ABSTRACT
Geminiviruses, in particular the members of the genus begomovirus, are considered to be a major phytosanitary problem for tomato crops production in the world. They are responsible for yield losses of up to 20 to 100% and Togo is not spared from this situation. The present study aimed to investigate the genetic diversity of the begomoviruses affecting tomato crops production in Togo and their relationship with other begomoviruses. To achieve these objectives, 307 samples of tomato leaves and wild plant species with typical virus symptoms were collected in the Maritime, Plateau, Central, Kara and Savannah regions and subjected to polymerase chain reaction (PCR) analysis. The results revealed the presence of begomovirus in 25.40% of the analyzed samples. The PCR products obtained were subjected to direct sequencing. Phylogenetic analysis of the sequences of the different regions of the DNA-A of the begomovirus identified in this study with that of other begomoviruses showed a nucleotide identity of 96% respectively to Tomato leaf curl Togo virus-Fontem, Tomato Leaf Curl Togo Virus, and Ageratum leaf curl Cameroon Alphasatellite; 98% respectively to Tomato leaf curl Nigeria virus, Ageratum leaf curl Cameroon virus, Tomato leaf curl Cameroon virus-Fontem, Ageratum leaf curl Cameroon virus and 99% respectively to Tomato leaf curl Kumasi virus, Pepper yellow vein Mali virus Bazegahot, and Pepper yellow vein Mali virus-Ouaga. These results suggest a high degree of genetic diversity of tomato begomoviruses identified in Togo.
Key words: Begomoviruses, genetic diversity, phylogenetic relationships, tomato, wild plants.
Begomoviruses, transmitted in persistent, circulative manner by Bemisia tabaci Gennadius (Homoptera: Aleyrodidae), are a major limiting factor for the production of many agricultural species in tropical and subtropical regions of the world (Brown et al., 2015). In particular, approximately 70 species of the genus Begomovirus (family Geminiviridae) have been identified as naturally infecting tomato (Solanum lycopersicum L.) in different parts of the world (Tsai et al., 2011; Van Brunschot et al., 2013) and even in some places, these diseases have eliminated the tomato as an economically viable crop (Zhou et al., 2008; Hanssen et al., 2010). The diversity of geminiviruses is reflected in seven genera (Becurtovirus, Begomovirus, Curtovirus, Eragrovirus, Mastrevirus, Topocuvirus and Turncurtovirus), which are defined on the basis of genome structure, host range and insect vector (Varsani et al., 2014 ; Adams et al., 2013). With 288 species currently recognized by the International Committee of Taxonomy of Viruses (ICTV), the genus Begomovirus is the largest genus of plant viruses in relation to the number of members it includes (http://www.ictvonline.org /virusTaxonomy.asp).
Their genome is a single-stranded circular DNA that can be monopartite (containing DNA-A) or bipartite (containing both genomic DNA-A and B) (Stanley et al., 2005).Based on genome organization, phylogenetic relationship and geographic distribution, begomoviruses have generally been divided into two groups: the begomoviruses of the Old World (Europe, Africa, Asia and Australia) and the begomoviruses of the New World (Americas) (Melgarejo et al., 2013). Most bipartite begomoviruses are found in the New World, while most monopartite species are found in the Old World. However, there are exceptions. Bipartite begomoviruses such as Tomato leaf curl New Delhi virus (ToLCNDV) (Padidam et al., 1995) and Tomato yellow leaf curl Thailand virus (TYLCTHV) (Rochester et al., 1994) are distributed in the Old World, while the monopartite Tomato yellow leaf curl virus (TYLCV) was introduced in the New World in the early 1990s and it is now widespread in the Caribbean basin and in the South America (Hawaii, Mexico and Guatemala) (Lefeuvre et al., 2010).
Contrary to the considerable genetic diversity that exists among begomoviruses infecting tomato crops, the symptoms induced by these viruses are relatively similar and include varying degrees of stunting, leaf curling, mottling and yellowing. In addition, symptoms vary depending on the cultivar, host plant species, age of the plant at the infection time, environmental factors and mixed infections with other viruses or pathogens. Thus, it is difficult, if not impossible, to identify the species involved in an outbreak based solely on symptoms, hence the importance of molecular analysis for the identification of these viruses. In Western Africa, begomoviruses have emerged recently and are caused by genetically distinct species that have evolved locally, hence these regions could be an important area for new evolutionary linkages of the species within the genus (Chen et al., 2009 ; Leke et al., 2011 ; Osei et al., 2008; Zhou et al., 2008). This work aimed the molecular characterization of genetic diversity of begomoviruses infecting tomato and associated wild plants in tomato fields in Togo.
