 2015); and need little or no registration measures in many countries (Lacey and Georgis, 2012).
In contrast, their disadvantages are that their broad host range can possibly include beneficial insects, are poorly tolerant to environmental conditions such as soil moisture content, UV radiation (Shapiro-Ilan et al., 2015) and have limited shelf-life (Lacey and Georgis, 2012). Entomopathogenic nematodes were first discovered during the 17th century (Nickle, 1984). However, it was only in the 1930s that serious consideration was assigned to them as controlling agents of insect pests (Glaser and Fox, 1930). This was a result from the discovery by Glaser and Fox in 1929, of a nematode infecting grubs of the Japanese beetle, Popillia japonica, at the Tavistock Golf Course in New Jersey; subsequently described as Neoaplectana (Steinernema) glaseri (Steiner, 1929). Previously, chemical-based pest control agents were utilised due to cheap prices and rapid effectiveness. However, these were recognised to possess negative effects on the environment, human and animals, which gradually prompted the need to search for biological alternatives (Adams and Nguyen, 2002; Stock, 2005). In the 1980s, research and use of EPNs as biocontrol agents were intensified (Bongers and Ferris, 1999; Adams and Nguyen, 2002). Thus, from numerous publications resulting from the plethora research efforts throughout the world and as more scientists are becoming trained in working with EPNs (Kaya et al., 2006) and the number of newly discovered EPN species is increasing (Dillman and Sternberg, 2012; Çimen et al., 2014; Nthenga et al., 2014; Cimen et al., 2015; Odendall et al., 2015; Cimen et al., 2016; Lephoto et al., 2016; Malan and Ferreira, 2017), there is an interest to conduct further research with these nematodes. Furthermore, scientists, apart from EPNs niche markets or greenhouse uses, have developed the use of EPNs in outdoor environments to control many insect pests in various crops, such as vegetable and fruit crops (Hazir et al., 2003; Stock and Hunt, 2005; Kaya et al., 2006). This is, however, restricted by the cost of production of such amount of EPNs to satisfy this demand (Spaull, 1992). This requires a competitive cost production price, which is reportedly met only by the scale-up of the liquid culture technology of efficacious isolate strains (Ehlers and Shapiro-Ilan, 2005). This review gives an overview of EPNs used for insect pest control. Main species of both EPNs and their symbiotic bacteria as well as their occurrence globally and particularly in South Africa, interaction and associated effects on insects’ host are described. In addition, their use and production are discussed.
Nematodes are organisms grouped in the phylum Nematoda (Humphreys-Pereira and Elling, 2014; Malan and Ferreira, 2017) that is among the most abundant groups of invertebrates on the surface layers of the earth, rivalling the Arthropoda in biodiversity and species abundance (Poinar, 2011; Humphreys-Pereira and Elling, 2014). Nematodes species range from 100 000 to 10 000 000 with about 20 000 species described (Poinar, 2011). They invade more habitats on land, or in fresh and salt water than any other group of multicellular animals due to their structure, physiology, diverse reproductive patterns and adaptability (Poinar, 2011; Humphreys-Pereira and Elling, 2014).
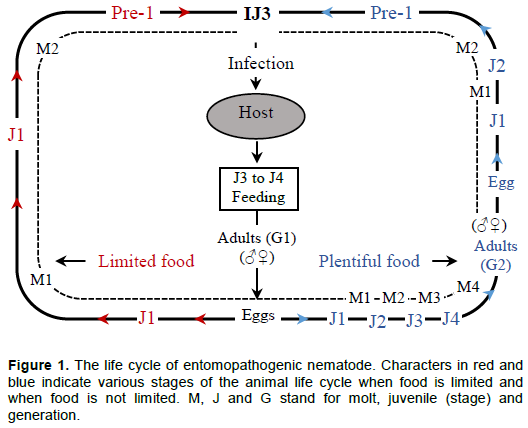
While most nematodes are free-living microbotrophs, numerous can be associated with invertebrates such as insects, mites and molluscs, ranging from casual to obligate parasitism and pathogenesis (Dillman et al., 2012; Malan and Ferreira, 2017). Parasitic forms are of great economic interest (Stock, 2005). Among these, are the entomopathogenic nematodes (EPNs), important families, commonly considered as having effective biocontrol agents of insects, are the Steinernematidae and Heterorhabditidae (Lacey and Georgis, 2012; Malan and Ferreira, 2017). They belong to the order Rhabditida (Malan and Ferreira, 2017). These families have many biological similarities though they are not closely related (Stock and Hunt, 2005; Malan and Ferreira, 2017). Their life cycle is illustrated in Figure 1. Steinernematidae has two genera, viz, Steinernema (with 100 species) and Neosteinernema (having only N. longicurvicauda) (Malan and Ferreira, 2017). Heterorhabditidae contains only one genus, viz, Heterorhabditis, with 20 species (Malan and Ferreira, 2017). However, this number is increasing as the number of novel species being described is growing every year (Malan and Ferreira, 2017). At the IJ3 stage, the nematode infects the host; then develops to J4 and G1 (adult 1st generation). Then produce eggs (after mating) that will develop to J1. In abundance of food, J1 will successively molt to J2, J3, J4 and G2 (2nd generation adult). This process will repeat until G3 depending on the availability of food. When food is limited, J1 will molt to J2, pre-I and IJ3 that will emerge from the cadaver to search for a new insect host (Brivio and Mastore, 2018).
