ABSTRACT
This study aimed at evaluating the health benefits of popular Moringa oleifera leaf. The aqueous and methanolic extracts of the leaf at two different concentrations (1:1 and 1:2) was used to determine the phytochemical screening and its antibacterial activity. Escherichia coli, Streptococcus pneumonia and Staphlococcus aureus were used in this study, applying agar diffusion methods. The phytochemical screening indicated presence of secondary metabolites such as alkaloid, flavonoids, anthraquinoines, tannins and phenol in both extracts making it to have antibacterial potentials. Both extract showed remarkable activity against the growth of the selected bacteria; nevertheless, the methanol extract had more antibacterial activity than the water extract, more so the extracts were discovered to be more active at higher concentration. The water extract was not active at low concentration, that is 1:1 but had diameter zone of inhibition of 10 mm each for 1:2 concentration. The minimum inhibition concentration (MIC) that inhibits these bacterial ranged between 1:4 and 1:16 and the minimum bactericidal concentration (MBC) that kills the growth of the bacterial isolates completely was 1:16. The result of this study showed that M. oleifera could be a valuable antibacterial drug in the treatment of infections caused by the test organisms.
Key words: Agar diffusion method, aqueous and methanol extracts, secondary metabolites, zone of inhibition, minimum inhibition concentration (MIC), minimum bactericidal concentration (MBC).
Moringa oleifera commonly called moringa, is regarded as one of the most popular and valuable trees in the world. It is known in India as drumstick, in Senegal as Nebedy, in Thailand as Marum, in Haiti as Benzolive tree and in Philippine as Malunggay. It is also well known in all parts of Nigeria, in the North, the Hausas refer to it as Zogale or Bagaruwarmakka, in the south-west; the Yorubas call it Ewe Igbale or Idagbomonoye and in the south-east, the igbos call it Ikwaoyibo. (Thilza et al., 2010). Virtually, all parts of Moringa tree are consumable (leaves, flower and pods). Studies from around the world revealed that Moringa leave have remarkable nutritional values such as vitamins, minerals and amino acids. As such tThe leaves have been used to fight malnutrition, mostly among pregnant woman, nursing mothers and infants (Anwar et al., 2007). They are considerable variations among the nutritional values of Moringa, which depends on factors such as genetic background, environment and cultivation method (Brisibe et al., 2009).
Infectious diseases are the leading cause of death worldwide. Antibiotic resistance has become a globe concern (Mahallingam et al., 2011). The issue of multi-drug resistant is continuously increasing at an alarming rate especially in developing countries where there are increases in the indiscriminate use of wide broad spectrum antibiotic as a result of self-medication, immunosuppressive agent and outbreak of epidemics (such as HIV, tuberculosis, etc) along with the use of adulterated drugs with side effects. Therefore, there is a need to research new infection compacting strategies to fight microbial infection. The potential of medicinal plants as source for new drugs is still widely unexplored. Among the estimated 250,000-500,000 plant species only a small percentage has been investigated phytochemically and the fraction submitted for biological or pharmacological screenings is even smaller. Therefore, any phytochemical investigation of a given plant will reveal only a very narrow spectrum of its constituents (Mahesh and Satish, 2008). Moringa and other medicinal plant has a long history of been traditionally used as a cure for illness such as cough, cold, asthma, nausea, fever, vomiting and diarrhea. But with development in science and technology, advancement has been made in the medical field with the discoveries of many natural and synthetic drugs (Preethi et al., 2010).
