ABSTRACT
The content and chemical composition of the essential oil may vary in certain species according to the climatic period. The aim of this study was to evaluate the influence of seasonality on the chemical composition of the essential oil from Hyptis dilatata flowers, to perform biological activities such as antimicrobial, inhibition of acetylcholinesterase enzyme and to evaluate the toxicity of the essential oil using for the test as indicator Artemia salina. H. dilatata flowers were collected in rainy and dry periods and extracted by hydro distillation using extractor of Clevenger condenser double Spell model. Analysis of essential oil resulted in 22 chemical components. The major constituents for dry and rainy periods were α-pinene (26.2 and 10.9%), 3-Carene (12.2 and 3.7%), fenchone (17% and 14.8%) and β-cariophyllene (16.36 and 30.9%), respectively. The essential oil inhibited the acetylcholinesterase enzyme in 93.4% (rainy period) and 92.4% (dry period). Between the dry and rainy periods, the best LC50 in microbial activity in vitro was obtained in the rainy period tested in Staphylococcus aureus bacterium (LC50 49.8 mg ml-1). The cytotoxic activity of A. salina in H. dilatata essential oil proved LC50 results below of 100 μg ml-1. Therefore, the chemical characterization and testing of biological activities of essential oils showed promising results in the search for new active substances and development of bioproducts of vegetable origin.
Key words: Hyptis dilatata, α-pinene, fenchone, β-cariofilene, 3-carene.
The genus Hyptis belongs to Lamiaceae family, consisting of approximately 580 species, many of them with great ethnopharmacological importance. They are distributed mainly in tropical America, southern United States, Caribe and Argentina. In Brazil, it is mainly found in Minas Gerais, Bahia, Goiás and Amazonas states (Lima, 2010). In this family of plants, the use of essential oil is common in the population of folk medicine as coding and remedy and they have been used as antioxidant, anti-inflammatory, antihypertensive, antitumoral, gastroprotective, insecticide, antibacterial, antifungal and antiherpetic (Barros et al., 2010; Hussain et al., 2011). Some biological activities have been associated to the content of phenolic compounds, present in the plant (Zgórka and Glowniak, 2001; Valant-Vetschera et al., 2003). In addition, owing to the diversity of volatile constituents found in essential oils of various species of the Lamiaceae family, these essential oils have aroused the interest of the perfume, cosmetics, food and pharmaceutical industries (Fernandez-Alonso and Rivera-Diaz, 2002). Species of genus Hyptis are mostly aromatic with great economic potential, due to its essential oil and presents important pharmacological action, such as anesthetic, anti-inflammatory (Botrel et al., 2010). Defense mechanisms of plants can vary in distinct environmental conditions, leading, consequently, to the production of different secondary metabolites. Factors such as cultivation conditions, soil type and parts of the plant analyzed, may influence the content and chemical composition of essential oils (Botrel et al., 2010). Botrel et al. (2009) studied the chemical composition and content of the essential oils of Hyptis marrubioides Epling flowers of two genotypes, white and purple. In this research, they identified that the content of some of the major principal compounds of the white genotype, such as β-caryophyllene, γ-muurolene and caryophyllene oxide, was higher compared to the purple genotype, and also observed that the essential oil content was the highest in the inflorescence of the white genotype. Researchers like Santos et al. (2008) studied the essential oils of Hyptis pectinata leaves and identified calamusenona as the major compound, which possess antimicrobial activity. Niero and Malheiros (2009) studied the essential oils of Hyptis suaveolens leaves and identified the antimicrobial compound sabinene as major constituent (antimicrobial activity) while from Hyptis crenata, α-pinene was the majoritary one (antioxidant activity). Urones et al. (1998), isolated, from a Hyptis dilatata species collected in Veraguas (Panama), the well-known compounds carnosol, rosmanol and methyl-rosmanol. Tafurt-Garcia et al. (2014) studying the flowers of H. dilatata from Arauca (Colombia) identifield δ-3-carene, camphor, bornyl acetate, E-caryophyllene and palustrol as the principal constituents. According to Lang and Buchbauer (2012), the δ-3-carene, camphor and bornyl acetate compounds have antimicrobial activity (Lang and Buchbauer, 2012; Misharina et al., 2009), and α-humuulene, caryophyllene and fenchone have antimicrobial and antioxidant activities. H. dilatata species is a perennial sub-bush known as field mint, in Tepequem saw, where it was collected in the municipality of Amajari, Roraima State, Brazilian Amazon rainforest. This species is usually used by the population for medicinal purposes, such as intestinal problems, influenza, and as insecticide. The aim of this work was to evaluate concentration of the chemical compounds present in the essential oil of H. dilatata flowers in two seasonal periods and to realize the biological tests for antimicrobial activity, fungicide, activity with acetylcholinesterase enzyme, as well as the cytotoxic activity using Artemia salina with essential oils.