The biological efficiency of the Steinernema and Heterorhabditis to kill an insect host is associated with symbiotic bacteria of the genera Xenorhabdus and Photorhabdus, respectively (Hazir et al., 2003; Ciche et al., 2006; Dillman et al., 2012). This association is highly specific (Hazir et al., 2003), although one bacterial species can be found associated with many species of EPNs. The best production of nematode occurs with their natural symbiont (Hazir et al., 2003). Although other nematode species (Oscheius chongmingensis and Oscheius carolinensis) can also be associated with symbiotic bacteria (Serratia spp.) in a parasitic form of insect hosts (Dillman et al., 2012), species from the genera Steinernema and Heterorhabditis are reportedly receiving more attention (Dillman and Sternberg, 2012; Lacey and Georgis, 2012; Malan and Ferreira, 2017). They have been effectively adapted to soil biological control agents of numerous insect pests (Malan and Ferreira, 2017).
Life cycle of entomopathogenic nematodes
The life cycle of both Steinernema and Heterorhabditis species comprises non-feeding, free-living infective juvenile (IJ) that infects the insect host in the soil environment, and develop into the adult life stage (Ehlers and Shapiro-Ilan, 2005; Dillman et al., 2012) as shown in Figure 1. The IJ stage is a form resulting from the depletion of food resources and adverse environmental conditions (Ehlers and Shapiro-Ilan, 2005). This stage is the only one that can occur outside of an insect (Dillman et al., 2012; Malan and Ferreira, 2017). Stock and Hunt (2005) describe Steinernematidae IJs as having collapsed stoma. The cuticle is annulated, lateral field with 6–8 ridges in middle of its body. Oesophagus and intestine collapse. Excretory pore distinct, anterior to nerve ring. Tail is conoid or filiform, with variable hyaline portion. Phasmids are prominent or inconspicuous. Heterorhabditidae IJ cuticle of second-stage juvenile has longitudinal ridges throughout most of the body length, and a tessellate pattern in anterior most region. It has lateral field with two ridges. It has prominent cuticular dorsal tooth. Excretory pore is located posterior to basal bulb. Tail is short, conoid, tapering to a small spike-like tip (Stock and Hunt, 2005).
These two families have a similar life cycle, but the Heterorhabditis first generation adults are hermaphroditic (Stock and Hunt, 2005). The Steinernema IJ second-stage cuticle can be easily lost, whereas the second-stage cuticle of the Heterorhabditis IJ is retained till when it is about to infect the host or shortly after the bacterial cells (200 to 2000 cells) are located in a special vesicle in the intestine of the Steinernema. In the Heterorhabditis they are located in the intestinal tract (Stock and Hunt, 2005; Ehlers and Shapiro-Ilan, 2005; Malan and Ferreira, 2017).
Nematode/bacterium interactions with hosts
The IJs locate their hosts by following cues such as host’s excreted CO2, or by attaching themselves to the host when it passes (Griffin et al., 2005). Infection occurs through natural openings, such as spiracles, thin areas of the host’s cuticle, mouth or anus (Kaya and Gaugler, 1993). The IJs use physical force (body thrusting) to pass through the cuticle or gut to get into the haemolymph.
