ABSTRACT
The ecological effects of oil spill on environmental media and organisms (pelagic, bottom dwelling organism) in the riverine areas of Odidi and Egwa in the Niger Delta ecological zone of Delta State was investigated approximately six months after the spill. The content of petroleum hydrocarbons and heavy metals (lead, copper, zinc, iron and chromium) in the water, sediment, fish (Clarias gariepinus) and snail (Pachymelania byronensis) was evaluated to determine the effect oil spill had on the environmental matrix and the organisms. The mean concentration of total petroleum hydrocarbon (TPH) in the sample matrix (water, sediment) and organisms (fish and snail) from Odidi and Egwa Rivers was above the Department of Petroleum Resources (DPR) limits of 50 mg/L and 5000 mg/kg. The control level of TPH in the media and organisms was less than 5 mg/kg. The mean water concentrations of iron and chromium were above their respective DPR limits of 1.00 and 0.03 mg/L. The concentrations recorded for iron in Odidi and Egwa waters was 2.96 ± 0.01 mg/L and 2.95 ± 0.08 mg/L while chromium was 0.33 ± 0.01 mg/L and 0.34 ± 0.01 mg/L respectively. Similarly, the mean concentrations of heavy metals in the water, sediment, cat fish (Clarias gariepinus) and snail (Pahymelania byronensis) were all significantly different from the control station (P<0.05). In other to prevent the adverse effects on environmental components and organisms resulting from oil spill, immediate clean-up of the environment and remediation measures should be carried out to prevent the bioaccumulative effects that could result from long term exposure to crude oil spill / pollution. This is with the view of safe guarding environmental media, aquatic fauna and subsequently man, who constantly feed on these organisms.
Key words: Crude oil, pelagic-fish, bottom dwelling organism-snail, spill, TPH, heavy metals
The ecological damage to the ecosystem as a result of oil spillage has been a major challenge affecting the Niger Delta area of Nigeria. The release of liquid petroleum hydrocarbon into the environment especially in aquatic environment have been persistent in most oil producing countries, causing serious negative impact on the environment, ecosystem and biodiversity, and destroying the very critical sources of livelihood.The liquid petroleum spilled in aquatic environment pose a threat not only to pelagic organisms but also to benthic organism, as they sink to the bottom of the sediment (Atlas, 1995; Blackburn et al., 2014).
Nigeria, which is the largest oil producer in Africa and the tenth largest producer in the world, has suffered environment degradation from oil spillage, especially in the Niger Delta region, since the discovery of oil in 1956. The Niger Delta region, famous for its rich oil deposit, has been identified as the world most severely petroleum impacted ecosystem, with spillage arising from both accidental and illegal discharges into both aquatic and terrestrial environment. An estimated 9 to 13 million barrels (1.5 million tons) of oil had been spilled in the Niger Delta ecosystem over the past 53 years representing about 50 times the estimated volume spilled in the Exxon Valdez oil spill in Alaska in 1989 (Weiner et al., 1997). Similarly, about 7000 spillage was recorded between 1975 and 2001. Some of these oil spillages had been linked to leaking pipelines and storage tankers due to lack of regular maintenance (Nwilo and Badejo, 2004). The Shell Petroleum Development Company (SPDC) since 1989 recorded an average of 221 spills per year in its operational area involving 7,350 barrels annually. From 1976 to 1996, a total of 4647 oil spill incidences spilling approximately 2,369,470 barrels of oil into the environment of which 1,820,410.5 (77%) were not recovered. Most of these oil spill incidences in the Niger Delta occur on land, swamp and the offshore environment (including Odidi and Egwa) (Nwilo and Badejo 2004, 2005; Twumasi and Merem, 2006; Uyigue and Agho 2007). The consequences of this have been enormous financial loss, extensive habitat degradation, and poverty leading to the continuous crises in the Niger Delta Area, The activities of the habitants of Odidi community in Warri South-West Local Government Area of Delta State, have been impeded due to several oil spills since 1998, and this have gone a long way to affect the lives of the people in such residence. On the 4th of April 2015, a fire outbreak from an oil blowout on the Riapele Forcados trunkline belonging to the Nigerian Petroleum Development Company (NPDC), a subsidiary of NNPC, reportedly killed a mother and 3 children in the community. Worried by the raging effect of these oil spills, the people of Odidi and Egwa communities threatened to disrupt company’s operations in the area, if the affected areas were not cleaned. Six months after the spill, the areas were yet to be cleaned up.
However, the severity of the impact of oil spill depends on a variety of factors including characteristics of the oil itself. Natural conditions, such as water temperature and weather, also influence the behavior of oil in aquatic environments. Such spillages have led to damage and loss of biodiversity, depletion of land and potable water, blockage of waterways. In such circumstances, the concentration of petroleum hydrocarbons and trace metals in water bodies are often observed to be elevated (Atlas and Bartha, 1992). This can have a disastrous consequences on the society; economically, environmentally, and socially.