Sample collection
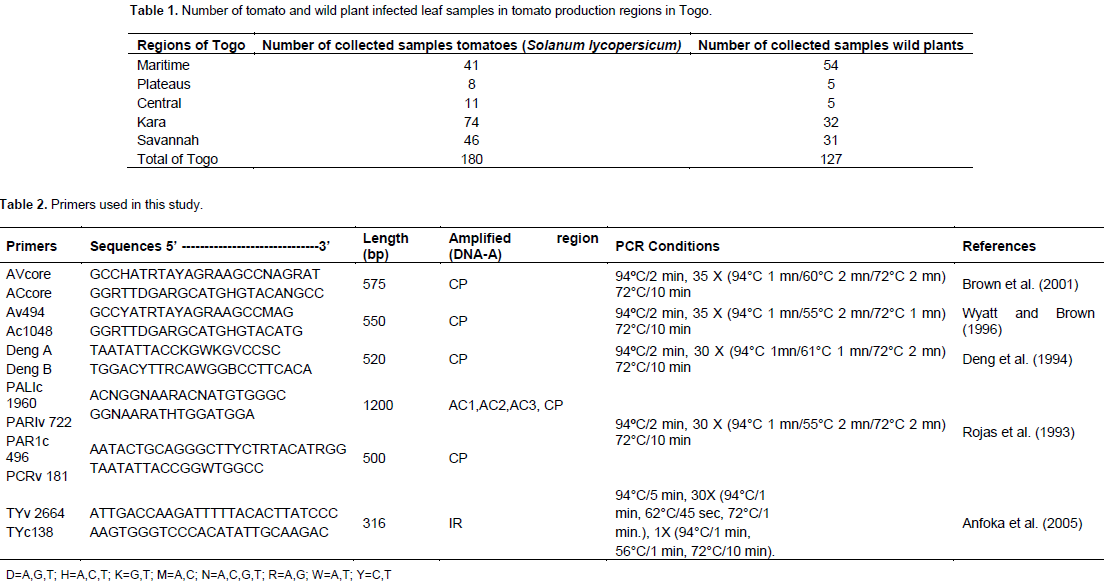
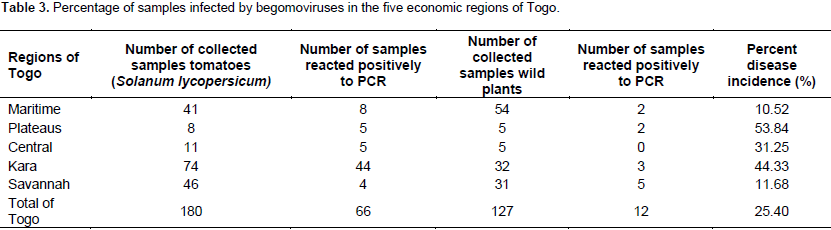
A total of 307 samples of tomato leaves and wild plants showing symptoms typical of begomoviruses ; leaf curling, yellowing, stunting and leaf thickening were collected in 2013, 2014 and 2015 from Maritime, Plateaus, Central, Kara and Savannah regions in Togo. The wild plants were collected in the field of tomato for research of host reservoir of begomoviruses. For the molecular analyses, the samples were dried in an oven at 40°C. This variability in sampling is explained by the fact that producers used to target the off-season periods that are most economically profitable. Moreover, this variability can also be explained by compliance with the sampling protocol which would require tomato fields to be separated by 10 km in order to take geo-referenced positions.
