ABSTRACT
Malva neglecta, a wild plant that grows in different parts of Lebanon, was noted by residents to have soothing effects if taken during episodes of respiratory tract infections. This study was designed to test for the ability of this plant to inhibit bacterial growth and biofilm formation of clinical bacterial isolates. The results showed that while the aqueous extract of the leaves of the plant did not show any antibacterial effect on the tested bacterial isolates, the methanol extract clearly demonstrated an ability to inhibit the growth of the isolates tested. The agar dilution method revealed that the lower concentrations of the methanol extract of M. neglecta inhibited some isolates, but the inhibition was noted to increase with an increase in the concentration of the extract until at a ratio of 0.3 (volume of extract to volume of the agar medium), the growth of all the tested organisms was completely inhibited. The methanol extract of the plant was also capable of inhibiting the formation of biofilms by many of the clinical isolates tested. The active component in the M. neglecta if identified, purified and proved safe for human consumption, may prove to be a new effective antibacterial agent.
Key words: Antibacterial agents, ethnobotany, biofilms, Malva neglecta, medicinal plants, plant extracts.
The advent of antimicrobial agents was probably the most important achievement in modern medicine. The lives of millions were saved as these were put in use to treat very acute and difficult infections like those following surgery and organ transplantation or those affecting preterm babies or the elderly. Some antimicrobial agents were even effective as cancer chemotherapeutic agents. However, these agents were abused and resulted in the emergence of drug resistant, multidrug resistant and exceptionally drug resistant organisms (Alanis, 2005; Kapil, 2005). Many infections started to become very hard to treat due to the spread of these aggressive pathogens. Thus, antimicrobial resistance, in addition to increasing the mortality rate of patient, increased healthcare expenses and threatened the achievement of the development goals even in advanced societies. In short, antimicrobial resistance, nowadays, is probably the most serious threat to global health.
What magnified the problem more were the reports that bacterial biofilms played a major role in initiating more than three quarters of microbial infections in the human body (O'Toole et al., 2000), including otitis media, osteomyelitis, native valve endocarditis, cystic fibrosis pneumonia as well as urinary tract infections (Costerton et al., 1999). A biofilm, which is a buildup of microorganisms in mainly, a polysaccharide environment (O'Toole et al., 2000; Sutherland, 2001), was shown to be involved in the change of organisms from a planktonic to a sessile mode of growth. Accordingly, the biofilm forming organisms were protected from any harsh surrounding conditions (Costerton et al., 1999), including exposure to antimicrobial agents. Mature biofilms (> 7 days) were demonstrated to be notably resistant to 500 to 5,000 times the concentrations of these agents than were necessary to kill free floating (planktonic) cells of the same organism (Khoury et al., 1992). Common biofilm formers are numerous and include Escherichia coli, Staphylococcus aureus, Streptococcus pyogenes, Klebsiella pneumonia, Proteus mirabilis and Pseudomonas aeruginosa (Donlan, 2001).
Finding new methods to combat these organisms including searching for new antimicrobial drugs including antibiotics has been a major concern to all public health authorities. Ethno-pharmacologists, botanists, microbiologists and natural-product chemists, have not seize to work on plants to find new phytochemicals that may serve as a solution for this wide spread and still magnifying threat (Ncube et al., 2008; Laxminarayan et al., 2013; WHO, 2016).
Traditional and even modern medicine depends fully or in part on medicinal plants that were and still are a very important bio-resource of drugs. Historically, many of these plants were used by regular people over time and were recognized for their positive effect (Neube et al., 2008). As reports indicated that the organic solvent extracts of both the leaves and flowers of other species of the same genus (Malva sylvestris and Malva moschata), had high bactericidal activity, anti-ulcerogenic and anti-inflammatory effects in patients when used for respiratory tract, GI tract and skin infections (Kumarasamy et al., 2002; ÇakılcıoÄŸlu et al., 2010; Razav et al., 2011; Mohammad Eini et al., 2014; Mirghiasi et al. 2015), the present study was performed using the plant, Malva neglecta, commonly used, in the Northern Bekaa region and other rural regions of Lebanon, as a medicinal plant for treatment of respiratory tract infection
M. neglecta is a wild plant that is also known as the dwarf mallow or common mallow and belongs to the mallow family – Malvaceae. Its flowers are regular (actinomorphic), 1.5-2.5 cm wide with 5 petals that are white, pinkish or red-veined with notched tips. The leaves of M. neglecta are alternate, long-stalked, stipulate and blade-kidney shaped (Nature Gate, 2017).