Plant and essential oil extraction
The flowers of H. dilatata were collected during the dry period (January to March) and in the rainy period (June to August) in 2015 in Paiva village at 634 m (meters above sea level), in Tepequem saw (RR 203), municipality of Amajari in Roraima State, Brazil. A voucher specimen was deposited in the INPA herbarium with registration number 263670 and another voucher specimen was deposited in the integrated museum of Roraima State (MIR 12754). The essential oil was extracted by hydrodistillation method, drag the water vapor for 2 h using extractor of Clevenger condenser double Spell model. The percentage of essential oil collected in the dry and rainy periods was measured in triplicate. The essential oil was placed in amber flask, weighed, and stored under nitrogen in a freezer until further analysis (Castro, 2006).
GC-FID analysis
The composition of the chemical constituents present in the essential oils was determined by gas chromatography after preparation of the methyl esters. The analyses were performed on a Gas Chromatograph HP7820A (Agilent). Column: HP5 30 m × 0.32 mm × 0.25 µm (Agilent). Temperature: Column: 50°C (0 min), 0°C min-1, up to 230°C. Injector: 250°C Split (1:30). Detector FID: 250°C. Vector gas: H2 at 3.0 ml min-1. Injection volume: 1 μl. Data acquisition software: EZChrom Elite Compact (Agilent). Samples diluted at 1% in chloroform.
GC-MS analysis
Identification of the chemical constituents was performed on GCMS-QP2010 ULTRA (Shimadzu) apparatus. Column: Rxi-1MS 30 m × 0.25 mm × 0.25 µm (Restek). Temperature Column: 50°C (3 min), 3°C min-1, up to 230°C. Injector: 250°C Split (1:10). Interface CG-MS a 250°C. Detector MS (electronic Impact at 70 eV) up to 250°C. Vector gas: He at 2.0 ml min-1. Injection volume: 1 μl. Samples diluted at 1% in chloroform. Data acquisition software: GCMS Solution (Shimadzu). Spectral library: NIST11.
Acetylcholinesterase (AChE) inhibition assay
Quantitative evaluation of acetylcholinesterase (AChE) inhibition activity was performed according to the methodology of Ellman (1961), modified by Rhee et al. (2001). This bioassay was performed on microplates of 96 wells. Eserine and galantamine (10 mg ml-1) were used as positive controls while the negative control was performed without inhibitor. In each well were added 25 μl of acetylcholine iodide (15 mM); 125 μl of 5.5'-dithiobis (2-nitro benzoic acid) (DTNB); 50 μl of tris-HCl pH 8 0.1% w/v buffer of bovine serum albumin and 25 μl of extract (10 mg ml-1) solubilized in Tween/DMSO (30:70). The tests were performed in triplicate. The plates were read nine times at 405 nm over a period of 10 min. Immediately after the first reading, 25 μl of acetylcholinesterase enzyme (Electrophorus electricus, Sigma Aldrich) (0.222 U mL-1) was added and nine readings were performed over a period of 10 min at 405 nm. The interference of spontaneous hydrolysis of the substrate was corrected from the subtraction of the average of the absorbance measured before the addition of the enzyme. The percentage inhibition of the enzyme was calculated from the following mathematical formula:
% inhibition = [(C- A) / C × 100]
where C = control containing enzyme and substrate; A = assay containing the extract, enzyme and substrate.