Also, they can secrete proteolytic enzymes to digest the midgut or use an anterior tooth (only present within Heterorhabditis spp.) to gain access into the hemocoel (Hazir et al., 2003). Once inside the hemocoel, the nematode/bacterium duo overcomes the host’s immune response (Hazir et al., 2003), as the latter may try to resist the infestation by metabolizing antibiotics or by initiating the phagocytosis to encapsulate them and thus inactivate them (Wang et al., 1995).
Once the IJs penetrate the host, they release their symbiotic bacterial cells through the anus or mouth. The released bacterium will multiply exponentially while producing toxic secondary metabolites that will kill the host (Dillman et al., 2012; Noguez et al., 2012), suppressing the growth of microbial competitors and, stimulating the macromolecular degradation by producing diverse antibiotics and exo-enzymes (Chaston et al., 2011; Bai et al., 2013). The IJs transform into feeding, a stage where they feed on the bacterial cells and host’s metabolized tissues, thus eventually developing to one or more generations depending on the size of the host (Hazir et al., 2003). After the depletion of food resources, Steinernema IJs re-associate with their bacteria and transform into IJ3 that will emerge into the soil to search for new hosts (Dillman et al., 2012) (Figure 1). The Heterorhabditis associated bacteria (Photorhabdus spp.) inhabit the gut of their nematode host during its development and are passed to IJ before it emerges (Ciche et al. 2008).
The EPN-bacterium relationship is mutual; the EPN protects the bacterium from external environment, vectors it into the host’s haemolymph and inhibits the host’s antibacterial proteins. In turn, the bacterium produces secondary metabolites that kill the insect host, create a suitable environment for the EPN development, produce, and serve as food source for the EPN (Dillman et al., 2012).
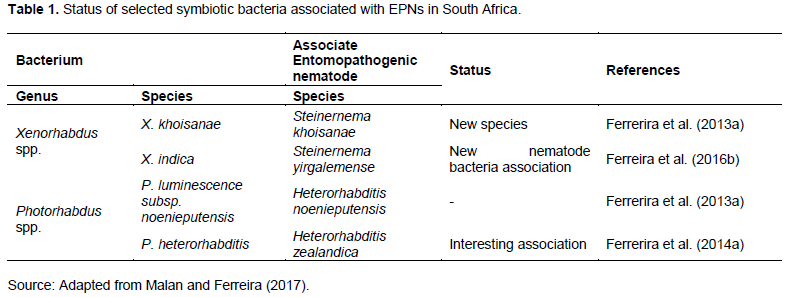
Both Xenorhabdus spp. and Photorhabdus spp. belong to the family Enterobacteriaceae. They are gram-negative, motile, facultative anaerobic rods and non-spore forming (Hazir et al., 2003). Twenty-one Xenorhabdus spp. are associated with the Steinernema spp., and three Photorhabdus spp. (P. luminescens, P. temperata and P. asymbiotica) are associated with Heterorhabditis (Thanwisai et al., 2012; Blackburn et al., 2016). Commonly, P. luminescens is associated with H. georgiana, H. indica and H. bacteriophora; while P. temperate with H. bacteriophora, H. downesi, H. georgiana, H. marelatus, H. megidis and H. zealandica and; P. aymbiotica with H. gerradi (Thanwisai et al., 2012). Xenorhabdus spp. are associated with numerous Steinernema spp. (Thanwisai et al., 2012). More bacterial symbionts from novel EPNs species are still to be characterized (Malan and Ferreira, 2017). Malan and Ferreira (2017) presented symbiotic bacteria (Table 1) associated with EPN that occur in SA. Bacteria are important in the commercial production of EPN as they have various growth phenotypic or phase variations.
Phenotypic variant
Phase I variant or primary form is the original bacterium phase variant, isolated from EPN. In contrast, Phase II variant or secondary form can arise after repeated in vitro sub-culturing (Ehlers and Shapiro-Ilan, 2005), during the bacterial stationary non-growth stage and when nematodes emigrate from the cadaver (Hazir et al., 2003; Ehlers and Shapiro-Ilan, 2005). This phenomenon of phase variation is reversible with the Xenorhabdus and has not been reported for Photorhabdus spp. (Han and Ehlers, 2001; Hazir et al., 2003; Ehlers and Shapiro-Ilan, 2005).