Aquatic invertebrate which plays an important role in water purification, habitat creation and shore line erosion control, could be affected from spill in the aquatic environment. This is of serious concern because they are critical food sources for a variety of aquatic wild life, they provide and sustain large commercial fisheries for humans, support lucrative tourist activities like coral reef snorkeling and fishing, and are important in the development of medicinal compounds. The negative impact of oil spill on aquatic invertebrate varies with the spill’s location and magnitude as well as invertebrate, life stage, habitat, sensitivity, feeding habits and ability to avoid or process contaminant. The effect of oil on aquatic invertebrate in general include: habitat degradation; smothering, fouling of gills structures; impaired reproduction, alterations of growth, development, feeding, immune response and respiration, and disturbance of food web (Blackburn et al., 2014).
Also, benthic invertebrate can be adversely affected by oil that is trapped and buried in sediment and or in mussels and oyster beds, where it can persist essentially unchanged for years. Benthic (bottom-dwelling) organisms are also killed if stranded oil accumulate and sink to the bottom, with possible tainting of commercial species. The low oxygen that characterizes mangrove ecosystems makes the oil that penetrates root systems to persist for long periods. Similarly, ingested and absorbed hydrocarbon can be transferred through the food web to a higher trophic level organism, raising serious concern for the loss and reduction of this important biota following an oil spill (Wanat et al., 2007).
Oil contains a lot of particulate matter, polycyclic aromatic hydrocarbons, hydrogen sulphide, acidic aerosols, and volatile organic compounds (VOCs), which are detrimental to human health including respiratory and heart diseases, allergic reactions and weakened immune systems. Lyons et al., (2002) in their study concluded that residence in oil-contaminated area (as a result of spillage) are significantly associated with greater anxiety and depression scores, worse mental health, self- reported headache, sore eyes and sore throat after adjusting for age, sex, smoking status, anxiety and the belief that oil had affected their health.
Measures which include; bioremediation (that is use of microorganism or biological agents to break down or remove oil, such as the bacteria (Alcanivorax)), controlled burning, and use of dispersants, have been used to clean- up oil spills from the environment (Kasia, 2002). However, laboratory experiment showed that dispersants increased toxic hydrocarbon levels in fish by a factor of up to 100 and may kill fish and fish eggs. Other studies also found that some dispersant are toxic and can increase the toxicity of oil up to 52 times (Barry, 2007). Increasing the awareness of potentially harmful effects of spilled oil present in affected rivers and protecting humans that depend on aquatic species, has created the impetus to carry out this research work on the evaluation of oil spill on Egwa and Odidi Rivers on pelagic and bottom dwelling organisms through the determination of total petroleum hydrocarbon (TPH) and heavy metals (lead, copper, chromium and zinc) in water, sediment, snail (Pahymelania byronensi) and catfish (Clarias gariepinus). The test organisms chosen for this assessment are highly prolific and relatively abundant all year round in the Niger Delta region of Nigeria. They are a major source of protein for the inhabitants in the region and also a principal prey of many larger vertebrates. They were also chosen due to their sensitivity since they are good bioindicators of environment pollution and can adapt to laboratory conditions (Beeby, 2001; DPR, 2002).
Study area
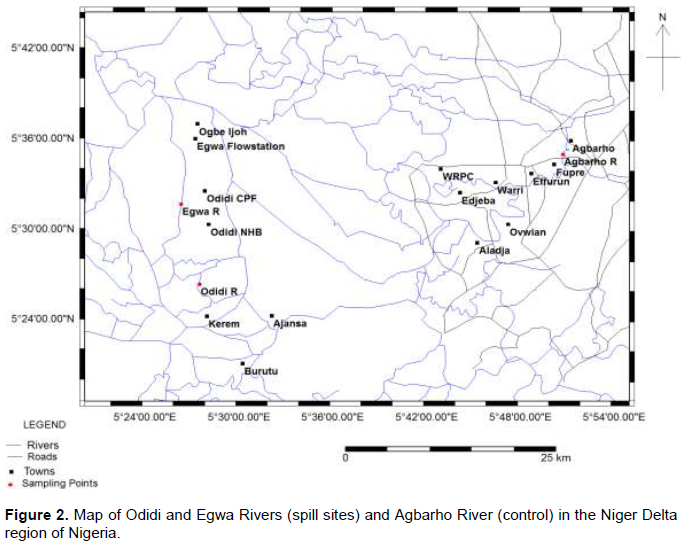
The study areas, Odidi and Egwa Rivers in Warri South-West Local Government Area of Delta State in the Niger Delta ecological zone of Nigeria lies within longitude 5°26’18”N; latitude 5°27’37”E and longitude 5°31’38N”; latitude 5°26’25”E respectively (Figures 1 and 2). The activities of the inhabitants of these areas consist mainly of fishing. There are a number of Exploration and Production facilities and activities located around these areas belonging to some major oil producing companies in the upstream sector of the oil industries, some of which includes oilfields, gas plants, flow stations. The rivers Odidi and Egwa and adjacent streams in the region have been constantly receiving different environmental pollutants or impact from Oil Exploration and Production activities over the years since crude oil was discovered in the regions. The control location for this study was collected from Agbarho River, which is presumably a semi-pristine environment. The major anthropogenic activities at Agbarho River include recreational (swimming), fishing and occasional sand mining.
