ABSTRACT
Gastro-duodenal ulcers are diseases that constitute a major public health problem all over the world and particularly in Côte d'Ivoire. The aim of this study was to evaluate the acute toxicity and gastric anti-ulcer activity of an aqueous extract of the leaves of Macaranga barteri (AEMb). Acute toxicity was carried out using the Organization for Economic Co-operation and Development (OECD) Guidelines 420. The anti-ulcer activity of AEMb was evaluated using four models of gastric ulcer induction which are HCl/ethanol solution, ibuprofen solution, pylorus ligation and cold restraint stress in rats. The parameters assessed were mucus production, ulcer surface, ulcer index, pH, acid concentration and volume of gastric contents. Cimetidine, aluminium hydroxide and ranitidine were used as anti-ulcer standard drugs. The results of this preventive gastric anti-ulcer study revealed that for doses ranging from 62.5 to 500 mg/kg body weight (b.w), AEMb dose dependently prevented gastric lesion formation (p<0.001) in the four models. The inhibition values were 98.96, 94.11, 90.73 and 96.89% on ulcerations induced respectively by HCl/ethanol, ibuprofen, pylorus ligation and cold restraint stress at the dose of 500 mg/kg b.w. This cytoprotective action was accompanied by a significant increase in gastric mucus production. These results suggested that the preventive anti-ulcer activity of AEMb may be due to a cytoprotective effect. The median acute toxicity LD50 value of AEMb was higher than 5000 mg/kg b.w. This extract was classified as nontoxic in the Globally Harmonized System of Classification and Labelling of Chemicals (GHS). Phytochemical compounds such as polyphenols, saponins, alkaloids, sterols and polyterpenes found out in AEMb could be responsible for its effects. In conclusion, the antigastric ulcer and the non-toxic effects of the aqueous extract of M. barteri could justify its use in traditional medicine for the treatment of gastro-duodenal ulcers.
Key words: Acute toxicity, gastric ulcer, rat, Macaranga barteri.
Gastric ulcers are heterogeneous disorders, which are manifested as a rupture in the wall of the gastric mucosa bathed by acid and pepsin (Harold et al., 2007). Gastric ulcers occur when there is a lack of balance between aggressive factors like acid, pepsin and local mucosal defense factors such as bicarbonate, mucus secretion and synthesis of prostaglandins (Harold et al., 2007). Gastric ulcers are mainly caused by Helicobacter pylori infection in 75 to 80% of the cases (Brown, 2000). However, non-steroidal anti-inflammatory drugs (Yeomans et al., 2009), alcohol and tobacco consumption can also trigger gastric ulcer (Soberg et al., 2010). In Côte d'Ivoire, epidemiological studies on gastro-duodenal ulcer diseases showed prevalence of up to 7% in 1999 (Kadjo et al., 1999) and 19.3% in 2016 (Soro et al., 2016). Many treatments are proposed by modern medicine for the eradication of gastric ulcers. Although, these medications are useful and effective, their success is overshadowed by their various side effects. Treatments are also too costly for populations, especially those living in developing countries. In addition, health centers are sometimes non-existent or too far (Keïta, 1990). Because of these problems, many people use herbs for their health care particularly in the treatment of gastric ulcers. However, the World Health Organization (WHO) recommends medicinal plants that scientific evidence is established for their harmless and therapeutic efficacy (OMS, 2002). Thus, according to WHO recommendations, Macaranga barteri (Euphorbiaceae) was chosen. M. barteri is usually a shrub or small thorny tree about 20 m high and 1.30 m in girth, sometimes with adventitious roots.