Phase I form is unlike phase II form by producing secondary metabolites with antibacterial activity, adsorbing certain dyes and by developing large intracellular inclusions composed of crystal proteins; whereas phase II form does not or weakly produce antibacterial secondary metabolites, does not adsorb dyes and, inefficiently produces intracellular inclusions (Dowds and Peters, 2002; Hazir et al., 2003). The primary form is also superior because it supports EPNs propagation during in vitro growth (Hazir et al., 2003). The complex nematode/symbiont bacterium is essentially monoxenic (Hazir et al., 2003).
USE OF NEMATODE-BACTERIUM AS BIOCONTROL AGENTS
The EPN-bacterium relationship for controlling insect pests has been the subject of intense laboratory and field-testing since 1930s (Glaser et al., 1940; Klein; 1990; Kaya and Gaugler, 1993; Shapiro et al., 2002; Grewal et al., 2005; Lewis and Clarke, 2012; Shapiro et al., 2012; Dillman and Sternberg, 2012; Rezaei et al., 2015). For instance, species such as S. scarabaei, H. bacteriophora strain GPS11, H. bacteriophora strain TF, H. zealandica, S. yirgalemense and H. zealandica strain X1 have been successfully used to control the Japanese beetle, Popillia japonica and the vine mealybug, Planococcus ficus (Signoret), (Grewal et al., 2005; Koppenhöfer, 2007; Klein et al., 2007; le Vieux and Malan, 2013a,b). Entomopathogenic nematodes are currently successfully used as pesticides worldwide (Dillman and Sternberg, 2012). For instance, EPNs are used to control invasive species of mole crickets, citrus root weevil in orange groves, and other damaging crop pests in Florida and California (Grewal et al., 2005; Dillman and Sternberg, 2012). Some, for instance H. bacteriophora and H. zealandica are commercially available for grub control (Grewal et al., 2005; Dillman and Sternberg, 2012). This surge of both scientific and commercial interest is the result of advances in mass-production and formulation technology of EPNs, compounded with the discovery of many efficacious isolate strains capable of reducing the use of chemical pesticides (Lacey and Georgis, 2012). Both scientists and companies are now focusing to improve both cost efficiency and better products to position themselves within the market.
MASS PRODUCTION
Entomopathogenic nematodes can be cultured easily either in vitro or in vivo (Ehlers and Shapiro-Ilan, 2005). The in vivo method is suitable method for laboratories, scientific expertise or infrastructures with no need of large investment. This is for instance to produce EPNs for niche markets, grower co-operatives or other commercial arenas lacking the capital outlay (Ehlers and Shapiro-Ilan, 2005). The in vitro method is suitable for EPNs production for commercial use (Ehlers and Shapiro-Ilan, 2005). It has a reasonable cost of production, and can supply high quality EPNs (Ehlers and Shapiro-Ilan, 2005), although some studies considered the quality of EPNs produced in vitro lower than that of EPNs produced in vivo (Yang et al., 1997).
In vitro liquid culture
The liquid fermentation method is the one chosen for EPNs mass-production in larger companies (especially those that have industries supplying multiple products). It has economies of scale and has the lowest cost of production. This is because its scale decreased labour proportion and capital costs (Hazir et al., 2003; Ehlers and Shapiro-Ilan, 2005). Liquid culture process involves the mixture of fluids, EPNs and symbiotic bacterium in bioreactors for up to 3 weeks (Ehlers and Shapiro-Ilan, 2005). Factors influencing the successful process include suitable medium, monoxenic conditions and adequate oxygen (Hazir et al., 2003). Typically, a medium comprises yeast extract (nitrogen source), carbon source (e.g. soy flour, glucose or glycerol), various proteins and lipids (of animal and plant origin), and salts (Han et al., 1995; Surrey and Davies, 1996; Ehlers et al., 1998; Hazir et al., 2003; Ehlers and Shapiro-Ilan, 2005). Its osmotic strength is not above 600 milliosmoles per kilogram (Ehlers and Shapiro-Ilan, 2005).