DNA extraction and PCR amplification
Total nucleic acids were extracted from symptomatic tomato plants and wild plants through the methods adapted from Lodhi et al. (1994); 150 mg of desiccated tissue was ground in 500 μL of extraction buffer (2% CTAB, 1.4 M NaCl, 20 mM EDTA, 100 mM Tris-HCl, 1% PVP, pH 8.0 and 0.2% β-mercaptoéthanol) and incubated at 60°C for 30 min. The mixture was kept on ice for 10 min followed by centrifugation at 13 000 rpm for 15 min. The supernatant was transferred to a new vial and diluted with distilled water before using in Polymerase Chain Reaction (PCR) reaction. The begomoviruses identification was performed by PCR using six pairs of universal primers able to amplify the different region of the component DNA-A according to protocols by Brown et al. (2001), Wyatt and Brown (1996), Deng et al. (1994), Rojas et al. (1993) and Anfoka et al. (2005) (Table 2). The universal primer pair Avcore/ACcore (Brown et al., 2001) amplify a 575 nucleotide fragment corresponding to the core region of coat protein (CP) gene of almost all begomoviruses. Two sets of begomovirus group specific universal primers Deng A/Deng B primers (Deng et al., 1994) and Av494/Ac1048 (Wyatt and Brown, 1996) which are capable of universally amplifying the core CP of many begomoviruses were used for viral detection.
The primer pair PALIc1960/PARI722 produces a 1.2 Kb band upon PCR and had been designed to amplify the bottom half region of the A genome component of most WTGs (Rojas et al., 1993). Primers PAR1c496/PCRv181 (Rojas et al., 1993) amplify ~ 300 bp of the A component that contains ~172 bp of region between the beginning of the loop and the beginning of the CP and~126 bp of the CP. Primers TYv2664/TYc138 were used to amplify the IR of TYLCV-Mld. The parameters for the PCR reaction were optimized for 25 μl. The final concentrations of reaction components were: 200 µM deoxynucleotide triphosphate (dNTPs), 1x Taq DNA polymerase buffer, 2.5 mM MgCl2, 0.8 units Taq DNA polymerase, 1 μM of each complementary and virus-sense primers and 2 μl of DNA. PCR cycle parameters were as described in Table 1. All PCR reactions were performed in a programmable thermocycler (Mastercycler ep gradient S, Eppendorf, Hamburg, Germany). The amplified products, along with 10 kb DNA ladder, were resolved in 1% agarose gel in Tris-borate EDTA (TBE), pH 8.0 buffer with 10 µl/100ml GelGreenTM. Gel electrophoresis was carried out at 70 V until tracking dye has reached the bottom of the gel. The DNA bands were viewed and photographed using a gel documentation system (BioRad, Hercules, CA, USA).
Sequencing and phylogenetic analysis
PCR products from amplifications were purified using the Agentcourt AMPure XP magnetic beads (Beckman Coulter, Inc. 250 S. Kraemer Blvd. Brea, CA 92821 USA) according to the manufacturer's protocol. The Illumina Nextera XT Index kit (Illumina Inc., San Diego, CA, USA) was used according to the manufacturer's instructions to assign a code to each sample prior to sequencing. The purity of the PCR products was verified at the Agilent Bioanalyzer 2100 (Agilent Technologies, Palo Alto, CA, USA) and the sequencing was performed at the UPJV Molecular Biology platform via the high throughput technique using the kit V2 of the Illumina Miseq (Illumina Inc., San Diego, CA, USA). The partial sequences were assembled using the FROG software(http://bioinfo.genotoul.fr/fileadmin/user_upload/FROGS_poster_Jobim_2015.pdf.) and aligned with the sequences available in the GenBank database using the BLASTn algorithm (http://www.ncbi.nlm.nih .gov). The multiple alignment of the sequenceswas obtained with the ClustalX software (ftp://ftpigbmc.u-strasbg.fr/pub/ClustalX/)(Larkin et al., 2007) with default parameters.