The leaves are simple, alternate, obovate oblong or obovate elliptic. The flowers are very small and are grouped along the axils in small glomerules. The fruits are small capsules, irregularly ovoid or globose (Burkill, 1985). M. barteri has several ethnobotanical uses. In Nigeria, the plant is used as a vermifuge and febrifuge. It is also used to cure coughs and bronchitis (Adesegun et al., 2007). In Sierra Leone and Cote d’Ivoire, the leaves are employed to treat gonorrhea, gastrointestinal ulcers, stomatitis, amnesia and anemia. A decoction of the bark is used against dysentery. It is chewed as an aperitif. It is also useful in the management of swelling, bruising, boils and headaches (Bouquet and Debray, 1974, Oliver-Bever, 1986). An ethnobotanical survey done by Gbadamosi and Erinoso (2015) revealed the use of M. barteri in the treatment of breast cancers. The choice of this plant was influenced by the fact that its traditional use to cure gastrointestinal ulcers is mentioned in many African Pharmacopoeia as stated by authors quoted above. Moreover, no scientific research work has been done concerning its anti-ulcer potential and the toxicity. Hence, the present study was undertaken to evaluate the acute toxicity, the preventive anti-ulcerogenic potential of the aqueous extract of M. barteri using rats models and also to screen the presence of phytochemical constituents which could be responsible for the pharmacological effects.
Plant material
Fresh leaves of M. barteri were collected from the forest of Nangui ¶Abrogoua University (Abidjan, Côte d'Ivoire) in August 2016. Taxonomic identification of these leaves was established by botanists from the National Floristic Center of Felix Houphouët Boigny University of Cocody-Abidjan, Côte d'Ivoire, with voucher no. 14735 in April 06, 1979 in Côte d'Ivoire National Herbarium and authentication was done by botanists of Nangui Abrogoua University (Abidjan, Côte d'Ivoire).
Animals
Albino Wistar rats of either sex weighing between 185 and 203 g of approximately 12 to 16 weeks old, were selected for gastric antiulcer experiments, those used for acute oral toxicity were seven weeks old and weighed between 120 and 125g. They were bred in the Animal House of Physiology, Pharmacology and Pharmacopeia Laboratory of the University of Nangui Abrogoua (Abidjan, Côte d’Ivoire) according to the principles for the care and use of laboratory animals of the Ethical Committee of the University (Nangui Abrogoua, Abidjan, Côte d’Ivoire). They were exposed to 12 h dark/light cycle and given standard food for rats and water ad libithum. Research was conducted in accordance with the internationally accepted principles for laboratory use and care as found in the European Community Guidelines (EEC Directives of 1986 86/609/EEC).
Drugs
The following reference drugs were used: Aluminium hydroxide (Maalox®, Sanofi Aventis, France), misoprostol (Cytotec®, Pfizer, Germany), USA), ibuprofene (ibuprofen®, Sanofi Aventis, France) ranitidine (Zantac®, Bristol Myers Squibb, and Ether (VWR International-Geldenaakfebaan 464-B-3001, Leuven, Belgium). Cimetidine, HCl and ethanol were purchased from Sigma Chemical Company (Saint Louis, MO, USA).
Preparation and administration of the leaf aqueous extract of M. barteri (AEMb)
The leaves of M. barteri were dried under shade and powdered with a machine (Mark RETSCH, type SM 100, Germany). One hundred grams (100 g) of the leaf powder of M. barteri were decocted in 1 L of distilled water for 15 min, filtered (Whatman no. 3) and then stored in desiccators (Mark Fruicell, France) at 45°C for two days. The powder obtained corresponding to the aqueous extract of M. barteri (AEMb) was dissolved in distilled water before oral administration to rats in all the experiments.