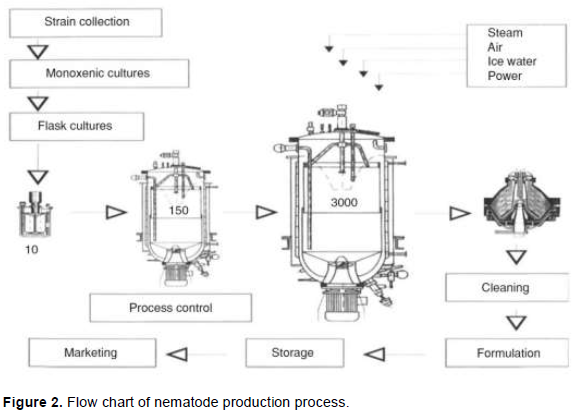
Conventional equipment such as bioreactor has been successfully used (Surrey and Davies, 1996; Ehlers et al., 1998; Hazir et al., 2003; Ehlers and Shapiro-Ilan, 2005). A configuration of the airlift bioreactor (Spier et al., 2011), internal loop bioreactors are reported to yield the highest IJ concentration (Ehlers and Shapiro-Ilan, 2005). The schematic outline of EPNs production process is presented in Figure 2. After monoxenic cultures are established they are scaled up to a 3000 L internal loop bioreactor. After 12 days, Infective juveniles are harvested with a separator. The nematode paste is then cleaned by passing through centrifugal sifters and formulated (Adapted from Ehlers and Shapiro-Ilan, 2005).
Cultures are always pre-incubated with the specific symbiont bacterium (0.5-1%, w/v) for 24-36 h before IJs are inoculated (5-10%, v/v of the culture), calculated based on optimum number of adults per millilitre (Ehlers and Shapiro-Ilan, 2005). Few data have been published on the optimum parameters of this process (Hazir et al., 2003; Ehlers and Shapiro-Ilan, 2005). Many nematode species, such as S. carpocapsae, S. feltiae, S. glaseri, S. kushidai, S. riobrave, S. scapterisci, H. indica, H. bacteriophora and H. megidis can be successfully mass produced in 7 to 8 litres liquid media in bioreactors (Hazir et al., 2003; Ehlers and Shapiro-Ilan, 2005). Yield capacity depends on the nematode species (Hazir et al., 2003; Ehlers and Shapiro-Ilan, 2005); reportedly, H. indica yield capacity of greater than 500,000 IJ/ml has been recorded (Ehlers and Shapiro-Ilan, 2005). After, nematodes are either bulk stored or formulated immediately (Georgis and Kaya, 1998; Grewal, 2000; Hazir et al., 2003), and finally commercialised after its quality being controlled (Gaugler and Han, 2002; Grewal, 2002; Hazir et al., 2003). It is worthwhile to notice that, the continuous scale-up of bioreactor volumes together with strengthening of the process stability and downstream processing, as well as increasing EPN shelf-life, improving transport logistics and marketing will bring along further reduction of production costs (Ehlers and Shapiro-Ilan, 2005).
EPN OCCURRENCE GLOBALLY AND IN SOUTH AFRICA
Entomopathogenic nematodes have been recovered worldwide (Hominick, 2002; Kaya et al., 2006). Species are likely to be globally distributed and are essentially ubiquitous (Hominick, 2002; Kaya et al., 2006). Although some species (S. rarum, S. kushidai, S. ritteri and H. argentinensis) appear, so far, to be more restricted to some regions, some others (S. carpocapsae and S. feltiae) are widely distributed in temperate regions, tropics and subtropics (H. indica), and some others (H. bacteriophora) in regions with continental and Mediterranean climates (Hominick, 2002). The research activities on the EPN in many countries throughout the world clearly demonstrate a great deal of interest. It is expected that the amount of published information will increase as more scientists are becoming trained in working with EPNs (Kaya et al., 2006).