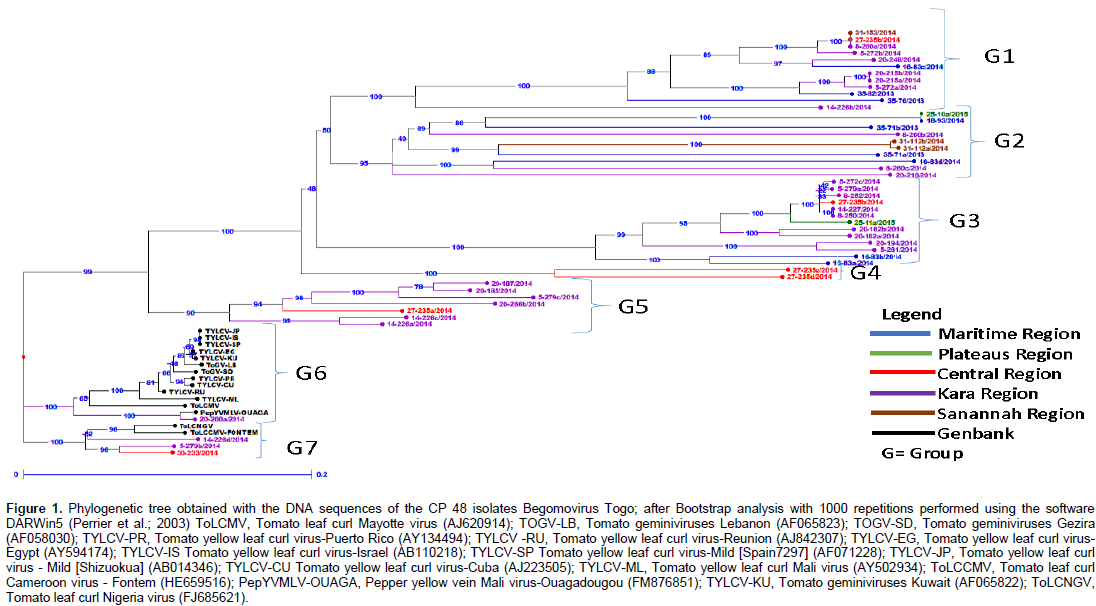
Phylogenetic analyze was performed by the Neighbor-Joining method using Darwin5. A thousand Bootstrap replicas were used to evaluate the robustness of the topology of the final tree. The nucleotide sequences of isolates Tomato geminiviruses Lebanon (TOGV-LB), Tomato geminiviruses Gezira (TOGV-SD), Tomato yellow leaf curl virus-Puerto Rico (TYLCV-PR), Tomato geminiviruses Kuwait (TYLCV-KU), Tomato yellow leaf curl virus-Reunion (TYLCV-RU), Tomato yellow leaf curl virus-Egypt (TYLCV-EG), Tomato yellow leaf curl virus-Israel (TYLCV-IS), Tomato yellow leaf curl virus-Mild [Spain7297](TYLCV-SP), Tomato yellow leaf curl virus - Mild [Shizuokua] (TYLCV-JP), Tomato yellow leaf curl virus-Cuba (TYLCV-CU), Tomato yellow leaf curl Mali virus (TYLCV-ML), Tomato leaf curl Nigeria virus (ToLCNGV) , Tomato leaf curl Cameroon virus - Fontem (ToLCCMV-Fontem) and Pepper yellow vein Mali virus-Ouagadougou (PepYVMLV-OUAGA) were included in the analysis.
Begomoviruses identification by PCR
A total of 307 samples, 180 tomatoes and 127 wild plants (Table 1) were collected and based on PCR analysis, 66 tomatoes and 12 wild plants were positive for the presence of a begomoviruses. In the Maritime Region, 10.52% of the samples analysed were positive, 53.84% in the Plateaus region, 31.25% in the Central Region, 44.33% in the Kara region and 11.68% in the Savannah region (Table 3). Surprisingly, no infection by begomoviruses was reported in Kpendjal Prefecture. This result suggests that the insect vector Bemisia tabaci is not present in the environment. The results obtained from PCR showed that the isolate TYLCV-Mild was not detected in all the collected samples. Different fragment sizes were obtained from five geminiviruses primer pairs.