Prior to this, this study was aimed at assessing and evaluating the health and economic benefit of Moringa leaf extract in the control of infections caused by the test organisms, taking advantage of its popularity, availability, cultural acceptability, cheaper alternative source for treatment, better compatibility to human body and low possibility of developing resistance and side effect by investigating the phytochemical composition of the plant extract, examining antibacterial activity of the leaf extract (Moringa leaf) on the pathogenic organism (which are Staphylococcus aureus, Escherichia coli and Streptococcus pneumonia) and determining the appropriate concentration required to inhibit and kill the organism in question through determination of minimum inhibiting concentration (MIC) and minimum bactericidal concentration (MBC) respectively.It is an established fact that the Gram- positive and negative bacteria react differently to antibacterial agents due to the differences in their cell wall component(peptidoglycan) and the ability of these agents to penetrate them. This influence the choice of E. coli (a Gram negative bacterium) and S. pneumonia (a Gram positive bacterial) to investigate the susceptibility of these bacteria to the plant extract. The inclusion of S. aureus was as a result of the need to find an alternative and effective cure to skin infections caused by this bacterium (that are developing more resistant to the conventional method of treatment) which is becoming very common in the environment of this study.
MATERIALS AND METHODS
Collection and processing of plant materials
Fresh leaves of M. oleifera were collected from Niger State Polytechnic botanical garden, Zungeru Campus, Wushishi Local Government Area of Niger State, Nigeria, on coordinates 9° 48’N 6° 9’E and elevation of 149 m (489 ft) in May, 2015. They were air-dried at room temperature (29 – 31°C) in the laboratory for one week, after which it was pounded into fine powder using a sterile laboratory mortar and pestle.
Extraction
Two solvents (aqueous and methanol) were used in the extraction of the antimicrobial component of the leave powder. One hundred grams was weighed using a weighing balance and soaked in 500 mL of methanol and water, respectively, in a liter capacity of conical flask for 48 h with intermittent stirring. Whatman No. 1 filter paper was used to filter the solution. The extracts were evaporated using a hot oven at 40°C (Abalaka et al., 2012). The dried extracts were aseptically stored in a sterile laboratory bottles till when they were used.
Phytochemical analysis
The phytochemical screening was the qualitative analyzed in accordance with Parekh and Chanda (2007) to determine the secondary metabolites present in each extract.
Steroids and terpenoids
Ten milligrams of extract was dissolved in chloroform. Two to three drops of acetic anhydride were added, followed by one mL of concentrated sulphuric acid. Blue colour in chloform layer which changes to green shows the presence of steroids, whereas the appearance of pink colour in chloroform layer shows the presence of terpenoids.
Alkaloids
Ten milligrams of extract was dissolved in concentrated HCl and filtered. Two to three drops of solution were poured into the centre of watch glass. Mayer reagent was added in the sides of the watch glass with the help of a glass rod. Formation of a gelatinous white precipitate at the junction of the two liquid shows the presence of alkaloids.
Flavonoid
Ten milligram of extract was dissolved in methanol. Magnesium turnings were added into it followed by concentrated HCl. A magenta colour shows the presence of flavonoids.
Saponins
Ten milligram of extract was dissolved in water and shaken well. Froth which last for a long time shows the presence of saponins.
Tannins
Ten milligrams of extract was boiled with one mile of water for 30 min. The extract is filtered clear and to this, 0.5 mL 2% gelatin was added. A curdy white precipitate indicates the presence of tannins.
Phenolic compound
Five milligram of extract was dissolved in alcohol and drop of neutral ferric chloride was added to it. The intense colour indicates the presence of phenolic compound.
Anthraquinone
To ten milligram of the dissolved extract, magnesium acetate solution was added. Pink colour developed indicates the presence of anthraquinone and no colour change indicates negative.
Test organism
One Gram negative (E. coli) and two Gram positive organisms (S. aureus and S. pneumonia) were used in this study. The stock culture of the organism were obtained from microbiology laboratory of Federal University of Technology Minna, Niger State, Nigeria, they had been stored on an agar slant and stored at 4°C until they were collected.
Standardization of the bacteria culture
A loopful of the test isolates were subcultured on a nutrient agar and inoculated at 37°C for 24 h to refurbish the organisms’ normal growth before they are used for susceptibility testing. The distinct colonies after 24 h of incubation were obtained with a sterile wire loop and inoculated in 10 ml of a freshly prepared nutrient broth and incubated for 3 h to standardize the culture.