Toxicity on A. salina
The toxicity on A. salina was carried out through the methodology adapted from Meyer et al. (1982). Artificial saline solution (40 g of coarse salt in 1 L of distilled water) was added in an aquarium that was used as an incubator. The pH of this solution was adjusted between 8 and 9 with sodium carbonate (Na2CO3 at 10%). The incubator was divided into two environments: an uncovered environment was artificially illuminated with a fluorescent lamp with aeration and the other environment was placed, approximately 100 mg of A. salina eggs, and covered with aluminum foil so that organisms at born remained isolated due to the difference in illumination, during an incubation period of 48 h. After hatching, the A. saline eggs were added in each test tube, 10 nauplii, containing serial concentrations (1000, 500, 250, 125, 62.5, 31.25 and 15.625 μl ml-1). After a period of 24 h, the number of live and dead nauplii in each test tube was counted, using macroscopic visualization. The tests were done, in triplicate, for each concentration. As a positive control, DMSO and also saline water were used without the essential oil. The probability of mortality was calculated using the Abbot formula:
M (%) = Amount of dead organisms / Total number of organisms in the tube × 100
A calibration curve was used to obtain the LC50 through the toxicity assessment test. Low toxicity was considered when the LC50 is greater than 500 μg ml-1; moderate when the LC50 was between 100 and 500 μg ml-1 and very toxic when the LC50 was less than 100 μg ml-1 (Amarante, 2010).
Antibacterial and yeast assay
The microorganisms used in the tests were two Gram-negative bacteria: Salmonella typhimurium (ATCC 13311) and Citrobacter freundii (ATCC8090), two gram-positive bacteria: Staphylococcus aureus (ATCC 25923) and Bacillus cereus (ATCC 11778) and a fungus (yeast) Candida albicans (ATCC 18804). The concentrations of essential oils in the tests were: 250, 125, 62.5, 31.25, 15.62, 7.81, 3.90 and 1.95 μg ml-1 (Zacchino and Gupta, 2007). The samples were weighed, dissolved in DMSO (500 mg ml-1) and added to Brain Heart Infusion broth (BHI) for bacteria and Sabouraud for yeasts to produce a solution with final concentration of 20 mg ml-1. A pre-inoculum was prepared in which the bacteria and yeast were transferred with a platinum ring to test tubes containing 3 ml of BHI broth. The tubes were incubated at 37°C for 24 h. The pre-inoculum (500 μl) was transferred to tubes containing 4.5 ml of distilled water. The tubes were homogenized and the concentration adjusted to 0.5 McFarland turbidity standard (108 UFC ml-1), thus obtaining the inocula used in the bioassays. The tests were performed on 96 microwell plates, in duplicate. Two controls were performed, one to monitor the growth of the microorganism, in which there was no addition of the working solution (to verify cell viability) and another one, in which the microbial inoculum was not added (in order to eliminate the effect of extracts color of the working solution). A control plate containing 100 μl of BHI culture medium and 100 μl of sterile distilled water was added to the experiment to control the sterility of BHI culture medium. Another control was prepared, containing the standard antibiotics: ampicillin (antibacterial), miconazole and nystatin (antifungals) to observe the activity of these antibiotics on the microorganisms. The microplates were incubated in an oven at 37°C and after 24 h the Elisa plates (492 nm) were read. The results were calculated as percent inhibition using the formula:
% Inhibition = 100 - AC1 - AC2 × 100AH - AM
where AC1 = absorbance of the sample; AC2 = absorbance of control sample; AH = absorbance in the control of microorganism and AM = absorbance of the control of the culture medium.
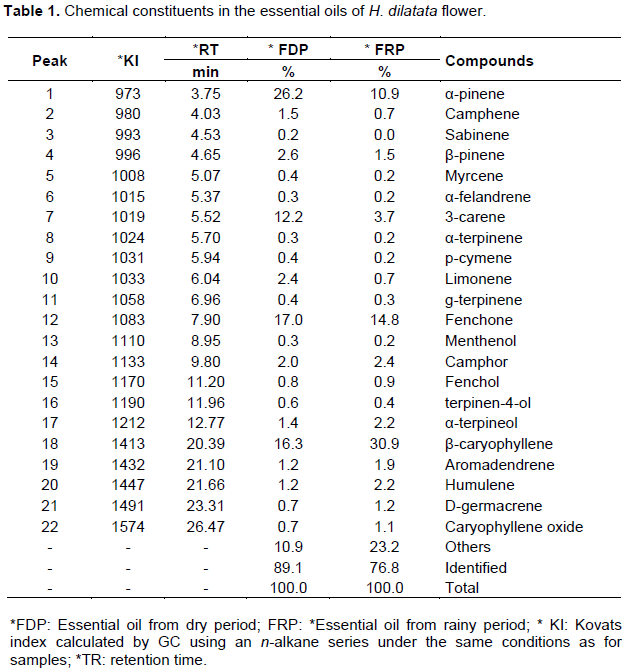
Analyses of GC-FID for quantification and GC-MS for identification of the essential oil components of H. dilatata flowers showed the presence of 22 constituents (Table 1).