In South Africa (S.A.), hundreds of invertebrate pests infest the agricultural industry (Hatting et al., 2018). To reduce this, the more than 500 pesticides actually allowed by the Act 36 of 1947 under the Fertilizers, Farm Feeds, Agricultural Remedies and Stock Remedies continue to pose a risk on humans, animals and the environment (AVCASA, 2018; Hatting et al., 2018). Their risk awareness has been increasing; since late 1970s, several insecticides including monocrotophos, chlorpyrifos, endosulfan, aldicarb and methyl bromide have been eliminated (or restricted) by the South African government (Hatting et al., 2018). This has encouraged the establishment of a "South African National Bio-Economy Strategy" (DST, 2013) and the use of alternatives biological pest control agents (Hatting et al., 2018). These have been further compounded by a synergetic effect of biological agents to chemical insecticide (by extending their active-life through reduced selection pressure), and by increasing regulatory (on chemical pesticides) and market pressures on industries to supply the newly discovered markets from the west (Hatting et al., 2018; Malan et al., 2018). Thus, agricultural industries have been compelled towards using biological pesticides and over the past few years, multinational agricultural chemical companies have been actively purchasing biopesticide companies (Moore et al., 2015). This had led to a dramatic growth of the biopesticide market (Hatting et al., 2018). For instance, from the year 2000 to 2010, a 20-fold growth of the global market of biopesticides was estimated (Ravensberg, 2011; Glare et al., 2012) and, this growth is likely to continue (Hatting et al., 2018).
Entomopathogens pest control agents in S.A. were first used in the late 1800s (Hatting et al., 2018). The first attempts involved the use of an entomopathogenic fungi Entomophaga grylli (Entomophthorales: entomophthoraceae) against the red locust, Nomadacris septemfasciata (Orthoptera: Acrididae) (Hatting et al., 2018). Report on EPNs were first recorded in the Eastern Cape Province in early 1950s; these were collected in a maize field from the black maize beetle, Heteronychus sanctae-helenae (Coleoptera: Scarabaeidae) (Harington, 1953; Hatting et al., 2018). Since then, few studies have been done in the effectiveness of endemic South African EPN species against insect pests (Kaya et al., 2006; le Vieux and Malan, 2013b; Malan and Ferreira, 2017). This is important because of strict regulations preventing the import of exotic organisms (amendment of Act 18 of 1989 under the Agricultural Pest Act, No, 36 1947) (Malan et al., 2006; Malan et al., 2011). Therefore, research on EPNs in SA is mainly focused on endemic South African strains against key insect pests such as Cydia pomonella (Lepidoptera: Tortricidae), Thaumatotibia leucotreta (Lepidoptera: Tortricidae) and Eldana saccharina (Lepidoptera: Pyralidae) (Malan and Hatting, 2015; Malan et al., 2018; Odendaal et al., 2016a,b; Hatting and Malan, 2017; Malan and Ferreira, 2017, Steyn et al., 2017). This is because of the economic damage that they may cause to agricultural industries.
Cydia pomonella is a key pest of apples and pears orchards (Addison, 2005). South Africa is considered as one of the biggest deciduous fruit producers in the Southern hemisphere due to its production area of 24156 and 1265 hectare (ha) of apples and pears, respectively (Addison 2005; Hortgro 2017). In 2017, it was ranked sixteenth and sixth in world apple and pear production; respectively (metric ton), and is considered among the top ten fresh apple and pear exporters in the world (Hortgro 2017). The main producing areas include the district of Ceres, Wolseley/Tulbagh, (pears only) Groenland, Villiersdorp/Vyebom and Langkloof East in the Eastern Cape Province (Hortgro 2017); representing approximately 88 and 84% of the country’s production area of apples and