Preparation of stock solution of the extracts of M. oleifera
The stock solution of the sample’s extracts were prepared by measuring 10 g of the extract dissolved in 10 mL of distilled water and methanol each which was labeled 1:1 and 20 g into 10 mL which was labeled 1:2, both were dissolved in the solvent used to obtain the extracts during its initial extraction resulting into two concentrations that is 1:1 and 1:2. It was shaken and stirred effectively to ensure it dissolved properly.
Antibacterial susceptibility testing of the extract on the selected bacterial isolates
Sterile nutrient agar was prepared and allows cooling to 40°C. One mL of the test organism from then incubated nutrient broth was mixed with 19 mL of the molten agar in sterile Petri dishes (agar diffusion method). Each Petri dish swirled carefully until the agar was spread evenly and allowed to cool. After the media has solidified, a sterile cork borer (5 mm) was used to bore well in each the Petri-dishes aseptically. Few drops of the different concentrations of the extract was dispensed into the well bored using a sterile pipette, one plate for each organism and concentration. Each plate was replicated three times (well diffusion method). The plates were incubated at 37°C for 24 h. The zone of inhibition of each plate was observed and recorded by measuring the clear zone at which growths had been inhibited from the edge of the growth inhibited (Kawo, 2007).
Determination of minimum inhibition concentration (MIC)
After the preparation and sterilization process of a nutrient broth, it was dispensed evenly into a test tubes (4 folds) in such a way that two rack stands for methanol and water extracts. From the zone of inhibition, the highest concentration of the sample that inhibited the bacteria isolates the most, was further diluted into four different concentration 1:2, 1:4, 1:8 and 1:16, respectively which were introduced aseptically on how they are been arranged. Five (5) drops of each test organisms were dropped into each test tube and were covered with a sterile aluminium foil wrapped with cotton wool, which were incubated at 37°C for 24 h, the concentrations at which these bacteria are inhibited completely is known and recorded as the minimum inhibition concentration (Akinyemi et al., 2005)
Determination of minimum bactericidal concentration (MBC)
The MBC was determined by observing the tubes which showed on growth during MIC determination. The growth-free test tubes were sub-cultured on a freshly prepared nutrient agar and incubated at 37°C for 24 h. The lowest concentration of the extract without colony growth on the media was recorded as the MBC.
Table 1 shows phytochemical screening result of both aqueous and methanol extract of M. oliefera leave. The result shows the presence of alkaloid, anthraquinones, tannins, flavonoids and phenol in both water and methanol extracts, meanwhile saponins was present in methanol extract but absent in water extract, whereas steroid was not discovered in both extracts.