The main compounds and their respective concen-trations in the essential oil of dried flowers harvested during the dry period (DP) and rainy period (RP) were α-pinene (26.2%), fenchone (17%), β-caryophyllene (16.3%), 3-carene (12.2%) for DP and α-pinene (10.9%), 3-Carene (3.7%), fenchone (14.8%), and β-caryophyllene (30.9%) for RP (Figure 1).
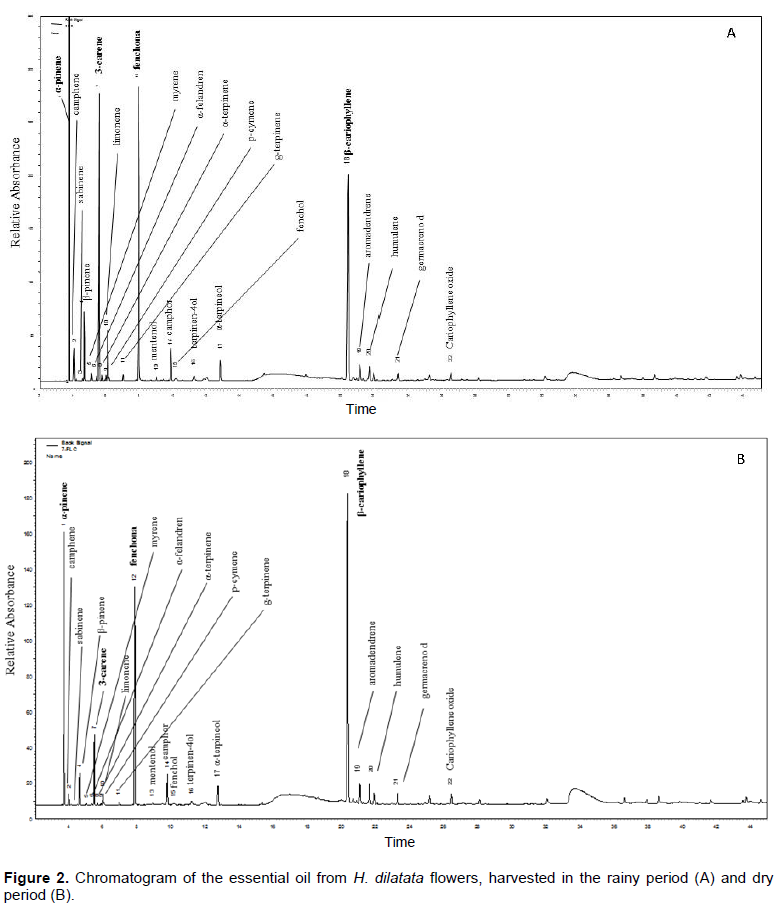
Figure 2A and B shows the chromatographic profile for H. dilatata essential oil. Twenty-two components corresponding to 89.1 and 76.8% of essential oil composition of the flower collected in the dry and rainy period, respectively, were identified. Four major compounds gave 71.7% of the essential oil collected in the dry period and 60.3% of the essential oil collected in the rainy period.
The chemical composition of the essential oils of H.dilatata was mainly constituted by monoterpenes, being fenchone, a bicyclic monoterpene compatible with literature data for volatile constituents of species of genus Hyptis (Martins et al., 2006). The values of α-pinene (0.07%) in the flowers of this species collected in Colombia (Tafurt-García et al., 2014) were much lower than those collected in the Tepequem saw in Roraima State, Brazil, according to the Table 1. In the work of Melo (2013), the 3-carene (9.5%) and camphor (16%) levels were above the levels found in the present work results of our research 3-carene (3.7%) and camphor (2.4%) ,respectively, but lower than the concentration of 3-carene (12.2%) obtained from Hyptis essential oil collected in dry period in this work. Brant et al. (2008) report that in different environments, there are differences in chemical composition of plant species due to different efficiencies in the production of active principles. They also emphasizes that the periods where there is greater production of oil usually do not match the largest production of chemical constituents.