pears, respectively (Hortgro 2017). T. leucotreta (false codling moth) is indigenous to S.A (Newton, 1998). It is also an agent of potential economic damage on several citrus, deciduous subtropical fruit and vegetable crops in most production areas (Prinsloo and Uys, 2015). South Africa is considered as the second largest exporter of citrus worldwide, with a total production area of 77708 ha located in all provinces (CGA, 2018). Its citrus oriented industry constitutes an important source of job creation and foreign income (CGA, 2018). In 2017, more than 40% of deciduous fruits produced were exported to Western Europe and UK, 25% to Asia, 20% to the Middle East, 9% to the Eastern Europe and 7% to the Northern America; with total export earnings of R17.7 billion (CGA, 2018). Eldana saccharina Walker is indigenous to South Africa; it was first reported in 1939 (Hatting et al., 2018). It is widespread in wetland sedges and indigenous grasses from KwaZulu-Natal and Mpumalanga Provinces, and it is the number one pest of South African sugarcane (Horton et al., 2002; Webster et al., 2006; Assefa et al., 2009). A 0.1% sucrose loss occurs per every 1% of sugarcane stalks damaged due to larval feeding; and a South African sugarcane damage of about US$10 million per annum has been estimated due to E. saccharina; (Black et al., 1995; Horton et al., 2002). Other important pests include the citrus mealybug P. citri (Hemiptera: Pseudococcidae), citrus codling moth Thawnatotibia leuxotreta (Lepidoptera: Tortricidae) (Malan et al., 2011), obscure mealybug Pseudococcus viburni (Hemiptera: Pseudococcidae), vine mealybug P. ficus (Hemiptera: Pseudococcidae) (Stokwe and Malan, 2016; Stokwe and Malan, 2017), white grubs from the family Scarabaeidae (Coleoptera) (Abate et al., 2017) and the woodwasp Sirex noctilio (Hymenoptera: Siricidae) (Tribe, 1995; Ismail et al., 2010). Other pests of interest include fruit flies Ceratitis rosa and C. capitata (Diptera: Tephritidae) (Malan and Manrakhan, 2009; James et al., 2018), stable flies, Stomoxys calcitrans (Diptera: Muschidae) (hatting and Malan, 2017), the banded fruit weevil Phyctinus callosus (Coleoptera: Curculionidae) (Ferreira and Malan, 2014), African bollworm, Helicoverpa armigera (Lepidoptera: Noctuidae) (Malan and hatting, 2015), and the fungus gnats, Bradysia spp. (Diptera: Sciaridae) (Katumanyane et al., 2018).
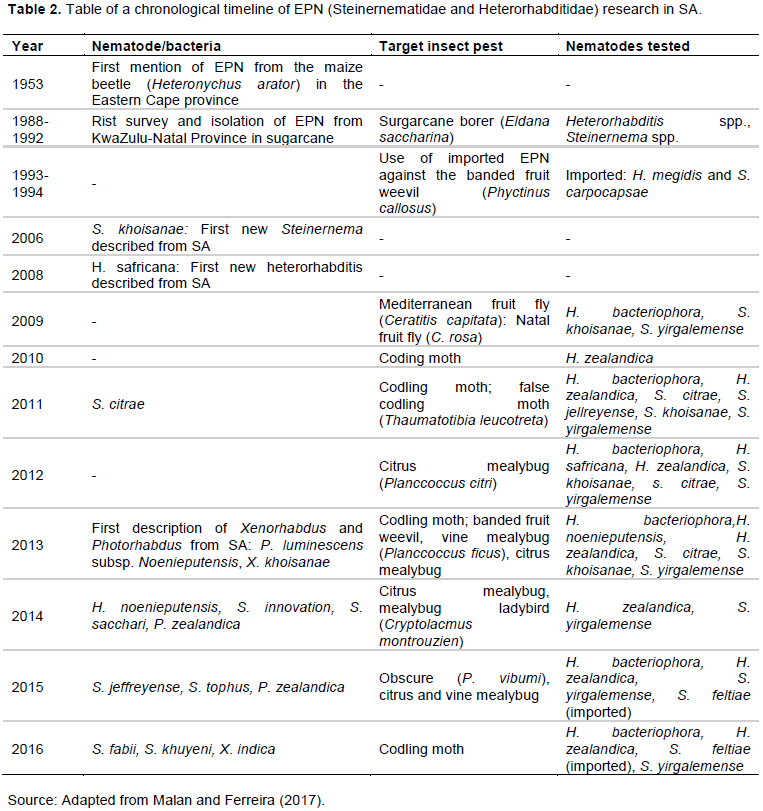
Increasing numbers of EPN species with biocontrol potential are being discovered, such as Heterorhabditis zealandica, H. Noenieputensis, H. zealandica, H. bacteriophora, H. safricana (Malan et al., 2006; Malan et al., 2008; Hatting et al., 2009; de Waal et al., 2011; Malan et al., 2011; Malan et al., 2014; Malan et al., 2016), and Steinernema citrae, S. khoisanae, S. yirgalemense, S. biddulphi, S. jeffreyense, S. sacchari, S. innovation, S. tophus (Malan et al., 2006; Nguyen et al., 2006; Hatting et al., 2009; de Waal et al., 2011; Malan et al., 2011; Stokwe et al., 2011; Nthenga et al., 2014; Çimen et al., 2014 ; Cimen et al., 2015; Cimen et al., 2016; Malan et al., 2016), and Oscheius sp. (TEL-2014), Oscheius safricana (Lephoto et al., 2016; Serepa-Dlamini and Gray, 2018). From surveys on indigenous EPN species (Hatting et al., 2009), such as a systematic survey throughout five provinces (Gauteng, Free State, KwaZulu-Natal, Mpumalanga and Western Cape) of S.A, with typical soil forms ranging from very sandy to sandy clay loam texture (Hatting et al., 2009), species were varied and adapted to various soil texture and environmental conditions (Hatting et al., 2009). Also, the authors have revealed an incredible richness of EPNs fauna with potential use as biocontrol agents and that the country is unexplored in terms of EPN diversity (Hatting et al., 2009; Hatting et al., 2018). Malan and Ferreira (2017) presented a Table (Table 2) of a chronological timeline of EPN research in SA up to 2016.