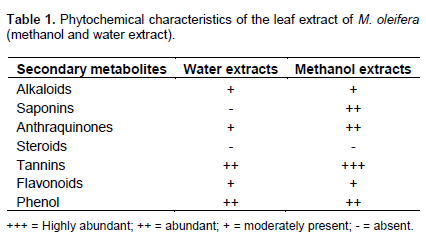
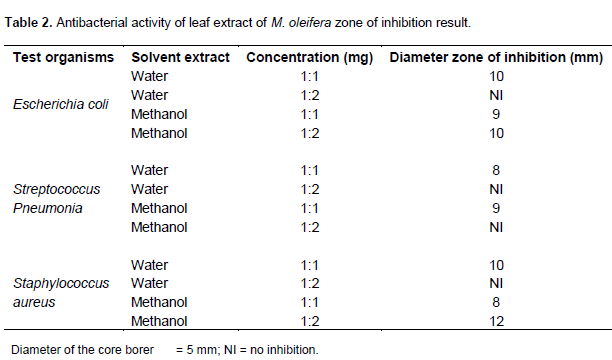
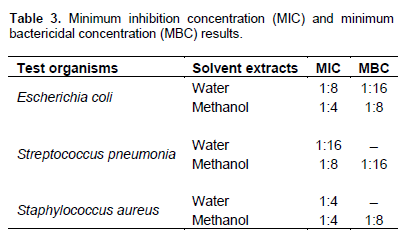
Table 2 shows the antibacterial activity of leaf extract of M. oliefera on the test organisms. The result in the table revealed that both aqueous and methanol extracts of the Moringa leave were active against the test organisms. The highest zone of inhibition was observed for methanol extract against S. aureus and the lowest zone of inhibition was observed for the water concentrations of 1:1 which was against all the three test organisms. Table 3 shows the result of the MIC and MBC investigation for aqueous and methanol extract of the leaf of Moringa. The result revealed that the MIC of water extract for E. coli, S. pneumonia and S. aureus were 1:8, 1:16 and 1:4, respectively. The MIC of methanol extract for E. coli, S. pneumonia and S. aureus were 1:4, 1:8 and 1:4, respectively. Table 3 also shows the result for MBC of water extract for E. coli to be 1:16 but no MBC of water extract on S. aureus and S pneumonia. It also shows the MBC of the methanol extract for E. coli, S. pneumonia and S. aureus were 1:8, 1:16 and 1:8, respectively.
The result of the preliminary phytochemical screening of MoringaM. oleifera presented in the Table 1 shows that alkaloids, anthraqunones, tannins, flavonoid and phenol are present in both water and methanol extract. Although, saponins was only determined in methanol extract while steroids was absent in both extracts. The absence of steroid may be as a result of the acid hydrolysis of the functional group in the phytoconstituents, which could have been dependent upon steroid structure and site of conjugation. As explained in the methodology of this research, most of the phytochemical results in Table 1 was read based on the colour formation or disappearance as in the case of flavonoids, phenolic compound and antraquinone or formation of precipitate as in the case of alkaloid and tannins or frothing production as in the case of saponins. Thus, the colour changes observed was as a result of the fact that a reducing sugar turns to deep green or blue on the addition of Fehling’s solution because copper sulphate is reduced to copper (II) oxide in the presence of Fehling’s reagent. However, non-reducing sugar does not covert copper sulphate. The result in this study corresponds to the findings of Theophilus et al. (2013). But Makanjuola et al. (2013) reported the absent of alkanoid in both aqueous and alcohol extracts, although they agreed with the presence of some of the extracts such as tanins, saponins and the absence of steroid. Bukar et al. (2010) also reported the absence of alkanoid in M. oleifera extract. This could be as a result of different climatic environment at which it was planted or the physiological and its maturity at the stage of harvesting (Taylor and Van Staden, 2001).
Apart from determination of nutritional value of the plant, Schneider and Wolfhing (2004) has reported the therapeutic effect of some phytochemical constituent such as tanins, cardiac glycoside against cardiovascular disease and digestive problems. It is important also to note that phytochemical constituent is an important factor that determines the antimicrobial properties of the leave extract. This explain why medicinal plants are used as antimicrobial drugs, several authors have linked the presence of bioactive compound to the antimicrobial properties of the plant extract (Afolabi et al., 2007).
Table 2 shows the result of different level of susceptibility of the bacterial to both aqueous and methanol extract of M. oleifera extract. It was observed that the test organisms were more susceptible at higher concentration. The extract showed a great concentration dependent inhibition, as it was observed that at concentration of 1:1 of the aqueous extract, there was no inhibition for the three isolate as the concentration increased, that is, 1:2 inhibition was spotted. This was because the quantity of extract’s constituent required to inhibit their growth was not enough at the lower concentration of 1:1.