In this research, the content of monoterpenes in essential oil of Hyptis flower presented in the dry and rainy periods were 69 and 39.5%, respectively. According to Passos et al. (2009), monoterpenes present in volatile oils have potential activities on the GABAergic neurotransmitters (gamma-aminobutyric acid), which are a good tool for the development of anxiolytic and anticonvulsant drugs.
The essential oils of H. dilatata showed high inhibition of acetylcholinesterase enzyme in the rainy (93.4%) and dry (92.4%) periods when compared with the standard drug used eserine (91.72%) and galatamine (94.36%) inhibitions. Extracts above 50% enzyme inhibition are indicated for isolation as potentially inhibitory substances of the enzyme; extracts ranging from 30 to 50% are considered moderate inhibitors and below 30% are weak inhibitors (Trevisan and Macedo, 2003; Vinutha, 2007). Inhibition of the acetylcholinesterase enzyme has demonstrated efficiency in the clinical treatment of Alzheimer's disease, which is associated with deficits of various brain neurotransmitters, such as acetylcholine, noradrenaline and serotonine. Symptomatic treatment of the disease primarily involves restoration of cholinergic function. It is suggested, therefore, that an increase in the level of acetylcholine could be useful to improve patients health (Trevisan and Macedo, 2003; Vinutha, 2007). Monoterpenes and sesquiterpenes, such as elemol, linalool and α-pinene have been reported to inhibit acetylcholinesterase enzyme (Miyazama et al., 1998; Perry et al., 2000). In the study by Miyazama and Yamafugi (2005), some bicyclic monoterpenes such as α-pinene and 3-carene showed inhibitory effect on acetylcholinesterase. The results obtained for essential oil of H. dilatata flowers in the present work, show that this oil is a potent acetylcholinesterase inhibition.
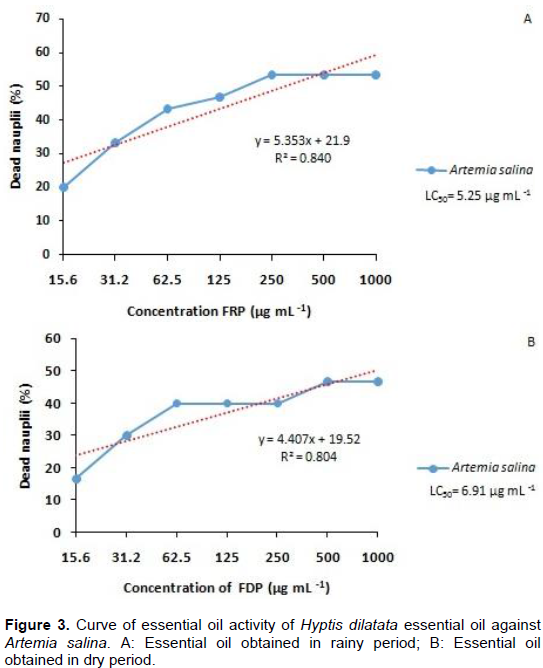
In the toxicity assay on A. salina, the samples were tested at concentrations of 1000, 500, 250, 125, 62.5, 31.25 and 15.625 μg ml-1. Survivors larval were counted after 24 h. The found for mortality percentages FRP (LC50=5.25) and FDP (LC50=6.91) are as shown in Figure 3.
According to the percentage of dead nauplii, in the lethality assay against A. salina, samples can be considered toxic when LC50 < 100 μg ml-1 (Amarante, 2010). In this assay, the control with DMSO (solvent) did not influence the results, because no larvae died in the presence of this solvent, in the same way as the control performed with salt water.
In the antimicrobial activity assay, essential oil of the H. dilatata flowers collected in both rainy and dry periods, were active against the pathogenic microorganisms. The controls used in the yeast test, showed LC50 of 7.73 μg ml-1 and the miconazole with LC50 <1.95 μg ml-1. The essential oil of the flowers collected in the dry period showed, greater effectiveness in relation to the inhibition of this microorganism by the essential oil harvested in the dry period, in the concentrations of 250 and 125 μg ml-1, with respective inhibitions of 51.9 and 59.7% as shown in Tables 2 and 3.