Acute oral toxicity study
The experiment was performed according to OECD (2001) Guideline 420. The median acute (LD50) toxicity value of AEMb was determined in rats using the limit test to 5000 mg/kg b.w. Ten female rats randomly selected, weighing between 120 and 125 g were used. They were seven weeks old and were divided into two groups (control and treated groups) after 12 h fasting. The rats of the control group received orally, distilled water (1 ml/100 g b.w.) while those of the treated group received orally, a single dose of AEMb. After AEMb administration, rats were fasted for 3h. Observations which began 30 min after drug administration were intensified for the first 4 h and continued for 14 days. The rats were observed for changes in their skin, fur and eyes and also for some other signs such as tremors, convulsions, salivation, diarrhoea, lethargy, sleep and coma. The weight of each rat was monitored before extract administration and every week thereafter. The formula (1) below was used to calculate variations of rats weights. After AEMb administration, animals were fasted for 3 h and then observed individually at least once during the first weights of the animals were determined shortly before the extract was administered and every week thereafter. At the end of the experiment, the animals were weighed and the weight changes were calculated using the following formula (1):
Weight gain (g) = Final weight (g) - Initial weight (g) (1)
Phytochemical screening
The aqueous extract of the leaves of Macaranga barteri (AEMb) were screened for the presence of polyphenols, tannins, flavonoids, saponins, alkaloids, sterols and ployterpenes, coumarines and quinones. The tests were carried out as described by Bekro et al. (2007). This experiment was repeated three times (n=3).¶
Anti-ulcer studies
The negative control group (Control 1) is the same for all the models. It is composed of 6 rats which received orally distilled water (1 ml/100 g b.w). It is considered as Group 1 in all ulcer-induced models.
Gastric lesions induced by a necrotizing agent (HCl/ethanol)
The method described by Hara and Okabe (1985) was used to induce gastric mucosal lesions. The animals were divided into 7 groups of 6 animals each. Group 2 received 1 ml/150 g b.w. of the necrotizing solution (150 mM of HCl in 60% ethanol) (Control 2). Groups 3 and 4 (positive controls) were pretreated with cimetidine (12 mg/kg b.w.) and aluminum hydroxide (50 mg/kg b.w.), respectively. Groups 5 to 8 were pretreated with the aqueous extract (AEMb) at doses of 62.5, 125, 250 and 500 mg/kg b.w. respectively. The drugs were administered orally. After 1 h drugs administration, 1 ml/150 g b.w. of the necrotizing solution was given orally to each rat except those of negative control group. The animals were sacrificed 1 h later using an over-dose of ether and then their stomachs were removed and incised along the greater curvature. The mucus covering the gastric wall of each rat was collected and weighed. The mucosal erosion was determined and scored by measuring the area of the lesions. The sum of the areas was expressed as ulcer index (mm2). The scoring of stomach lesions was established according to the method described by Robert et al. (1983). The percentage of inhibition (I) was calculated using the following formula (2):
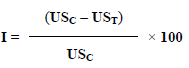
With USC = ulcer surface area in control animals and UST = ulcer surface area in treated animals.
Gastric lesions induced by Ibuprofen
The method described by Parmar and Desai (1993) was used in this study with slight modifications. Forty two (42) rats were randomly divided into seven groups of six animals each. Group 2 received ibuprofen orally (500 mg/kg b.w.) (Control 2). Groups 3 and 4, considered as positive controls, were pretreated with misoprostol (0.012 mg/kg b.w.) and aluminium hydroxide (50 mg/kg b.w.), respectively. Groups 5 to 8 were pretreated with AEMb at doses of 62.5, 125, 250 and 500 mg/kg b.w. The drugs were administered by oral route. After 30 min drug administration, each animal received orally for the first time, a dose of ibuprofen (500 mg/kg b.w.) except rats of the negative control. Fifteen hours later, the same dose of ibuprofen was given to the animals for the second time. Six hours later after the second dose, the animals were sacrificed by over-dose of ether. The stomachs were removed and excised, rinsed with normal saline and examined for ulceration. The mucus covering the gastric wall of each rat was collected and weighed. The ulcerations were scored as described by Kunchandy et al. (1985). The ulcer index and the percentage of inhibition were estimated as described above.