It could be perceived that methanol extract was more effective against the test organism as compared to the aqueous extract. This could be because, water is considered to have dipole molecules and a high dielectric constant, while methanol though classified as a polar solvent, is not as polar as water. This means methanol has a better dissolving capability than water. This observation is in agreement with the study of Moyo et al. (2012). They suggested that water extracts differ from other solvent because it has numerous compounds that may interact antagonistically in their overall activities.
The result revealed no inhibition for water extract at 1:1 concentration but methanol extract had 9 mm for both E. coli and S. pneumonia and 8 mm for S. aureus for the same 1:1 concentration. Voravuthikunchai et al. (2004) and Parekh et al. (2005) also discovered that alcohol extracts showed greater activity than the water extracts and implied that antimicrobial component resided more in methanol concentrations.
Table 3 revealed the MIC and MBC result of M. oliefera aqueous and methanol extract, the result proposed that Moringa leave extract was more active against Gram negative bacteria (e.g. E. coli) than Gram positive bacteria (e.g. S. pneumonia and S. aureus). It could be noticed from the table that there is no MBC for the water extract used against Gram positive bacterial in this study. It could be that the toxin produced by the plant is more active against Gram negative than Gram positives. The result obtained by Moyo et al. (2012) also agreed with this study. Moyo et al. (2012) suggested that the difference in the bacterial species was responsible for this discovery. However, most research findings disagreed with this (Aiyegoro et al., 2008; Bousaade et al., 2008; Ashafa and Afolayan, 2009). Their results was best explained with the nature of the cell well of a bacterial, which gives room to Gram positive bacterial to be more susceptible to antimicrobial compounds as compared to the Gram negatives which have a thick peptidoglycan cell well.
Methanol extract of M. oleifera leaves have a potential antibacterial activity against bacterial pathogens and to cure infections caused by bacterial such as E. coli, S. aureus and S. pneumonia. Nevertheless, the result in this study could also serve as a basis for more investigations on the plant, MoringaM. oleifera and its antimicrobial capabilities.
The authors have not declared any conflict of interest
REFERENCES
|
Abalaka ME, Daniyan SY, Oyeleke SB, Adeyemo SO (2012). The antibacterial evaluation of Moringa oleifera leaf extract on selected bacterial pathogens. J. Microbiol. Res. 2(2):1-4
Crossref
|
|
|
|
Afolabi CA, Ibukun EO, Afor E, Obuofor EM, Farombi EO (2007). Phytochemical constituent and antioxidant activity of extracts from the leaves of Ocimum gratissimum. Sci. Res. Essay 2(5):163-166
|
|
|
|
|
Afolabi FE (2007). Chemical composition and antibacterial activity of Gongrenema latifolium. J. Zhejilang Univ. Sci. 8(5):352-358.
Crossref
|
|
|
|
|
Aiyegoro OA, Akinpelu DA, Afolayan AJ, Okoh AI (2008). Antibacterial activities of crude stem bark extracts of Distemonathus benthamianus Baill. J. Biol. Sci. 8(2):356-361.
Crossref
|
|
|
|
|
Akinyemi KO, Oladapo O, Okwara CE, Ibe CC, Fasure KA (2005). Screening of crude extracts of six medicinal plants used in South-west Nigerian unorthodox medicine for antimethicillin resistant S. aureus activity. BMC Complement. Altern. Med. 5:6-8.
Crossref
|
|
|
|
|
Anwar F, Sajib L, Muhammed A, Anwarul HG (2007). Moringa oleifera, a food plant with multiple medicinal uses. Phytol. Res. 21:17-25
Crossref
|
|
|
|
|
Ashafa AOT, Afolayan AJ (2009). Screening the root extract from Biden pilosa L. Var. radiate (Asteraceae) for antimicrobial potentials. J. Med. Plant Res. 3(8):568-572.
|
|
|
|
|
Boussaada O, Ammar S, Saidana D, Chriaa J, Chraif J, Daami M, Helel AN, Mighri Z (2008). Chemical composition and antimicrobial activity of volatile components from captitula and aerial parts of Rhaponticum acaule DC growing wild in Tunisia. Microbial. Res. 163:87-95.