Among the 12 Steinernema species (spp.) recovered (Malan et al., 2006; Hatting et al., 2009; Malan et al. 2011), only S. yirgalemense is not novel (Hatting and Malan, 2017); and among the 7 Heterorhabditis spp. recovered, two are novel H. safricana and H. noenieputensis (Malan and Ferreira, 2017). These were recovered more likely in citrus orchards (17% recovery rate) than in deciduous fruit orchards (5–7%) (Malan and Ferreira, 2017). From their tests in controlling insect pest (Hatting et al., 2009; Hazir, 2009; Stokwe, 2009; Stokwe and Malan, 2010; de Waal et al., 2011; Van Niekerk and Malan, 2012; Odendall et al., 2015; Malan and Ferreira, 2017), they showed promising results to control T. leucotreta and B. impatient (Diptera: Sciaridae) (only tested in laboratory) in both laboratory and field plots under optimized conditions (Malan et al., 2011; Malan and Moore, 2016; Katumanyane et al., 2018). There are excellent results in C. pomonella in laboratories, but variable results in the field. This is due to suboptimal environmental conditions such as cryptic habitats (bark and pruning wounds on apple trees), low relative humidity and temperatures, wind and unpredictable rainfall in the Mediterranean climate of the Western Cape Province (De Waal et al., 2010; De Waal et al., 2011; Odendaal et al., 2016a,b; De Waal et al., 2018). There were unsuccessful against woolly apple aphid Eriosoma lanigerum (Hemiptera:Aphididae) due to their inability to develop in the soil stage insect’s haemolymph (Stokwe and Malan, 2017).
Researches on the optimisation of EPNs hostile field conditions have been conducted. These include the application of irrigation system for EPNs application (Mason et al., 1999); the development of low-volume spray application system, such as the spinning disc spray application system (Mason et al., 1999) and its improved version in terms of IJs carriage in droplets presenting a better deposition of IJs per cm2 (Piggott et al., 2003); the use of appropriate adjuvants for the spray application to facilitate the infectivity (mortality and intensity of infection) of IJs; for instance, a spinning disc spray Micron Ulva and the adjuvant Micron Herbaflex significantly increased the IJs deposition (IJs/cm2) (Mason et al., 1998) and also studies on EPNs (Rhabditida: Steinernematidae and Heterorhabditidae) water loss and survival to desiccation (Patel et al., 1997; Mason and Wright, 1997; Spence et al., 2011). Some species, such as S. carpocapsue have a slow rate of water lost among the Steinernema spp. and can survive up to 20 min (min) at a relative humidity of 0% (Patel et al., 1997). But Heterorhabditis spp. were reportedly the most promising species to be used in condition of desiccation (Mason and Wright, 1997).
Only flask-cultures of H. zealandica (Ferreira et al., 2014), S. innovationi (Ramakuwela et al., 2016) and S. yirgalemense (Ferreira et al., 2015) have been obtained despite several attempts for in vitro mass culturing (Hatting et al., 2018). Currently, only an imported formulation based on H. bacteriophora is produced and commercialized under the name Cryptonem® (Hatting et al., 2018). In all, there is a need for more intensive surveys in natural areas and geographic regions, including regions where surveys have not yet been done. There is need for more research efforts on EPN field application especially in areas with hostile environmental conditions and; more effort toward EPNs mass production for a broader application in agriculture. Substituting chemical pesticides will stabilize agricultural environments and crop yields (Ehlers and Shapiro-Ilan, 2005). Thus, progressing in EPNs research will be an important move toward sustainable agricultural practices.