Water sampling
A total of twenty four (24) water samples were randomly collected at a depth of half meter from the water surface in triplicates from different spot of the two spill points, namely Odidi and Egwa rivers. The water samples were stored in 1 L polyethylene bottles and 1 to 2 mL of 1:1 nitric acid was added to the samples to preserve them for heavy metals determination (APHA, 2005). The nitric acid was added to the water samples because it would lead to a drop in pH, therefore the loosely bonded ions can be released for determination.
Sediment sampling
The top few centimeters of sediment samples from the spill and control site were randomly collected using a hand held van Veen grab. The sediment were collected in Teflon bags and kept at 4°C for TPH analysis while samples for the determination of heavy metals were air-dried in laboratory. The sediment samples were sieved and fractions with particles diameter less than 2 mm were retained for metal treatment and analysis since this particle size is the fraction that interacts mainly with the species (Whale and Worden, 1999).
Species sampling
Fish (C. gariepinus) and snail (P. byronensis) samples were collected from the spill site of the two rivers (Odidi and Egwa) and the control site in Agbarho.
The fish samples were collected with dip nets and transferred into Teflon bags and transported in ice chest to the laboratory and the species tissues were sorted prior to organics and heavy metal analysis. The snail samples were collected by gently hand sorting from the spill locations and place in jute bags (coarse, strong fibre of the East India plant used to make bags, mats, paper). In the laboratory, the organisms were sacrificed by freezing at 4°C until it is required for organics and heavy metal analysis. The samples for TPH were air-dried and extracted while for heavy metals, the samples were dried at 310°C. The mean weight and length of fish used was 5.30 ± 0.04 g and 9.00 ± 0.07 cm while the snail had a mean weight of 30.40 ± 0.06 g and length of 6.5 ± 0.03 cm respectively. The samples were analyzed within the holding time for each parameter.
Determination of metals in water
Water samples were digested using concentrated nitric acid (AR). Each sample was mixed and 100 mL was transferred to a beaker to which 5 mL concentrated nitric acid was added and brought to a boil on hot plate to lowest volume possible (15 to 20 mL). Filtration was done after digestion to remove insoluble particles. The filtrate was then diluted to volume with distilled water in a 50 mL volumetric flask (APHA, 2005). The concentration of heavy metals (lead, copper, chromium, iron and zinc) was determined by running the samples on Atomic Absorption Spectrophotometer (AAS)
(Shimadzu 6710F).
Determination of metals in sediment
Two (2) g of the sieved air-dried sediment samples was digested in a mixture of 7 mL concentrated nitric acid (HNO3) and 2 mL sulphuric acid (H2SO4). The mixture was heated on a hot plate until brown fumes of nitric acid were gone. The digestion was stopped when white fumes started coming out. The digest was allowed to cool and the wall of the beakers was washed down with distilled water and then filtered to remove insoluble materials. The filtrate was then diluted to mark in a 100 mL volumetric flask. The concentrations of heavy metals (lead, copper, chromium, iron, and zinc) were analyzed using Atomic Absorption Spectrophotometer (AAS) Shimadzu 6701F.
Determination of heavy metals in tissue of species (fish and snail)
The species (fish and snail) were dried at 310°C for 6 h and the dried tissues were ground thoroughly to achieve homogeneity. Five (5) g of the sample was weighed into a beaker. Ten (10) mL of nitric acid and 5 mL of perchloric acid was then added, this was warmed on a hot plate until the tissue solubilized. The temperature of the hot plate was increased to near boiling until the solution turned brown. Then it was allowed to cool and 2 mL of nitric acid was added and heated until the volume of the sample was heated to 10 to 15 mL. Then five (5) mL of 30% hydrogen peroxide was added and heated to 10 to15 mL. Filtration was done after digestion and the filtrate was diluted to volume with distilled water in a 50mL volumetric flask. The concentration of heavy metals was determined by running the samples on an AAS.
Determination of TPH in water
100 mL of the water sample was measured into a separatory funnel. Thirty (30) mL of n-hexane was added, and was swirled for 10 to 15 min with venting at intervals. Five (5) g of sodium sulphate was then added. The mixture formed two layers with the n-hexane at the upper region since it was lighter than water. The water layer was collected first and volume noted. The upper n-hexane layer which now contains the extract was collected in a sample vial (small glass bottles). The extract was concentrated to one (1) mL by allowing it to evaporate in a fume cupboard using compressed air. The extract was re-dissolved in 5mL tetrachloroethylene and an Agilent 5890 GC-FID was used to ascertain the concentration of TPH in the water.