Pylorus-ligated rats
Seven groups of six animals each were used. Group 2 was pylorus-ligated and did not receive any solution (Control 2), while Groups 3 and 4 (positive controls) were pretreated with cimetidine (12 mg/kg b.w.) and aluminium hydroxide (50 mg/kg b.w.), respectively. Groups 5 to 8 received the aqueous extract at doses of 62.5, 125, 250 and 500 mg/kg b.w. administered orally. Pylorus ligation was made under ether anesthesia one hour after treatment except rats of Control 1. The rats were sacrificed six hours after pylorus ligation. The stomachs were removed, their contents collected and the volumes measured. One milliliter of the centrifuged gastric contents from each pylorus ligated rat was analyzed for titratable acidity against 0.01 mol/l NaOH at pH 7 using a pH meter (HANNA Instruments HI 8010, Romania). The weight of the collected mucus covering the gastric wall of each rat was determined. The ulcers produced were scored as described by Shay et al. (1945). The ulcer index, the percentage ulcerated surface and the percentage of inhibition were estimated as described above.
Hypothermic restraint stress-induced ulcers
The method described by Gupta et al. (1985) was used with slight modifications. Seven groups of 6 animals each were used. Group 2 was hypothermic restraint stress-induced ulcer without receiving a treatment (Control 2). Groups 3 and 4 (positive controls) were pretreated orally with misoprostol (0.012 mg/kg b.w.) and ranitidine (50 mg/kg b.w.), respectively. Groups 5 to 8 were pretreated with the aqueous extract at doses of 62.5, 125, 250 and 500 mg/kg b.w. One hour after the oral administration of AEMb, the rats were placed in cold water and kept for 1 h 30 min at temperature of 3 to 5°C (Gupta et al., 1985) except the rats of Group 1. The animals were then sacrificed and the stomachs were excised. The weight of the collected mucus covering the gastric wall of each rat was determined. They were examined for ulceration and the severity of intraluminal bleeding according to the scale described by Chiu et al. (1984).
Data analysis
Data are presented as means ± SEM. Comparisons between treated groups versus controls were made using ANOVA and values of p<0.05 were considered statistically significant using Graph Pad Prism 5.01 (San Diego, California, USA) software. The analysis was followed by multiple comparisons of the average values of the different parameters using the Tukey-Kramer post-hoc test, if significant differences were revealed between the tested averages.
Acute oral toxicity study
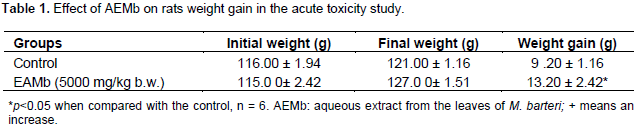
The effect of the extract on the body weight of the control and treated animals is shown in Table 1. There was significant increase (p<0.05) in the body weight of treated group as compared to the control¶. No acute toxicity after oral administration of AEMb was observed to the limiting dose of 5000 mg/kg. ¶All the animals survived till the end of the 14-day observation period. This implies that the LD50 of AEMb is higher than 5000 mg/kg b.w.
Phytochemical screening
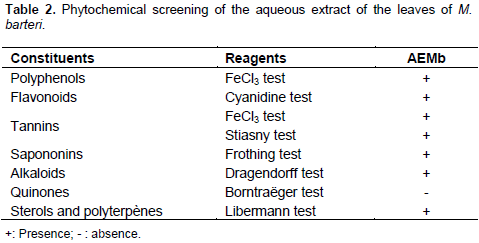
Phytochemical screening of AEMb showed positive results for polyphenols, tannins, flavonoids, saponins, alkaloids, sterols and polyterpenes. No quinones were found in the extract (Table 2).