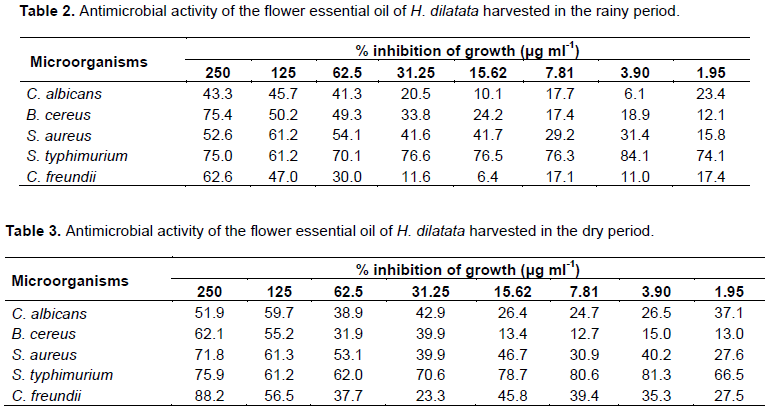
It was observed that they inhibited more than 50% at concentrations of 250 and 125 μg ml-1 both essential oils of all bacteria (Table 2). These results are very important and suggest future studies in vivo, which will contribute to a better knowledge of the potential of this species for producing bioproducts of medicinal interest. Tables 2 and 3 present the percentage of bacterial and yeast inhibition by essential oils of H. dilatata harvested in the rainy and dry period.
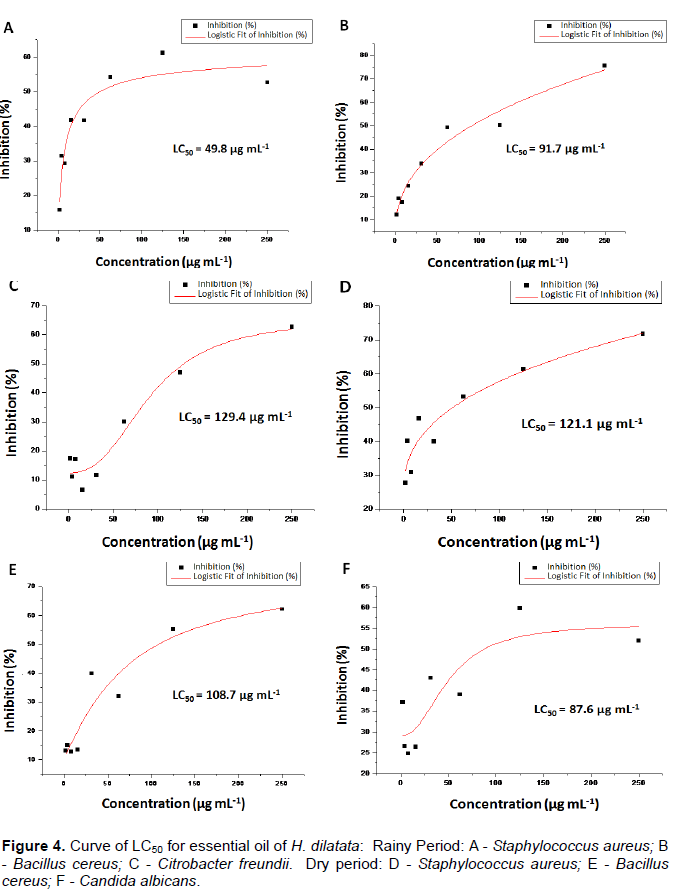
The LC50 values of the essential oils were calculated using software origin 8.0 (Figure 4). The control used in the bacterial assays was ampicillin that showed a LC50 value < 1.95 μg ml-1. Although, the inhibition of S. aureus bacterium was higher when the oil was collected during the dry period, the values of 250 μg ml-1 of LC50 were better for the essential oil collected in the rainy period with LC50 = 49.8 μg ml-1 while when harvested in the dry period furnished a LC50 = 121.1 μg ml-1.
According to Alvarez et al. (2015), a H. dilatata species, collected in San Jose Del Graviane (Colombia), presented monoterpenoid hydrocarbons represented by 2-β-pinene (12.29%), camphor (5.67%) and 1-β-pinene (4.21%) and between sesquiterpenoids and aromadendrene (6.48%), the main components. However, working with complex extracts, it is not possible to attribute the antimicrobial effects to a single component, since the major components can make a significant contribution to the biological activity. In this research, some of the chemical constituents of essential oil of H. dilatata collected in Tepequem saw, Amajari, Roraima, Brazil, in the dry and rainy periods were the same than those detected by Alvarez et al. (2015), but with different concentrations, such as β-pinene (2.6 and 1.5%), camphor (2.0 and 2.4%) and aromadendrene (1.2 and 1.9%). On the other side, there are some differences in the percentages of some major constituents such as α-pinene, 3-carene, fenchone and β-caryophillene.