Determination of TPH in sediment
Five (5) g of sediment was accurately weighed into a glass extraction bottle and dried with sodium sulphate. The sample was then extracted in a shaking bath with 20 mL of tetrachloroethylene for 3 h. The extract was then transferred into a clean bottle through a glass funnel stripped with glass wool and sodium sulphate at the aperture. The eluate was then concentrated to 1mL, placed in a vial and stored in a cool environment. The extract was re-dissolved in 5 mL tetrachloroethylene and the concentration of TPH in the sample was then analyzed with an Agilent 5890GC-FID.
Determination of TPH in species (fish and snail)
The harvested fish and snail samples from the study area were air-dried at room temperature, in a well aerated room free from hydrocarbon contaminant. The tissue of the dried specie samples was grinded and homogenized into a mortar with pestle. Ten (10) g of the homogenized tissue sample was accurately weighed and transferred into a soxhlet extractor. The hydrocarbon content in the fish and snail sample was extracted using 100 mL of dichloromethane and hexane mixture (3:1) for 3 h on a heating mantle, and was then allowed to cool. The extract was concentrated to 1 mL by allowing it to evaporate at room temperature in a fume cupboard using compressed air. The extract was then re-dissolved in 5 mL tetrachloroehylene and concentrations analyzed on an Agilent 5890GC-FID.
Bioconcentration factor (BCF)
Bioconcentration factor (BCF) is defined as the concentration of a chemical in an organism’s tissues divided by the concentration in the medium (water, soil, sediment). That is the degree to which a chemical / substance will bioconcentrate in an organism in the medium in which it inhabits (Heng et al., 2004). Bioconcentration is the specific accumulation process by which the concentration of a chemical in an organism becomes higher than its concentration in the medium around the organism. In water for instance, bioconcentration is focused on the net accumulation of a chemical / substance from water into and unto an aquatic organism, which results from uptake and depuration. Although the process is the same for both natural and man-made chemicals, the term bioconcentration usually refers to chemicals foreign to the organism. For fish and other aquatic animals, bioconcentration after uptake through the gills (or sometimes the skin) is usually the most important bioaccumulation process.
A bioconcentration value greater than 1000 implies that the chemical / substance have the tendency to bioaccumulate in the organism exposed to it. The level is usually evaluated using sublethal (chronic) exposures at relatively lower concentrations on a longer-term basis, which gives a true picture of the effects of chemicals on the exposed organisms (Hall and Golding, 1998). sublethal effects could result in growth inhibition, impaired reproduction, behavioural changes, immobilization, lack of sensitivity, endocrine disruption, deoxyribonucleic acid (DNA) damage, other metabolic changes and eventually death of species.
The degree or tendency of a chemical to bioaccumulate is very important in protecting humans and other organisms from the deleterious effects of chemical exposure, and it should be regarded as a critical consideration in the regulation of chemicals (Dimitrov et al, 2002; DRP, 2011). In this study, the bioconcentration values in the organisms were determined by dividing the concentration in the tissue of the organism by the concentration in the medium of the specific specie.
The results of TPH and heavy metals in the water, sediment, pelagic organism (cat fish – C. gariepinus) and bottom dwelling organism (snail – P.a byronensis) from the spill locations in Odidi and Egwa Rivers are presented in Tables 1 - 5 with further illustration in Figures 3 - 4.
Water characteristics
The concentration of TPH reported for Odidi and Egwa Rivers were 97592 ± 46 mg/L and 91590 ± 51 mg/L respectively, which were far above the DPR regulatory limit of 10 mg/L (DPR, 2011). This was evident as observed on the water samples that had scum of crude oil in it. The mean concentrations of iron and chromium were above their respective DPR limits of 1.00 and 0.03 mg/L. They were also found to be higher than their respective control values (Table 1). The physico-chemical composition of the water from the affected rivers indicated that the waters were slightly acidic, turbid and brackish (Table 2).
Sediment
Total petroleum hydrocarbon (TPH) in the sediment from
Odidi (215730 ± 81 mg/kg) and Egwa (215700 ± 77 mg/kg) exceeded the DPR permissible range of 50 to 5000 mg/kg. Since sediment is known to act as sink for most hydrocarbons, most of the crude oil would have settled to the bottom bed over time, hence the high concentrations are reported. However, the concentrations of heavy metals were within the various DPR target and intervention
values (Table 3).
Pelagic organisms - catfish (Clarias gariepinus)
The mean concentration of heavy metals in the fish muscle from the spilled site varied. In comparison with the FAO/WHO permissible limits, all the metals except chromium complied with the set standard (Table 4).
Theconcentrations of petroleum hydrocarbon was very high in both spill stations Odidi (58334 ± 32 mg/kg) and Egwa (58314 ± 35 mg/kg). Some dead fish and other pelagic organisms were seen floating on the water with scum of crude on their body surfaces after the spill. The TPH from the control location does not indicate any hydrocarbon pollution since values obtained was less than 5mg/kg while the heavy metals concentration were relatively low.
Bottom dwelling organism - snail (Pachymelania byronensis)
The level of TPH in the snail was 103180 ± 99 mg/kg and 103380 ± 98 mg/kg for Egwa and Odidi Rivers, respectively. The high concentration of the hydrocarbon residue in the tissue of the organisms could possibly be as a result of the oil spill. The concentrations of heavy metals (iron, zinc, chromium, and copper) were found to be above their respect control values (Table 5).