M. neglecta was also reported to have many additional benefits. It demonstrated anti-ulcerogenic and anti-inflammatory activities in patients with osteoarthritis, and was used as an expectorant, laxative and for the maturation of abscesses, stomach ache, menstrual disorders, abdominal pain, sore throat, vaginitis and constipation (ÇakılcıoÄŸlu et al., 2010; Mirghiasi et al., 2015).
This study aimed at determining whether the leaves of M. neglecta, had antibacterial effects and/or had the ability to inhibit the formation of biofilms by different pathogenic bacteria.
Plant used
Fresh samples of the leaves of the plant, M. neglecta, which grows in the wild, were obtained from the Deir El Ahmar region of the Governorate of Bekaa in Eastern Lebanon, identified by our reference botanist and then immediately sent to the microbiology laboratory for processing.
Bacterial isolates
The bacterial isolates used in the study were clinical isolates courteously provided by the Clinical Microbiology Laboratory of the Lebanese American University Medical Center- Rizk Hospital (LAUMC- RH). Specifically, the isolates used were the following: 3 isolates of Pseudomonas aeruginosa (designated in the study as isolates 1, 2 and 3) and one isolate of each of the following organisms: Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis, Staphylococcus aureus and Streptococcus pyogenes. The identity of the isolates was confirmed by Gram staining and standard tests (Jorgensen and Pfaller, 2015) were performed. For the definitive identification of the Gram negative bacilli, API 20E kits ((Biomerieux-France) were used.
Aqueous extraction and testing for anti-bacterial activity
Preparation of the aqueous extract
The fresh M. neglecta leaves were boiled in water until the liquid became thick. A portion of the extract was autoclaved while another was used without autoclaving.
Testing for anti-bacterial activity of the aqueous extract
The antibacterial effect of the aqueous extracts was performed by using the disc agar diffusion and the well agar diffusion methods. The recommended Mueller-Hinton agar (MHA) was prepared and used in both methods as recommended (CLSI, 2014).
The disc agar diffusion method: The MHA plates were seeded with a 0.5 McFarland equivalent turbidity of the fresh test organisms (S. aureus, E. coli and P. aeruginosa isolate 1). The discs used were filter paper discs soaked with the aqueous extract (20 µl of the extract were added to each disc) and were inserted on the surface of the agar as recommended (CLSI, 2014). The plates were incubated at 35°C for 24 h after which the appearance of zones of inhibition around the discs were noted and measured (when present). The test for each organism was done using the autoclaved and the unautoclaved aqueous extract.
The well agar diffusion method: The MHA plates were seeded with a 0.5 McFarland equivalent turbidity of the fresh test organisms (S. aureus, E. coli and P. aeruginosa isolate 1). Using a cup borer, an 8.5 mm well in the middle of the plate was filled with 100 µl of the extract (Perez et al., 1990). The plates were then incubated at 35°C for 24 h after which the appearance of zones of inhibition around the wells were noted and measured (when present). The test for each organism was done using the autoclaved and the unautoclaved aqueous extract.
Methanol extraction and testing for anti-bacterial activity
Preparation of the methanol extract
The fresh M. neglecta leaves were chopped and mixed with 80% methanol in a blender. The extract was then transferred to an Erlenmeyer flask and kept in an orbital shaker for 1 week, after which the extract was filtered using vacuum filtration and was ready for use.