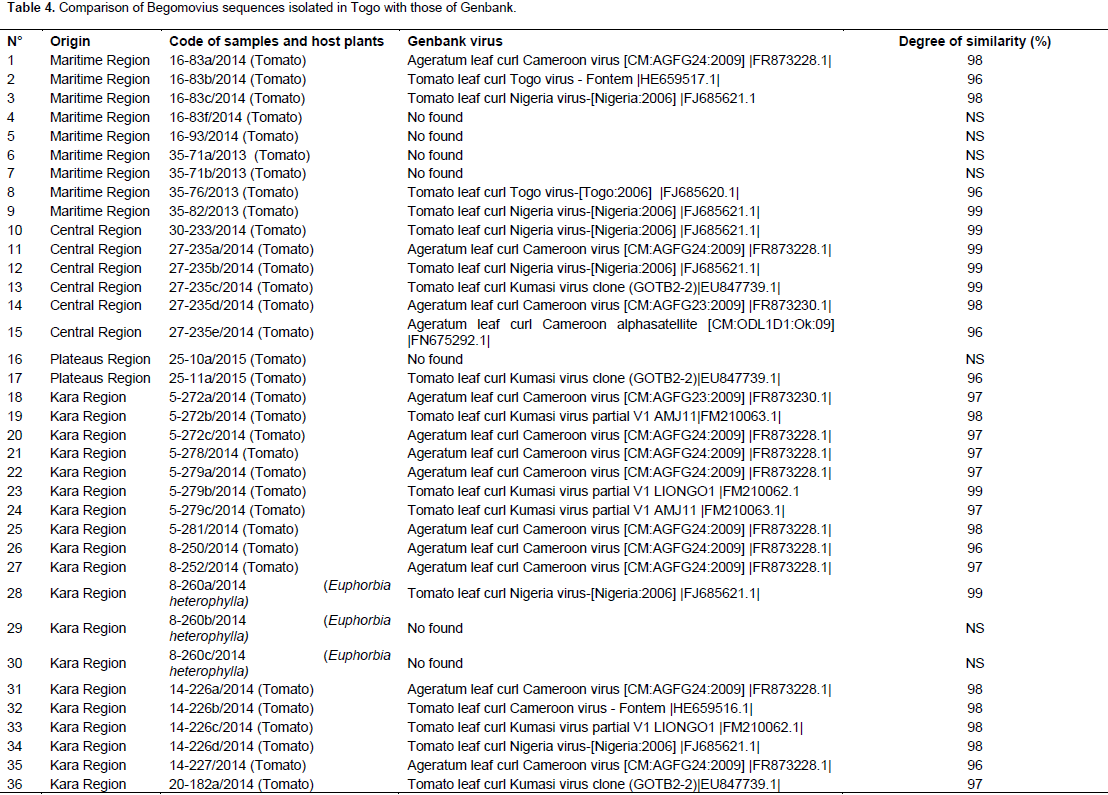
Sequence analysis and identification of new viruses A total of 123 PCR products including 111 from tomato and 12 from wild plants were fully sequenced to identify the different species of the genus Begomovirus. Partial sequences up to 500 nucleotides were obtained from each reaction and processed with the FROG software (http://bioinfo.genotoul.fr/fileadmin/user_upload/FROGS_poster_Jobim_2015.pdf.). These partial sequences were compared to sequences published in GenBank using the BLASTn algorithm (http://www.ncbi.nlm.nih.gov). This comparison showed that the degree of similarity was between 95 and 100%. Moreover, the alignment showed that 11 sequences were close to Tomato leaf curl Nigeria virus (FJ685621.1), 15 to Ageratum leaf curl Cameroon virus (FR873228.1; FR873230; FN675292.1), 7 to Tomato leaf curl Kumasi virus (EU847739.1; FM210063.1; FM210062.1), 2 to Tomato leaf curl Togo virus (FJ685620.1 ; HE659517.1), 1 from Tomato leaf curl Cameroon virus-Fontem (HE659516.1), 1 to Pepper yellow vein Mali virus- Bazegahot (FM876848.1) and 1 to Pepper yellow vein Mali virus -Ouaga (FM876851.1). 123 PCR products were fully sequenced but only 49 sequences available were obtained among which 10 did not show any similarity with Genbank sequences (Table 4).
Phylogenetic analysis
Phylogenetic analysis was performed with the partial sequences of Togo isolates and control sequences available in GenBank using ClustalX multiple alignment software. The phylogenetic tree was designed using the DARWin5 program and the Neighbor-Joining method. Of the positive PCR samples, 44 sequences from tomato and 4 from wild plants were selected for phylogenetic analysis. This analysis indicated that the begomoviruses isolated in Togo form seven groups (Group 1 to 7) (Figure 1). Groups 1 and 2 contain begomovirus isolates from tomato and two from wild plants Solanum macrocarpon L. and Euphorbia heterophylla L. Groups 3, 4 and 5 contain only Begomovirus isolates from tomato. Most of GenBank isolates and Group 7 contain both GenBank and tomato isolates.