Bioconcentration of TPH and heavy metals in species
The results for bioconcentration were relatively low in the species from the spilled locations. The values are presented in Figures 3 and 4 for fish and snails respectively. The bioconcentration factor (BCF) for TPH in fish varied between 0.60 and 0.64 in Odidi and Egwa Rivers while the control was 0.0 since there was relatively less than 5 mg/kg of hydrocarbon in control site.For copper, the BCF was 18.5 in Odidi and 13.5 in Egwa, while the control was 9.0. Zinc biocentrated is a factor of 12.0 in the fish muscle in Odidi and 10.26 in Egwa and the control was 7.0 (Figure 3).
In the snail, the BCF for TPH was 0.48 from the two spill sites while a factor of 0.0 was recorded in the control. A factor of 1.7 was reported for zinc in Odidi and Egwa while a BCF of 1.06 was obtained in the control location (Figure 4).
Total petroleum hydrocarbon (TPH) in matrix and organisms
The mean concentrations of total petroleum hydrocarbon (TPH) were significantly higher than the DPR and FAO/WHO standards for the environmental matrix (water, sediment) and organisms (fish, snails) (FAO/WHO, 1999; DPR, 2011). In the environmental matrix, the recommended DPR intervention and target limit is 50 and 5000 mg/kg respectively (DPR, 2011). The high concentration of TPH observed in the two locations could be due to the crude oil spill that occurred in the area. Thus, the impact of the spill was still evident approximately five months after the spill occurred and sampling carried out. This was because no clean-up of the spill sites was done either by National Oil Spill Detection and Response Agency (NOSDRA) or the local communities.
In the Niger Delta area, due to protests and agitations from the immediate communities, they would not allow the agencies and other stakeholders to enter the environment, to carry out a thorough clean-up so as to safe guard aquatic organisms in the areas and the entire ecosystem.
The concentrations observed in the two organisms varied probably due to their mobility and habitat use (Albers 2003). Catfish uses pelagic habitat and could swim away from the pollution, while snails are benthic organisms because of their lower mobility, they are restricted to a type of habitat. Oil spilled on water forms a layer on the water surface and prevents oxygen from dissolving in it, thus affecting aquatic organisms by interfering with the functioning of various organs of these species and subsequently death. Hydrocarbon pollution on water results in an anoxic condition that affect the breathing function of the organisms since there is no longer a steady supply of dissolved oxygen. Thus, this would lead to death of these viable species in the affected environment (Dublin-Green et al., 1997).
Due to the ability of oil to float on the surface of water and disperse within the ocean/rivers as it weathers, most pelagic organisms are exposed to both floating oil, slicks and small droplets of oil in the water column (Almeda et al., 2013). Some of these organisms at the air-sea interface are thought to be especially sensitive to oil spill due to their proximity to high concentrations of dissolved oil and to the additional toxicity of photo graded hydrocarbon products at this boundary. In addition, the chemicals used after a spill to break up oil slick, may exacerbate the effect of oil exposure.
A review by Lee et al. (2012) found that pelagic organisms were more severely impacted by a mixture of dispersant and crude oil than the crude oil alone. Feeding and growth rates in the common sea star fish (Evasteria stroscheli) were impaired during a 28 day exposure to 0.13, 0.97 and 0.2 ppm crude oil and dispersant and the organisms with impaired feeding suffered from loss of arms and death (O’clair and Rice, 1985). This is because emulsified oil / dispersant mixture was ingested and internalized in the invertebrates. The surface active nature of these dispersants causes interaction with cell surfaces of organisms, as well as the gills.
Furthermore, chemical dispersant that emulsify the oil create smaller droplets within the water column and may allow further diffusion into the tissue than oil alone (Chase et al., 2013). Thus, resulting in higher body burden which may have impacts on behavior, reduced fitness, impact on predators, and ultimately disrupting the role they play as ecosystem engineers and eventually death. Benthic organisms are usually adversely affected since oil penetrates sheltered beaches, which are homes for sediment-dwelling bivalves, resulting in long-term oil persistence (Culbertson et al., 2008).
However, the hydrocarbon availability may decrease as it bounds to sediments (Neff, 2002). The water had high concentration of petroleum hydrocarbon because of the longer time it takes for it to sink into the riverbed, and most marine organism accumulate these hydrocarbons due to their sedentary and bottom feeding habit (Jack et al., 2005). Animal studies have shown that TPH have some toxicological effect on the blood, immune system, spleen, kidneys, lungs, reproductive system and even developing foetus (USEPA, 2000).
Heavy metals in water and sediment
The mean concentrations of heavy metals in water from spilled site were all higher than the control station Agbarho River. The contamination of the water with heavy metals could possibly be attributed to the crude oil spill as well as oil Exploration and Production discharges from flow stations, leaching from protection plates of transportation and fishing boats due to the salty nature of the water (Hamed, 1998).