Testing for anti-bacterial activity of the methanol extract
The antibacterial activity of the methanol extract was tested using the agar dilution method (Ncube et al., 2008). Trypticase soy agar (TSA) plates were prepared and autoclaved as recommended by the manufacturer. A portion of the agar was poured into the first half of a bi-Petri dish (to be used as control) in each plate that will be used in the experiment. The Erlenmeyer flask containing the remaining melted TSA agar was then put in a water bath at 70°C, and a volume of M. neglecta methanol extract was added to the agar to obtain a ratio of 0.14 of the methanol extract volume to the volume of the TSA agar. The procedure was then repeated twice to prepare TSA agar media containing a ratio of methanol extract volume to that of TSA agar of 0.16 and 0.30, respectively. The melted agars were kept in the water bath (at 70°C) for 15 min to allow for the evaporation of the methanol, after which each of the 3 melted agars were poured in the second half of each of the bi-petri dishes that were used in the testing of the antibacterial effect of each of the different organisms used in the study.
After the turbidity of each of the tested organisms (P. aeruginosa, isolates 1, 2 and 3, E. coli, K. pneumoniae, P. mirabilis, S. aureus and S. pyogenes) was adjusted to that of a 0.5 McFarland standard (in saline), the prepared bi-plates were each seeded by the test organisms (individually) using different sterile swabs, for each part of each plate, incubated at 35°C for 24 h and then checked for the growth of the organisms on the surface.
Effect of the methanol extract on biofilm formation
Preparation of the bacterial isolates
From fresh agar plates, each of the test organisms was used to inoculate a 10 ml trypticase soy broth (TSB) tube with 1% glucose. The inoculated TSB tubes were left in the incubator at 37°C for 24 h after which, the culture tubes were diluted 100 times with fresh media.
Effect of the extracts on biofilm formation
To test for the formation of biofilms by the isolates and possible inhibition of the process by the methanol extract of M. neglecta, a method very slightly modified from that used by Mathur et al. (2006) was used. The methanol extract was added to the test wells of the 96 well flat-bottom tissue culture plates and the plates were left to dry in the incubator under aseptic conditions. Upon drying, 200 µl of sterile TSB were added to the wells of the plates with 10 µl of the diluted cultures (previous section) and incubated at 35°C for 24 h. The contents of the wells were then gently discarded by repeated soft tapping, after which the wells were washed with phosphate buffered saline (PBS, pH of 7.2) several times. Then, 0.2% sodium acetate was added to fix any biofilms that may have formed and a 0.1% solution of crystal violet was finally added to stain the biofilms, when present. Excess stain was then removed with deionized water and the plates were left to dry. The optical densities were later determined by using a microplate auto-reader at 570 nm wavelength. To have a precise result, each of the test samples (and controls) was performed in 16 wells. The reported optical densities in the study were the averages of the 16 readings of each sample.
Antibacterial effect of the aqueous extract
Using both the disc agar diffusion and well agar diffusion methods, the aqueous extract of M. neglecta did not demonstrate any antibacterial effect. No zones of inhibition of growth were detected in any of the test plates for any of the 3 bacterial isolates used: E. coli, P. aeruginosa (isolate 1) and S. aureus.
Antibacterial effect of the methanol extract
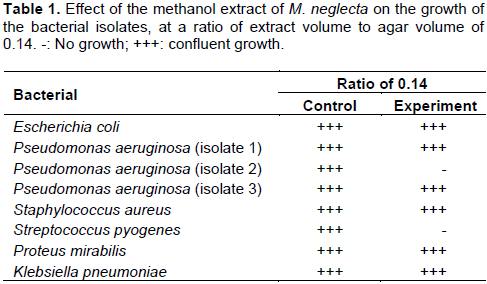
Unlike the aqueous extract, the methanol extract showed obvious antibacterial characteristics. At a ratio of extract volume to agar volume of 0.14, the extract of M. neglecta inhibited the growth of the P. aeruginosa (isolate 2) and S. pyogenes, but had no effect on the other isolates (Table 1).