Differences were observed on the components of the essential oil of H. dilatata flowers, harvested in rainy and dry periods, as well as in the biological activities towards aceticolinesterase enzyme, bacteria and yeast inhibitions in addition, the bioassays results showed that the species under study has potential to be used in the development of alternative emedicine for treatment of neurode generative diseases and against pathogenic microorganisms.
The authors have not declared any conflict of interests.
REFERENCES
|
Alvarez F, Tello E, Bauer K, Diaz LE, Rodriguez J, Jimenz C (2015). Cytotoxic and antimicrobial diterpenes isolated from Hyptis dilatata. Curr. Bioactive Compounds 11(3):189-197.
Crossref
|
|
|
|
Amarante CB (2010). Phytochemical study biomonitored by toxicity tests front of Artemia salina and antiplasmodial activity aninga stem (Montrichardia linifera). Acta Amaz. 41(3):431-434.
Crossref
|
|
|
|
Barros L, Heleno SA, Carvalho AM, Ferreira I (2010). Lamiaceae often used in portuguese folk medicine as a source of powerful antioxidants: vitamins and phenolics. LWT-Food Sci. Technol. 43:544-550.
Crossref
|
|
|
|
Botrel PP, Pinto JEBP, Figueiredo FC, Bertolucci SKV, Ferri PH (2009). Essential oil content and chemical composition in Hyptis marrubioides Epl. of different genotypes. Rev. Bras. Plantas Med. 11(2):164-169.
Crossref
|
|
|
|
Botrel PP, Pinto JEBP, Araujo ACC, Bertolucci SKV, Figueiredo FC, Ferri PH, Costa DP (2010). Variations in the content and volatile composition of Hyptis marrubioides EPL. Cultivated in the field and in greenhouse. New Chem. 33(1):33-37.
|
|
|
|
Brant RS, Pinto JEBP, Bertolucci SKV, Albuquerque CJB (2008). Water content of the essential oil of Aloysia triphylla (L'Hér.) Britton as a function of the seasonal variation. Federal University of Lavras, Department of Agriculture, Lavras-MG. Rev. Bras. Plant Med. 10(2):83-88.
|
|
|
|
Castro DP (2006). No preference of Spodoptera frugiperda (Lepidoptera Noctuidae) for essential oils of Achilleamille folium L. and Thymus vulgarius L. Braz. J. Med. Plants Botucatu. 8(4):27-32.
|
|
|
|
Ellman GL (1961). A new and rapid colorimetric determination of acetylcholinesterase activity. Bioch. Pharm. 7:88-95.
Crossref
|
|
|
|
Fernandez-Alonso JL, Rivera-Diaz O (2002). The lipped. Red book of flowering plants of Colombia. Bromeliads, lipids and laspasi floras. The lipped. Alexander von Humboldt Biological Resources Research Institute. Inst. Nat. Sci-Univ. Colombia-Bogotá 3:385-679.
|
|
|
|
Hussain AI, Anwar F, Nigam OS, Sarker SD, Moore JE, Rao, JR, Mazumdar A (2011). Antibacterial activity of some Lamiaceae essential oils using resazurin as an indicator of cell growth. LWT-Food Sci. Technol. 44:1199-1206.
|
|
|
|
Lang G, Buchbauer G (2012). A review on recent research results (2008–2010) on essential oils as antimicrobials and antifungals. A review. Flavour Fragr. J. 27:13-39.
Crossref
|
|
|
|
Lima KSB (2010). Contribution to the chemical knowledge of Hyptis carvalhoi Harley. Federal University of Ceará, Graduate Program in Chemistry. Fortaleza - CE Brazil.
|
|
|
|
Martins FT, Santos MH, Polo M, Barbosa LCA (2006). Chemical variation of the essential oil of Hyptis suaveolens (L.) Poit, under cultivation conditions. New Chem. 29:1203-1209.
|
|
|
|
Melo JLM (2013). Bioprospecting of the Antimicrobial and Anticholinesterase Activity of essential oils of species occurring in western Amazonia: Title of the project: Botanical, Chemical and Biological Evaluation of Plants. Bachelor's Degree in Biotechnology, Faculty of Biotechnology, Institute of Biological Sciences-PA. Summaries of the XXIV Scientific Initiation Seminar of UFPA: ISSN 2176-1213.
|
|
|
|
Meyer BN, Ferrigni NR, Putnam LB, Jacobsen DE, Nichols, DE, McLaughlin JL (1982). A convenient general bioassay for active plant constituents. Plant Méd. 45:31-34.