Thus, the contamination of waterways with heavy metals may not only affect the water, but also the aquatic organisms that live in it. This is because heavy metals do not biodegrade and are regarded as causing cytotoxic, mutagenic and carcinogenic effects in animals. Similarly, the contamination resulting from the oil spills may have devastating effects on the ecological balance of the recipient environment as well as the diversity of aquatic organisms.
The availability of the metals in sediment could be attributed to the same reasons as noted for the water. The presence of heavy metals in sediment can alter the physical and chemical properties of the sediment thus affecting the organisms that reside in it. This could lead to accumulation of the heavy metals in the organisms and likely biomagnification along the food chain. Sediments are known to be the major sinks of trace metals in aquatic ecosystem and serve as an indication of trend and profile of pollution (Gundacker, 1999). The high concentration of heavy metals observed may be linked to the crude oil spill in the area, in addition to runoffs and effluent discharge from oil Exploration and Production activities. Since bioconcentration of metals in benthic organisms (snail) is strongly dependent on the amount of trace metal in the sediment and the regulatory capacity of the organism (snail), snails can thus be useful in bioindicators of trace metals in the aquatic sediment.
Heavy metals in fish (Clarias gariepinus)
The heavy metal concentrations in cat fish (C. gariepinus) samples from spilled site were closely to the metal content of water from spill site. It was detected in the following order Fe > Cr > Zn > Cu > Pb, which agrees with the research of Saleh, (1982) that the amount of pollutant in fish was directly proportional to the degree of heavy metal pollution in aquatic environment. Values obtained from his research for heavy metals (Cu, Fe and Zn) were (0.17 to 2.08, 31.9 to 743 and 45.5 to 86.1 mg/kg) respectively. Similar observations were noted for other studies carried out with various fish species (Guerrin et al., 1990; Saeed and Sakr, 2008).The metal concentrations reported in the spill area were higher than the control site, which could possibly be due to the exploration and other industrial activities in the study area as compared to non-industrial activities in the control site. High level of cadmium in fish enhances susceptibility to disease due to innate immune response (Ghiasi et al., 2010), while zinc at low levels adversely affect hatchability, survival and haematological parameters of the specie.
Zinc has its primary site of accumulation as the liver and kidney and may persist for many years without decomposition (Murugan et al., 2008). The bioavailability of these metals could lead to respiratory problem, reproduction / birth defect and even disrupt the organism’s ability to fight disease and function. Copper enters into fishes though gills, liver, stomach and intestine and combine with other contaminants such as ammonia mercury or zinc to produce additive or synergistic toxic effects on the fish. Similarly, it have been reported that high concentrations of heavy metals could adversely affect aquatic organisms resulting in retarded growth, reduced reproduction and abnormal response to the opposite sex (Rompala et al., 1984; Nussey et al., 2006).
Heavy metals in snail (Pachymelania byronensis)
The effects of heavy metals in snails was observed to be similar to that reported for pelagic organisms, although bottom dwellers are usually worse hit by effects of pollution, since sediment acts as sink for all types of pollutants (Adams et al., 1992). These heavy metals have been identified with the crude oil spill and laboratory studies further confirmed the acute and long term impacts on gastropods physiology and behaviour. After the Laura D’ Amato spill, the intertidal gastropod showed significant mortality after 96h exposure to crude oil with lethal concentration (LC50) of 11.7 ppm. A concentration that will kill 50% of organisms was exposed to the crude oil. This was found to affect gastropod predating on uncontaminated mussel prey and was three times higher than predation on the one exposed to sublethal concentration of crude oil, the study also showed that reproduction in organisms was also affected (Eisler, 1975).
Bioconcentration of TPH and heavy metals in species
Due to hydrophilic nature of the tissues of an organism, there is the tendency to bioaccumulate some chemicals and substances. Bioaccumulation is the building-up of a chemical to a toxic level in an organism's body. Bioaccumulation becomes an environmental problem when chemicals accumulated are toxic. Bioaccumulation of chemicals is an important factor in the assessment of environmental hazard or risk (Beek, 1991; Heng et al., 2004). The degree to which a contaminant will concentrate in an organism is expressed as a bioconcentration factor (BCF), which is defined as the concentration of a chemical in an organism’s tissues divided by the exposure concentration. A chemical / substance are said to bioaccumulate, if the BCF is greater than 1000 (EPA, 2000).
In this study bioconcentration potentials varied between the two rivers (Odidi and Egwa) and the parameters did not indicate any level of bioaccumulation in the tissues of the organisms since the BCF for the parameters were relatively low that is less than 1000. However, long term exposure of the species to such substances / chemicals (TPH and metals) could occur and this could lead to detrimental effects on pelagic and bottom dwellers. Noting that this study was done six months after the spill, the low concentration of heavy metals values in the water and sediment could raise considerably if analysis is done after a year or two of the spill. It is therefore imperative to ensure immediate clean up to avoid potential bioaccumulation effects (Dimitrov et al., 2002).