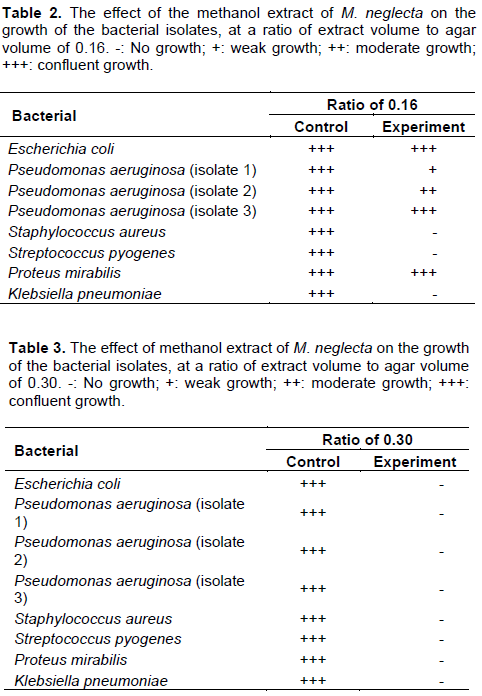
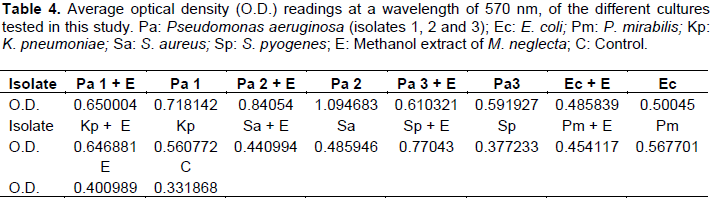
When the ratio of extract volume to agar volume was slightly increased to 0.16, the growth of S. pyogenes S. aureus and K. pneumoniae was inhibited totally, while the growth of P. aeruginosa (isolate 1) was partially inhibited. It was noted, however, that the P. aeruginosa (isolate 2) which was totally inhibited at the ratio of 0.14 (v/v) grew moderately at the slightly higher ratio of 0.16 (v/v) (Table 2). Upon raising the ratio of extract volume to agar volume to 0.3, the growth of all the tested clinical isolates was totally inhibited (Table 3).
Effect of the extracts on biofilm formation
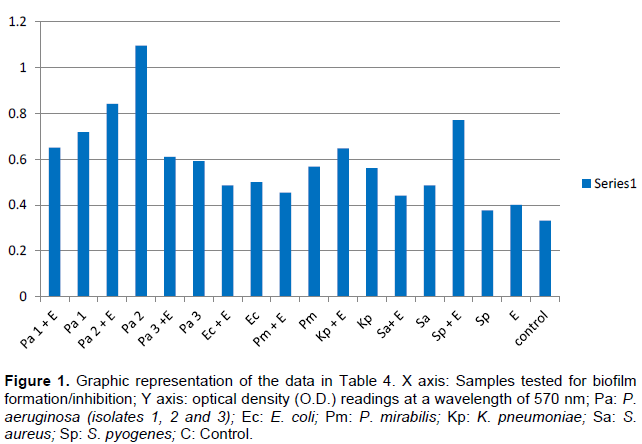
Table 4 represents the optical densities read for each of the samples tested and controls. The same results are represented graphically in Figure 1. The results clearly indicate the ability of all the chosen clinical isolates to form biofilms on the bottom of the tissue culture plates. Whereas it was clear that the M. neglecta methanol extract was not capable of inhibiting the formation of biofilms by: P. aeruginosa (isolate 3), E. coli, K. pneumoniae and S. pyogenes, it was clearly evident that it was efficient in inhibiting biofilm formation by two of the P. aeruginosa isolates (1 and 2), P. mirabilis isolate and the S. aureus isolate (Figure 1).