In West Africa, several viruses infecting tomato crops have been reported, including strains of begomoviruses such as: Tomato leaf curl Cameroon virus (ToLCCMV), Tomato yellow leaf curl Mali virus (TYLCMLV), Tomato leaf curl Nigeria virus (ToLCNGV), Tomato leaf curl Ghana virus (ToLCGHV), Tomato leaf curl Kumasi virus (ToLCKuV) and Tomato leaf curl Togo virus (ToLCTGV) (Kon et al., 2009 ; Leke et al., 2011 ; Osei et al., 2008 ; Zhou et al., 2008) belong to the West African tomato infecting begomoviruses (WATIBs) clade. The result in this study has shown that the PCR with virus-specific primers for the Mediterranean TYLCV isolates used by Anfoka et al. (2005), did not succeed in detecting the TYLCD-associated viruses in symptomatic tomato plants and wild plants collected in fields in Togo. However, the degenerate primers of Avcore/ACcore, Deng A/Deng B, Av494/Ac1048, PALIc1960/PARI722 and PAR1c496/PCRv181 designed respectively by Brown et al. (2001), Deng et al. (1994), Wyatt and Brown (1996) and Rojas et al. (1993) for geminiviruses succeeded in amplifying the expected band size of geminiviruses of the tested infected samples.
The identification of Tomato leaf curl Cameroon virus-Fontem (Leke et al., 2014) first in Cameroon and now in Togo suggests that this virus is widely distributed in Central and West Africa. Furthermore, since cultivated tomato is not indigenous to Africa and begomoviruses are not seed transmissible (Inoue-Nagata et al., 2016), it is more likely that WATIBs originated from an endemic alternate host. The identification of Ageratum leaf curl Cameroon virus first in Ageratum conyzoides and now on tomato in Togo suggests that this begomovirus can infect more than one host. The identification of ALCCMA first in Ageratum conyzoides and now in tomato suggests that ALCCMA may infect more than one host and may be trans-replicated by other begomoviruses. The identification of Tomato leaf curl Nigeria virus (Kon and Gilbertson, 2012) on the wild Euphorbia heterophylla in our study suggests that it is a reservoir plant for this virus and that there would be a transfer at the time of place a tomato crop. This hypothesis remains to be verified.
Analysis of the genetic diversity of begomoviruses infecting tomato was carried out by direct sequencing of the PCR products. This strategy, after the PCR parameters were optimized, was extremely efficient for analyzing a large number of sequences in a short period of time. The sequenced region comprises the 5 'end of the CP gene. This is the most variable region of the CP gene and according to Padidam et al. (1995), is representative of the variability of the nucleotide sequence of the viral genome. Therefore, phylogenetic analysis based on the region is usually sufficient to establish the taxonomic position of a given isolate of begomovirus. From the results obtained from the phylogenetic analysis, sequences from the begomovirus CP gene that infect tomato in Togo form seven large groups of begomoviruses. Thus, in groups 6 and 7 there are isolates from Togo mixed with GenBank isolates. But it should also be noted that the elements of the Maritime Region, Central Region, Savannah Region and those of the Kara region can be found together in the same group (G1) and so on.
It should be noted that, according to our study, the isolates of begomovirus infecting tomato in Togo are specific to Togo (as shown in the tree of Figure 1 except for two cases where Togo isolates are mixed with isolates from GenBank). The proximity of Pepper yellow vein Mali virus-Ouagadougou (Tiendrébéogo et al., 2011) with a high degree of similarity of 99% with one of our isolates (20-266a/2014) from the Kara region suggests that this begomovirus has been introduced into this area through the vector insect or trade. Although new distinct species of begomovirus were identified in the early 2000s, this identification and the determination of begomoviruses as emerging viruses and the discovery of new species by viral genome sequencing is the major concern nowadays. Ha et al. (2008) have suggested that the South-East of continental Asia could be an important center of diversity for begomoviruses based on the great diversity of strains and species of local monopartite begomoviruses and associated betasatellite molecules identified in these regions.
It appears from this study that geographically separated viruses meet and are in mixed infection in Togo. So for the future it would be important to sequence more begomovirus isolates from tomato and wild plants to monitor viral genotypes and to be able to track possible changes in the population structure of these virus. This should be considered when screening programs for virus resistance are established because recombination is permanent with begomoviruses and it is known that when there is recombination, the new recombinants are usually more infectious than their parents.