The high concentrations of total hydrocarbon and heavy metals in the sample matrix (water, sediment) and organisms (fish and snail) from the spill environment could be attributed to the oil spill that occurred in the sampling locations since concentrations were significantly higher than the control from Agbarho River. The concentration of heavy metals and petroleum residues in aquatic organisms may become higher over time, which may lead to lethal and sublethal consequences in aquatic fauna and clinical poisoning to man and the environment. Thus, a site that had been damaged or destroyed by an oil spill calls for immediate response and restorative/ remediation measures since natural attenuation cannot clean up very high concentration of petroleum residues as revealed from this research.
The authors have not declared any conflict of interests.
REFERENCES
|
Adam WJ, Kimerle RR, Bazaett JW (1992). Sediment quality and aquatic life assesment. Environ. Sci. Technol. 26(10):1864-1875.
Crossref
|
|
|
|
Albers P (2003). Petroleum and Indivdual polycyclic aromatic hydrocarbons. Chapter 14. In BR. edited by Hoffman DJ. Handbook Ecotoxicol. pp. 341-371.
|
|
|
|
|
Almeda R, Wambaugh Z, Wang Z, Hyatt C, Liu Z, Buskey EJ (2013). Interactions between zooplankton and crude oil: Toxic effects and bioaccumulation of polycyclic aromatic hydrocarbons. Plos ONE 8(6):e67212.
Crossref
|
|
|
|
|
America Public Health Association (APHA) (2005). Standard methods for examination of water and waste water (20th ed.). Washington DC: American Water Works Association and Water Pollution Control Federation.
|
|
|
|
|
Atlas RM (1995). Petroleum biodegradation and oil spill bioremediation. Mar. Pollut. Bull. 31:175-182.
Crossref
|
|
|
|
|
Atlas RM, Bartha R (1992). Hydrocarbon biodegradation and oil spill bioremediation. In K.C. Marshall (ed). Adv. Microb. Ecol. 12:287-338.
Crossref
|
|
|
|
|
Barry C (2007). Slick Death: Oil-spill treatment kills coral. Science News. 172:67.
Crossref
|
|
|
|
|
Beeby A (2001). What do sentinels stand for? Environ. Pollut. 112:285-298.
Crossref
|
|
|
|
|
Beek B (1991). Bioaccumulation in aquatic systems. In Bioaccumulation in aquatic systems: Contributions to the assessment. Nagel R, Loskin R. (Ed.), VHC. Germany.
|
|
|
|
|
Blackburn MC, Mazzacano AS, Fallon C (2014). Oil in our oceans: A review of impact of oil spills on marine invetebrates. Portland. P. 152.
|
|
|
|
|
Chase DA, Edwards DS, Qin G, Wages MR, Willming MM, Anderson TA, Maul JD (2013). Bioaccumulation of petroleum hydrocarbons in fiddler crabs (Uca minax) exposed to weathered MC-252 crude oil alone and in mixture with an oil dispersant. Sci. Total Environ. 444:121-127.
Crossref
|
|
|
|
|
Culbertson JB, Valiela I, Olsen YS, Reddy CM (2008). Effects of field exposure to 38 year old residual petroleum hydrocarbons on growth, condition index, and filtration rate of ribbed mussel, Geukensia demmissa. Environ. pollut. 154:312-319.
Crossref
|
|
|
|
|
Department of Petroleum Resources (DPR) (2011). Environmental Guidelines and standards for the petroleum indsutry in Nigeria (EGASPIN). Revised edition. Universal Press, Lagos, Nigeria. pp. 276-297.
|
|
|
|
|
Dimitrov SD, Dimitrova NC, Walker JD, Veith GD, Mekenyan OG (2002). Predicting bioconcentration factors of highly hydrophobic chemicals. Effects of molecular size. Pure Appl. Chem. 74:1823-1830.
Crossref
|
|
|
|
|
Dublin-Green CO, Awo Bamise A, Ajao EA (1997). Large marine Ecosystem Project for the Gulf of Guinea (coastal profile of Nigeria). Lagos: Nigeria Institute of Oceanography.
|
|
|
|
|
Eisler R (1975). Toxic, sublethal, and latent effects of petroleum on Red Sea macro fauna. International Oil Spill Conference Proceedings. pp. 535-540.
Crossref
|
|
|
|
|
Environmental Protection Agency (EPA) (2000). Bioaccumulation
|
|
|
|
|
Ghiasi F, Mirzargar SS, Bdakhshan H, Shamsi S (2010). Effects of low concentrations of cadmium on the level of lysozyme in serum, leukocyte count and phagocytic index in Cyprinus carpio under the wintering conditions. J. Fish. Aqua. Sci. 5:113-119.
Crossref
|
|
|
|
|
Guerrin F, Burgat-Sacaze V, Saqui-Same P (1990). Levels of heavy metals and organochlorine pesticides of cyprinid fish reared four years in wastewater treatment pond. Bullet. Environ. Contam. Toxicol. 44:461-467.