The inability of the aqueous extract of M. neglecta to show any effect on the growth of the 3 bacterial isolates tested (E. coli, P. aeruginosa (isolate 1) and S. aureus), may have indicated that the inhibitory bioactive compound may have been thermolabile and was destroyed during the boiling process. However, traditional healers depended primarily on aqueous extracts of the plant, although organic extracts were found to yield more consistent results regarding antimicrobial activity (Parekh et al., 2005). The result may have been caused, however, by what was noted by Silva et al. (2005), that the disk agar diffusion assay can lead to unreliable results in case mixtures containing different constituents, because of different diffusion rates. Another reason may have been not soaking the disks with the aqueous extract for hours as as suggested by Mbata et al. (2006).
A previous report by Parekh et al. (2006) noted that most of the identified antimicrobial chemicals from plants were water insoluble, a reason why extracts by organic solvents were more effective. Moreover, Imtiaz et al. (2012) found that the M. neglecta organic solvent extract was much more effective in inhibiting the growth of E. coli, K. pneumoniae, Salmonella Typhi and B. subtilis than the aqueous extract of the plant. Taking the results of these studies into consideration, methanol was selected and was a very appropriate organic solvent for this study.
The methanol extract proved to have a very notable effect on the growth of the bacterial isolates tested. This effect increased with the increase in the volume of the methanol extract added to the melted agar. Whereas, there was complete inhibition of only two isolates at a ratio of 0.14 (Table 1), there was complete inhibition of 3 isolates and partial inhibition of 2 others when the ratio was increased to 0.16 (Table 2). At a ratio of 0.3, the methanol extract completely inhibited the growth of all the clinical bacterial isolates used in this study (Table 3).
Although, at higher concentrations of the extract, the inhibition of growth was clear, yet the effect obviously started at lower concentrations. The growth of bacteria at lower concentrations does not indicate the absence of an effect as it has been verified previously (Zhanel et al., 1992) that the sub-inhibitory antimicrobial concentrations may have produced numerous effects including altering bacterial morphology and growth, affecting bacterial virulence factors and altering bacterial susceptibility to host immune defenses. However, further studies are needed to discover whether this happened in our case or if the extract exerted any effect at the molecular levels.
The use of the agar dilution method in this study proved to be more suitable for our purposes than either the agar diffusion or well diffusion methods. Mathur et al. (2006) and the European Committee for Antimicrobial susceptibility testing – EUCAST (2003) recommended the use of Muller-Hinton agar for similar tests, some researchers reported the use of nutrient agar (Meyer and Afolayan, 1995; Grierson and Afolayan, 1999). TSA was chosen for use in this study as it proved to be very suitable for the growth of the test isolates, with an inoculum density equivalent to the 0.5 McFarland standard as recommended in the standard procedure (Costerton et al., 1999; CLSI, 2014).
Antibacterial assays showed that the methanol extracts of both the leaves and flowers of other species of the same genus, M. sylvestris had inhibitory activity against human pathogens like, E. coli, Listeria monocytogenes and Streptococcus agalactiae. A comparison between the antibacterial effects of the aerial and the root organs of M. sylvestris showed that the main effect belonged to the aerial parts and very strongly against Pasteurella multocida (Eini et al., 2014). Also, the methanol extract of the seeds of M. moschata showed antibacterial activity against certain bacteria (Kumarasamy et al., 2002).
The antibacterial effect that was demonstrated in the methanol extract of M. neglecta and not in the aqueous extract of the plant in this study, was also reported by Jasim (2006), who found out that the aqueous extract of M. neglecta did not have antibacterial activity against S. aureus, S. pneumoniae, Haemophilus influenzae and Moraxella catarrhalis, but the petroleum ether extract of the same plant inhibited the growth of these organisms.
This study is believed to be the first to demonstrate the ability of M. neglecta methanol extract to also inhibit biofilm formation of several clinically important bacterial strains (Table 4 and Figure 1). This finding is extremely important as one of the most challenging problems in antibacterial therapy remains difficult in targeting bacteria in already formed biofilms (Khoury et al., 1992).