The authors declare that there is no conflict of interest.
The authors wish to express their thanks to the Program of Agricultural Productivity in West Africa for Togo (PPAAO / TOGO) for the financing of this project.
REFERENCES
|
Adams MJ, King AMQ, Carstens EB (2013). Ratification vote on taxonomic proposals to the International Committee on Taxonomy of Viruses. Arch. Virol. 158(9):2023-2030.
Crossref
|
|
|
|
Anfoka GH, Abhary M, Nakhla MK (2005). Molecular identification of species of the tomato yellow leaf curl virus complex in Jordan. J. Plant Pathol. 87:61-66.
|
|
|
|
|
Brown JK, Idris A, Torres-Jerez I, Banks GK, Wyatt SD (2001). The core region of the coat protein gene is highly useful for establishing the provisional identification and classification of begomoviruses. Arch. Virol. 146:1581-1598.
Crossref
|
|
|
|
|
Brown JK, Zerbini FM, Navas-Castillo J, Moriones E, Ramos-Sobrinho R, Silva JF, Fiallo-Olivé E, Briddon R, Hernández-Zepeda C, Idris A (2015). Revision of Begomovirus taxonomy based on pairwise sequence comparisons. Arch. Virol. 160:1593-1619.
Crossref
|
|
|
|
|
Chen LF, Rojas M, Kon T, Gamby K, Xoconostle-Cazares B, Gilbertson RL (2009). A severe symptom phenotype in tomato in Mali is caused by a reassortant between a novel recombinant begomovirus (Tomato yellow leaf curl Mali virus) and a betasatellite. Mol. Plant Pathol. 10:415-430.
Crossref
|
|
|
|
|
Deng D, Mcgrath PF, Robinson DJ, Harrison BD (1994). Detection and differentiation of whitefly-transmitted geminiviruses in plants and vector insects by the polymerase chain reaction with degenerate primers. Ann. Appl. Biol. 125:327-336.
Crossref
|
|
|
|
|
Ha C, Coombs S, Revill P, Harding R, Vu M, Dale J (2008). Molecular characterization of begomoviruses and DNA satellites from Vietnam: additional evidence that the New World geminiviruses were present in the Old World prior to continental separation. J. Gen. Virol. 89:312-326.
Crossref
|
|
|
|
|
Hanssen IM, Lapidot M, Thomma B (2010). Emerging viral diseases of tomato crops. Mol. Plant Microbe Interact. 23:539-548.
Crossref
|
|
|
|
|
Inoue-Nagata AK, Lima MF, Gilbertson RL (2016). A review of geminivirus (begomovirus) diseases in vegetables and other crops in Brazil: current status and approaches for management. Hortic. Bras. 34:8-18.
Crossref
|
|
|
|
|
Kon T, Gilbertson RL (2012). Two genetically related begomoviruses causing tomato leaf curl disease in Togo and Nigeria differ in virulence and host range but do not require a betasatellite for induction of disease symptoms. Arch. Virol. 157(1):107-120.
Crossref
|
|
|
|
|
Kon T, Rojas MR, Abdourhamane IK, Gilbertson RL (2009) Roles and interactions of begomoviruses and satellite DNAs associated with okra leaf curl disease in Mali, West Africa. J. Gen. Virol. 90:1001-1013.
Crossref
|
|
|
|
|
Larkin MA, Blackshields G, Brown NP, Chenna R, Mcgettigan PA, Mcwilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG (2007). Clustal W and Clustal X version 2.0. Bioinformatics 23(21):2947-2948.
Crossref
|
|
|
|
|
Lefeuvre P, Martin DP, Harkins G, Lemey P, Gray AJ, Meredith S, Lakay F, Monjane A, Lett JM, Varsani A, Heydarnejad J (2010). The spread of tomato yellow leaf curl virus from the Middle East to the World. PLoS. Pathog. 6(10):e1001164.
Crossref
|
|
|
|
|
Leke WN, Brown JK, Ligthart ME, Sattar N, Njualema DK, Kvarnheden A (2011). Ageratum conyzoides: A host to a unique begomovirus disease complex in Cameroon. Virus Res. 163:229-237.