Crossref
|
|
|
|
|
Gundacker C (1999). Comparison of heavy metal bioaccumulation in freshwater molluscs of urban river habitats in Vienna. Environ. Pollut. 110(2):61-71.
|
|
|
|
|
Hall JA, Golding LA (1998). Chronic toxicity test protocol. standard methods for whole effluent toxicity testing: development and application. Report no. MFE80205. NIWA report for the Ministry for the Environment, Wellington, New Zealand.
|
|
|
|
|
Hamed MA (1998). Distribution of trace metals in the River Nile ecosystem, Damietta branch between Mansoura city and Damietta Province. J. Egypt. Germ. Soc. Zool. 27(A):399-415.
|
|
|
|
|
Heng LY, Mokhtar MB, Rusin S (2004). The bioaccumulation of trace essential metals by the freshwater snail Turritella sp. found in the rivers of Borneo East Malaysia. J. Biol. Sci. 4(4):441-444.
Crossref
|
|
|
|
|
Jack IR, Fekarurhorho JK, Igwe FU, Okorosaye OK (2005). Determination of total hydrocarbon levels in some marine organisms from some towns within river state in Nigeria. J. Appl. Sci. Environ. Manage. 9(3):59-61.
|
|
|
|
|
Lee RF, Koster M, Paffnhofer GA (2012). Ingestion and defecation of dispersed oil droplets by pelagic tunicates. J. Plankton Res. 34(12):1058-1063.
Crossref
|
|
|
|
|
Lyons BP, Pascoe CK, McFadzen IRB (2002). Phototoxicity of pyrene and benzo(a)pyrene to embryo-larval stages of the pacific oyster Crassostrea gigas. Mar. Environ. Res. 54:627-631.
Crossref
|
|
|
|
|
Murugan SS, Karuppasamy R, Poongodi K, Puvaneswari S (2008). Bioaccumulation pattern of zinc in fresh water fish Channapunctatus (Bloch) after chronic exposure. Turk. J. Fish. Aquat. Sci. 8:55-59.
|
|
|
|
|
Neff JM (2002). Bioaccumulation in marine organisms: Effect of contaminants from oil well produced water. Amsterdam: Elsevier, Ltd.
|
|
|
|
|
Nussey G, Vuren J, Preez H (2006). Bioaccumulation of chromium, manganese, nickel and lead in the tissues of moggel, Labeo umbratus (Cyprinidae), from Withbank Dam, Mpumalanga. Water South Africa 26(2):269-284.
|
|
|
|
|
Nwilo PC, Badejo TO (2005). Oil spill problems and management in Niger Delta. International oil spill conference. Miami, Florida, USA.
Crossref
|
|
|
|
|
Nwilo CP, Badejo TO (2004). Management of oil dispersal along the Nigerian coastal areas. Department of Survey and Geoinformatics, University of Lagos, Lagos, Nigeria.
|
|
|
|
|
O'Clair CE, Rice SD (1985). Depression of feeding and growth rates of the seastar Evasterias troschelii during long-term exposure to the water-soluble fraction of crude oil. Mar. Biol. 84(3):331-340.
Crossref
|
|
|
|
|
Rompala JM, Rutosky FW, Putnam DJ (1984). Concentrations of environmental contaminants from selected waters in Pennsylvania. Pennsylvania, USA. United States Fishery and Wildlife Service Report. Volume 2:18.
|
|
|
|
|
Saeed SM, Sakr SF (2008). Impact of cage-fish culture in the river Nile on physicochemical characteristics of water, metals accumulation, histological and some biochemical parameters in fish. Abbassa Int. J. Aquat. 1(A):179-202.
|
|
|
|
|
Saleh HH (1982). Fish liver as an indicator for aquatic environmental pollution. Bull. Inst.Oceanogr. Fish. 8(1):69-79.
|
|
|
|
|
Twumasi Y, Merem E (2006). GIS and remote sensing applications in the assessment of change within a coastal environment in the Niger Delta region of Nigeria. Int. J. Environ. Res. Public Health. 3(1):96-106.
Crossref
|
|
|
|
|
United States Enivronmental Protection agency (USEPA). (2000). Interpretation for the purpose of sediment quality assessment status and need. Bioaccumulation testing and Bioaccumulation Analysis Workshop. Washington DC. P 33.
|
|
|
|
|
Uyigue E, Agho M (2007). Coping with climate change and environmental degradation in the Niger Delta of Southern Nigeria. Community Research and Development Centre Nigeria (CREDC). pp. 24-27.
|
|
|
|
|
Wanat Y, Jin XH, Jin F (2007). Trophic dilution of polycyclic aromatic hydrocarbon (PAHs) in marine food web from Bohai Bay, North China. Environ. Sci. Technol. 41:3109-3114.
Crossref
|
|
|
|
|
Weiner A, Berg C, Gerlach T, Grunblatt J, Holbrook K, Kuwanda M (1997). The Exxon Valdez oil spill; Habitat protection as a restoration strategy. Restor. Ecol. 5(1):44-55.
Crossref
|
|