M. neglecta, being a plant that grows in the wild, seems to be a powerful medicinal plant as it proved to have the ability to inhibit the growth and biofilm formation of many clinically significant bacteria and may be a very promising source of new antibacterial medications. Further work is still needed, however, to determine, as recommended for previous similar studies (Iwu et al., 1999), the active compounds in the plant, purify them, and evaluate if they can be used as new medications that do not cause any harm to the host or the normal microbiota.
The authors have not declared any conflict of interests.
REFERENCES
|
Alanis A (2005). Resistance to antibiotics: Are we in the post-antibiotic era?. Arch. Med. Res. 36:697-705.
Crossref
|
|
|
|
ÇakılcıoÄŸlu U, Åžengün MT, TürkoÄŸlu I (2010). An ethnobotanical survey of medicinal plants of Yazıkonak and Yurtbaşı districts of Elazığ province, Turkey. J. Med. Plants Res. 4(7):567-572.
|
|
|
|
|
Clinical and Laboratory Standards Institute (CLSI) (2014). Performance Standards for Antimicrobial Susceptibility Testing; Twenty-Fourth Informational Supplement. M100-S24. Wayne, PA: Clinical and Laboratory Standards Institute.
|
|
|
|
|
Costerton JW, Stewart PS, Greenberg EP (1999). Bacterial biofilms: a common cause of persistent infections. Science 284(5418):1318-1322.
Crossref
|
|
|
|
|
Donlan RM (2001). Biofilms and device-associated infections. Emerg. Infect. Dis. 7(2):277.
Crossref
|
|
|
|
|
EUCAST Discussion Document (2003). Determination of minimum inhi-bitory concentrations (MICs) of antibacterial agents by broth dilution. Clin. Microbiol. Infect. 9(8):1-7.
|
|
|
|
|
Grierson DS, Afolayan AJ (1999). Antibacterial activity of some indigenous plants used for the treatment of wounds in the Eastern Cape, South Africa. J. Ethnopharmacol. 66:103-106.
Crossref
|
|
|
|
|
Imtiaz B, Fozia W, Abdul Rehman A, Ullah H, Iqbal H, Wahab A, Almas M, Ahmad I (2012). Antimicrobial activity of Malva neglecta and Nasturtium microphyllum. Int. J. Res. Ayurveda Pharm. 3(5):808.
|
|
|
|
|
Iwu MW, Duncan AR, Okunji CO (1999). New antimicrobials of plant origin. In: Janick J (editor), Perspectives on new crops and new uses. ASHS Press. Alexandria. pp. 457-462.
|
|
|
|
|
Jasim TM (2006). Study of the antibacterial activity of Malva neglegta. Tikrit J. Pharm. Sci. 2(1):15-18.
|
|
|
|
|
Jorgensen JH, Pfaller MA (2015). Manual of Clinical Microbiology. Volumes 1 and 2. 11th edition. In: Carroll KC, Landry ML, Funke G, Richter SS, Warnock DW (eds,). American Society for Microbiology: ASM Press. Washington, D.C
|
|
|
|
|
Kapil A (2005). The challenge of antibiotic resistance: Need to contemplate. Indian J. Med. Res. 121(2):83-91.
|
|
|
|
|
Khoury AE, Lam K, Ellis B, Costerton JW (1992). Prevention and control of bacterial infections associated with medical devices. ASAIO J. 38(3):174-178.
Crossref
|
|
|
|
|
Kumarasamy Y, Cox PJ, Jaspars M, Nahar L, Sarker SD (2002). Screening seeds of Scottish plants for antibacterial activity. J. Ethnopharmacol. 83(1):73-77.
Crossref
|
|
|
|
|
Laxminarayan R, Duse A, Wattal C, Zaidi AK, Wertheim HF, Sumpradit N, Vlieghe E, Hara GL, Gould IM, Goossens H, Greko C, So AD, Bigdeli M, Tomson G, Ombaka E, Peralta AQ, Qamar FN, Mir F, Kariuki S, Bhutta Z, Coates A, Bergstrom R, Wright GD, Brown ED Cars O (2013). Antibiotic resistance - the need for global solutions. Lancet Infect. Dis. 13(12):1057-1098.