Anti-ulcer effects of AEMb
Effects of AEMb on gastric lesions induced by a necrotizing agent (HCl/ethanol)
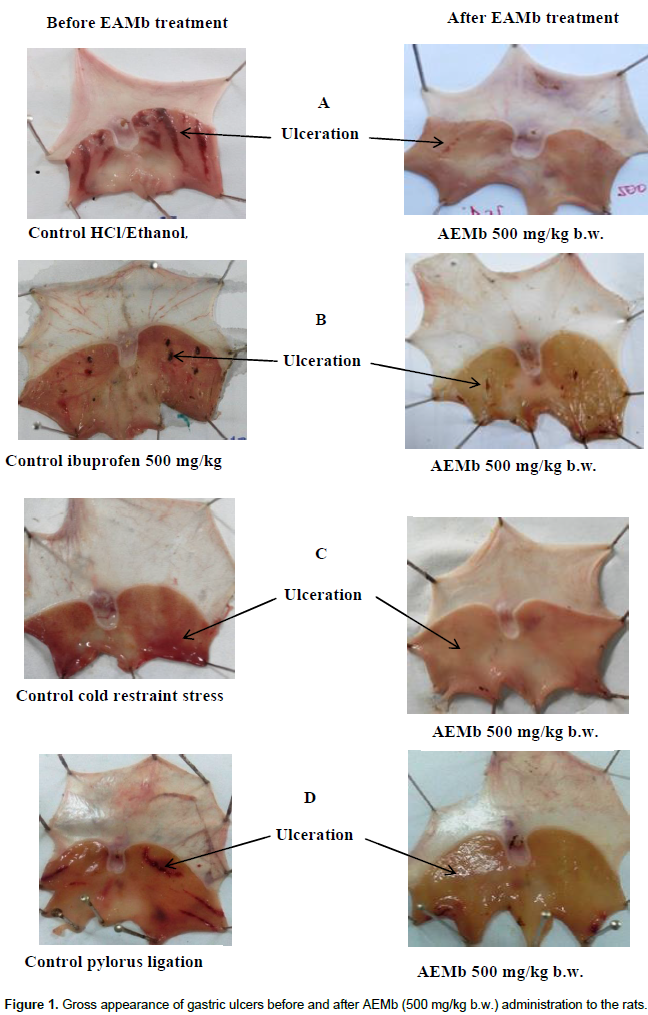
As shown in Figure 1A, HCl/ethanol produced extensive gastric lesions in rats’ glandular stomach. The leaf aqueous extract of M. barteri (62.5, 125, 250 and 500 mg/kg) dose-dependently offered a significant (p<0.001) cyto-protection to the stomach mucosa of rats against the HCl/ethanol solution from 52.46 (62.5 mg/kg) to 98.96% (500 mg/kg) corresponding to an ulcer surface area ranging from 117 ± 3.97 (62.5 mg/kg) to 2.56 ± 1.04 mm2 (500 mg/kg) as compared to the Control 2 (Table 3). The mean ulcer index score was reduced from 5.16±1.03 in Control 2 to 0.3±0.2 for the rats receiving 500 mg/kg of extract. Cimetidine (12 mg/kg) and Maalox (50 mg/kg) also significantly (p<0.001) inhibited gastric ulceration as compared to Control 2 (Table 3). The mucus produced by rats of the Control 2 group (106.7±2.19 mg) was significantly (p < 0.05) decreased as compared to those of Control 1 (148.02±1.6 mg) (Table 3). Cimetidine, Maalox and the doses of AEMb (from 62.5 to 500 mg/kg b.w.) significantly (p<0.001) and dose-dependently increased the mucus weight as compared to Control 2 (Table 3).
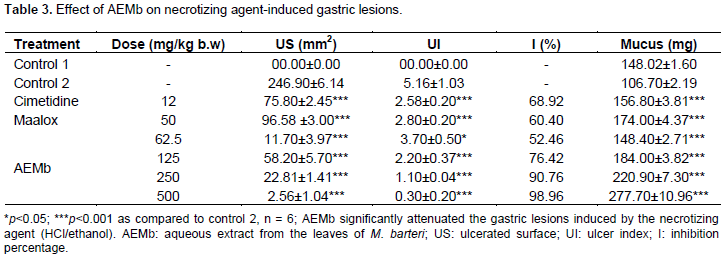
Effects of AEMb on ibuprofen induced ulcer model
Aqueous extract of M. barteri (62.5, 125, 250 and 500 mg/kg) induced significant (p<0.001) decrease in ulcer surface area (from 18.59 ± 0.37 to 1.90 ± 0.89 mm2), ulcer index (from 1.8 ± 0.3 to 0.38 ± 0.1). It elicited increase in the percentage of protection (from 42.44 to 94.12%) and an augmentation of mucus production (from 112.2 ± 2.31 to 178.6 ± 11.2 mg) as compared to Control 2 (Table 4), while oral administration of ibuprofen induced damage in the rat glandular stomach (Figure 1B). Results showed that standard drugs such as ranitidine (50 mg/kg) and misoprostol (0.012 mg/kg) also decreased significantly (p<0.001) the ulcer surface area and the ulcer index but increased the percentage of protection and mucus production as compared to Control 2 (Table 4).