Crossref
|
|
|
|
|
Brisibe EA, Umoren UE, Brisibe F, Magalhaes PM, Ferreira JFS, Luthria D, Wu X, Prior RL (2009). Nutritional characterization and antioxidant capacity of different tissues of Artemisia annua L. Food Chem. 115:1240-1246.
Crossref
|
|
|
|
|
Bukar A, Uba A, Oyeyi TI (2010). Antimicrobial profile of extract Lam extract against some food-borne microorganisms. Bayero J. Pure Appl. Sci. 3(1):43-48.
|
|
|
|
|
Kawo AH (2007). Water purification potentials and in vivo toxicity evaluation of the aqueous and petroleum either of catotropisprocera (Ait.F) Ait.F Latex and Moringa oleifera lam seed powder. PhD thesis, Microbiology Unit, Department of Biological sciences, Bayero University Kano. P 184.
|
|
|
|
|
Mahalingam R, Bharathidasan R, Ambikapathy V, Panneerselvam A (2011). Study on antibacterial activity of some medicinal plant against human pathogenic microorganism. Asian J. Plant Sci. Res. 1(3):86-90.
|
|
|
|
|
Mahesh B, Satish S (2008). Antimicrobial activity of some important medicinal plant against plant and human pathogens.World j. Agric. Sci. 4(5):839-843.
|
|
|
|
|
Makanjuola OO, Dada EO Ekundayo FO (2013). Antibacterial activities of Moringa oleifera (Lam) on coliforms isolated from some surface water in Akure Nigeria. FUTA J. Res. Sci. (1):63-71.
|
|
|
|
|
Moyo B, Masika PJ, Muchenje V (2012). Antimicrobial activities of Moringa oleifera lam Leaf extract. Afr. J. Biotechnol. 11(11):2797-2802.
Crossref
|
|
|
|
|
Parekh J, Chanda SV (2007). In vitro antimicrobial activity and phytochemical analysis of some Indian medicinal plants. Turk. J. Biol. 31:53-58.
|
|
|
|
|
Parekh J, Jadeja D, Chanda S (2005). Efficacy of aqueous and methanol extracts of some medicinal plants for potential antibacterial activity. Turk. J. Biol. 29:203-210.
|
|
|
|
|
Preethi RM Devanathan VV, Longanathan M (2010). Antimicrobial and antioxidant efficacy of some medicinal plant against food borne pathogens. Adv. Biol. Res. 4:122-125.
|
|
|
|
|
Schneider G, Wolfhing J (2004). Sherriesmedical microbiology. An introduction to infectious disease 4th ed. pp. 261-273.
|
|
|
|
|
Taylor JLS Van Staden J (2001). The effect of age season and growth conditions on anti-inflammatory activity in Eucomisautumnalis(mill). Chitt.plant extracts. Plant Growth Regul. 34(1):39-47.
Crossref
|
|
|
|
|
Theophilus UO, Omojate GC Anowi CF (2013). Phytochemical screening and investigation of antibacterial activities of various fractions of the ethanol leaves extract of Moringa oleifera Lam (Moringaceae). J. Pharm. Chem. Biol. Sci. 3(3):962-973.
|
|
|
|
|
Thilza IB, Sanni S, Zakari AI, Sanni FS, Muhammed T, Musa BJ (2010). In vito antimicrbial activity of water extract of Moringao leifera leaf stalk on bacteria normally implicated in eye diseaces. Acad. Arena. 2(6):80-82.
|
|
|
|
|
Voravuthikunchai S, Lortheeranuwat A, Jeeju W, Sririrak T, Phongpaichit S, Supawita T (2004). Effective medicinal plants against Enterohaemorrhagic Escherichia coli O157,H7. J. Ethanopharmacol. 94:49-54.
Crossref
|
|