Crossref
|
|
|
|
Misharina TA, Terenina MB, Krikunova NI (2009). Antioxidant properties of essential oils. Appl. Biochem. Microbiol. 45:642-647.
Crossref
|
|
|
|
Miyazama M, Watanabe H, Umemoto K, Kameoka H (1998). Inhibition of acetylcholinesterase activity by essential oils of Mentha species. J. Agric. Food Chem. 46(9):3431-3434.
Crossref
|
|
|
|
Miyazawa M, Yamafguji C (2005). Inhibibition of acetylcholinesterase Activity by Bicyclic Monoterpenoids. J. Agric. Food Chem. 53:1765-1768.
Crossref
|
|
|
|
Niero R, Malheiros A (2009). Main chemical and biological aspects of terpenes: Chemistry of natural products, new drugs and modern pharmacognosy. Itaj. Univ. 2:259-278.
|
|
|
|
Passos CS, Arbo MD, Rates SMK, Poser LV (2009). Terpenóides with activity in the Central Nervous System (SNC). Braz. J. Pharm. 19(1A):140-149.
|
|
|
|
Perry NSL, Houghtonp J, Theobald A, Jenner P, Perry EK (2000). In-vitro inhibition of human erythrocyte acetylcholinesterase by Salvia Lavandula efolia essential oil and constituents terpenes. J. Pharm. Pharmacol. 52(7):895-902.
Crossref
|
|
|
|
Rhee IK, Van der Meen M, Ingkaninan K, Verpoorte R (2001). Screening for acetylcholinesterase inhibitors from Amararyllidaceae using silica gel thin-layer chromatography in combination with bioactivity staining. J. Chromat. A. 915:217-223.
Crossref
|
|
|
|
Santos PO, Costa MJC, Alves JAB, Nascimento PFC, Melo DLFM, Barbosa-Junior AM, Trindade RC, Blanki AF, Arrigoni-Blank MF, Alves PB, Nascimento, MPF (2008). Chemical composition and antimicrobial activity of the essential oil of Hyptis pectinata (L.) Poit. New Chem. 31(7):1648-1652.
Crossref
|
|
|
|
Tafurt-García G, Mu-oz-Acevedo A, CALVO AM, Jiménez LF, Delgado WA (2014). Volatile componentes of Eriope crassipes, Hyptis conferta, H. dilatata, H. brachiata, H. suaveolens y H.mutabilis (Lamiaceae): Volatil e compounds of analysis of Eriope crassipes, Hyptis conferta, H. dilatata, H. brachiata, H. suaveolensy, H. mutabilis (Lamiaceae). Latin Am. Caribbean Bull. Med. Aromatic Plants 13(3):254-269.
|
|
|
|
Trevisan MTS, Macedo FVV (2003). Selection of Plants with Anti-Acetylcholinesterase Activity for Treatment of Alzheimer's Disease. New Chem. Fortaleza-Brazil. 26(3):301-304.
|
|
|
|
Urones JG, Marcos IS, Diez D Cubilla LR (1998). Tricyclic diterpenes from Hyptis dilatata. Phytochemistry 48(6):1035-1038.
Crossref
|
|
|
|
Zacchino AS, Gupta MP (2007). Manual of in vitro techniques for the detection of antifungal compounds. Corpus Editorial and Distributor: Rosario. 85.
|
|
|
|
Zgórka G, Glowniak K (2001). Variation of free phenolic acids in medicinal plants belonging to the Lamiaceae family. J. Pharm. Biom. 26:79-87.
Crossref
|
|
|
|
Valant-Vetschera KM, Roitman JN, Wollenweber E (2003). Chemo diversity of exudate flavonoids in some members of the Lamiaceae. Biochem. Syst. Ecol. 31:1279-1289.
Crossref
|
|
|
|
Vinutha B (2007). Screening of Selected Indian Medicinal Plants for Acetylcholinesterase Inhibitory Activity. J. Ethnopharm. 109(2):359-363.
Crossref
|