Crossref
|
|
|
|
|
Mathur T, Singhal S, Khan S, Upadhyay DJ, Fatma T, Rattan A (2006). Detection of biofilm formation among the clinical isolates of staphylococci: an evaluation of three different screening methods. Indian J. Med. Microbiol. 24(1):25.
Crossref
|
|
|
|
|
Mbata TI, Debiao L, Saikia A (2006). Antibacterial activity of the crude extract of Chinese Green Tea (Camellia sinensis) on Listeria minocytogenes. Afr. J. Biotechnol. 7(10):1571-1573.
|
|
|
|
|
Meyer JJM, Afolayan AJ (1995). Antibacterial activity of Helichrysum aureonitens (Asteraceae). J. Ethnopharmacol. 47:109-111.
Crossref
|
|
|
|
|
Mirghiasi SM, Akhzari M, Vassaf M, Akbari A, Baghi SM (2015). The Effect of Malva neglecta on the Reduction of Inflammatory Agents in Patients with Osteoarthritis. Mol. Biol. 4(4):135.
Crossref
|
|
|
|
|
Mohammad Eini A, shayegh J, Moharrami Fard M (2014). Comparison of Antibacterial Effect of Malva Sylvestris L (Aerial and Root Organs) by MIC. JSSU 21(6):816-822.
|
|
|
|
|
Nature Gate (2017). Dwarf Mallow: Malva neglecta. Info sheet. LuontoPortti. Available at: http://www.luontoportti.com/suomi/en/kukkakasvit/darf-mallow
|
|
|
|
|
Ncube NS, Afolayan AJ, Okoh AI (2008). Assessment techniques of antimicrobial properties of natural compounds of plant origin: current methods and future trends. Afr. J. Biotechnol. 7(12):1797-1806.
Crossref
|
|
|
|
|
O'Toole G, Kaplan HB, Kolter R (2000). Biofilm formation as microbial development. Ann. Rev. Microbiol. 54(1):49-79.
Crossref
|
|
|
|
|
Parekh J, Jadeja D, Chanda S (2005). Efficacy of aqueous and methanol extracts of some medicinal plants for potential antibacterial activity. Turk. J. Biol. 29:203-210.
|
|
|
|
|
Parekh J, Karathia N, Chanda S (2006). Screening of some traditionally used medicinal plants for potential antibacterial activity. Indian J. Pharm. Sci. 68(6):832-834.
Crossref
|
|
|
|
|
Perez C, Paul M, Bazerque P (1990). An Antibiotic assay by the agar well diffusion method. Acta. Biol. Med. Exp. 15:113-115.
|
|
|
|
|
Razav SM, Zarrini G, Molavi G, Ghasemi G (2011). Bioactivity of Malva Sylvestris L., a Medicinal Plant from Iran. Iranian J. Basic Med. Sci. 14(6):574-579.
|
|
|
|
|
Silva MTG, Simas SM, Batista TGFM, Cardarelli P, Tomassini TCB (2005). Studies on antimicrobial activity, in-vitro, of Physalis angulata L. (Solanaceae) fraction and physalin B bringing out the importance of assay determination. Memorias Instituto Oswaldo Cruz. 100(7):779-782.
Crossref
|
|
|
|
|
Sutherland IW (2001). Biofilm exopolysaccharides: a strong and sticky framework. Microbiology 147(1):3-9.
Crossref
|
|
|
|
|
World Health Organization (WHO) (2016). Antimicrobial resistance WHO Media Centre: Fact sheet. WHO. Available at: http://www.who.int/mediacentre/factsheets/fs194/n/
|
|
|
|
|
Zhanel GG, Hoban DJ, Harding GK (1992). Subinhibitory antimicrobial concentrations: a review of in vitro and in vivo data. Can. J. Infect. Dis. Med. Microbiol. 3(4):193-201.
Crossref
|
|