Crossref
|
|
|
|
|
Leke WN, Kvarnheden A (2014). Mixed infection by two West African tomato-infecting begomoviruses and ageratum leaf curl Cameroon betasatellite in tomato in Cameroon. Arch. Virol. 159(11):3145-3148.
Crossref
|
|
|
|
|
Lodhi MA, Ye GN, Weeden NF, Reisch BI (1994). A simple and efficient method for DNA extraction from grapevine cultivars and Vitis species. Plant Mol. Biol. Rep. 12(1):6-13.
Crossref
|
|
|
|
|
Melgarejo TA, Kon T, Rojas MR, Paz-Carrasco L, Zerbini FM, Gilbertson RL (2013). Characterization of a new world monopartite begomovirus causing leaf curl disease of tomato in Ecuador and Peru reveals a new direction in geminivirus evolution. J. Virol. 87:5397-5413
Crossref
|
|
|
|
|
Osei MK, Akromah R, Shih SL, Lee LM, Green SK (2008). First report and molecular characterization of DNA A of three distinct begomoviruses associated with tomato leaf curl disease in Ghana. Plant Dis. 92(11):1585.
Crossref
|
|
|
|
|
Padidam M, Beachy RN, Fauquet CM (1995). Tomato leaf curl geminivirus from India has a bipartite genome and coat protein is not essential for infectivity. J. Gen. Virol. 76:25-35.
Crossref
|
|
|
|
|
Rochester DE, Depaulo JJ, Fauquet CM, Beachy RN (1994). Complete nucleotide sequence of the geminivirus tomato yellow leaf curl virus, Thailand isolate. J. Gen. Virol. 75:477-485.
Crossref
|
|
|
|
|
Rojas MR, Gilbertson RL, Russell DR, Maxwell DP (1993). Use of degenerate primers in the polymerase chain reaction to detect whitefly-transmitted geminiviruses. Plant Dis. 77:340-347.
Crossref
|
|
|
|
|
Stanley J, Bisaro DM, Briddon RW, Brown JK, Fauquet CM, Harrison BD, Rybicki EP, Stenger DC (2005). Family Geminiviridae. In. Fauquet CM, Mayo MA, Maniloff J, Desselberger U, Ball LA (Eds.), Eighth report of the International Committee on Taxonomy of Viruses. Els. Aca. Press. Pp. 301-326.
|
|
|
|
|
Tiendrébéogo F, Lefeuvre P, Hoareau M, Traoré VS, Barro N, Péréfarres F, Reynaud B, Traoré AS, Konaté G, Lett JM, Traoré O (2011). Molecular and biological characterization of Pepper yellow vein Mali virus (PepYVMV) isolates associated with pepper yellow vein disease in Burkina Faso. Arch. Virol. 156(3):483-487.
Crossref
|
|
|
|
|
Tsai WS, Shih SL, Venkatesan SG, Aquino MU, Green SK, Kenyon L, Jan F-J (2011). Distribution and genetic diversity of begomoviruses infecting tomato and pepper plants in the Philippines. Ann. Appl. Biol. 60:787-799.
Crossref
|
|
|
|
|
Van Brunschot SL, Bergervoet JH, Pagendam DE, de Weerdt M, Geering AD, Drenth A, Van der Vlugt RA (2013). A bead-based suspension array for the multiplexed detection of begomoviruses and their whitefly vectors. J. Virol. Methods198:86-94.
Crossref
|
|
|
|
|
Varsani A, Navas-Castillo J, Moriones E, Hernandez-Zepeda C, Idris A, Brown JK, Murilo Zerbini F, Martin DP (2014). Establishment of three new genera in the family Geminiviridae: Becurtovirus, Eragrovirus and Turncurtovirus. Arch. Virol. 159:2193-2203.
Crossref
|
|
|
|
|
Wyatt SD, Brown JK (1996). Detection of subgroup III geminivirus isolates in leaf extracts by degenerate primers and polymerase chain reaction. Phytopathology 86:1288-1293.
Crossref
|
|
|
|
|
Zhou YC, Noussourou M, Kon T, Rojas M, Jiang H, Chen LF, Gamby K, Foster R, Gilbertson RL (2008). Evidence of local evolution of tomato-infecting begomovirus species in West Africa: characterization of tomato leaf curl Mali virus and tomato yellow leaf crumple virus from Mali. Arch. Virol. 153:693-706.
Crossref
|
|