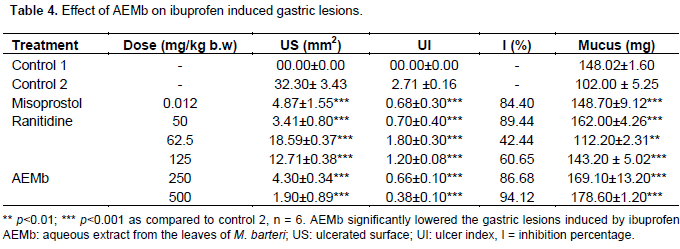
Effect of AEMb on pylorus ligation-induced gastric lesions
Figure 1C and Table 5 show the results obtained when the animals were subjected to pylorus ligation. Treatment with the aqueous extract of M. barteri at doses ranging between 62.5 and 500 mg/kg significantly (p<0.01) reduced the ulcer index from 1.6 ± 0.36 (62.5 mg/kg) to 0.35 ± 0.61 (500 mg/kg); the ulcer area from 33.25 ± 1.5 (62.5 mg/kg) to 5.44 ± 2.10 mm2 (500 mg/kg) corresponding to 43.35 (62.5 mg/kg) and 90.73 (500 mg/kg) percent inhibition. The ulcer index and the ulcer area were compared with Control 2 (2.73 ± 0.32 and 58.7 ± 7.41 mm2 respectively). This significant reduction (p<0.001) of the ulcerated surface was followed by a significant increase (p<0.001) of mucus secretion. For 43.2 ± 2.37 mg in Control 2, the mucus content of rats treated with M. barteri at doses ranging from 62.5 to 500 mg/kg was increased from 44.2 ± 2.1 to 62.5 ± 1.89 mg. Cimetidine and Maalox increased the pH and the mucus weight. Six hour after pylorus ligation, a considerable amount of basal gastric juice was recorded (6.30 ± 0.29 ml) in Control 2 (Table 5). When rats were treated with the extract of M. barteri (62.5, 125, 250 and 500 mg/kg), a significant (p < 0.001) dose dependent decrease in gastric juice and titratable acidity was produced while a significant (p<0.05) increase in the pH of gastric juice was recorded as compared to Control 2 (Table 5).
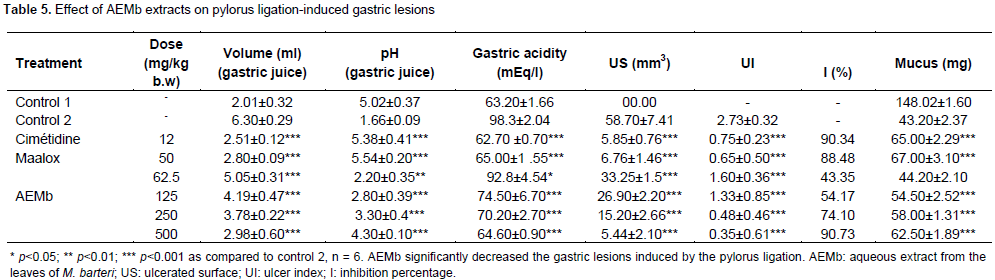
Effect of AEMb on hypothermic restraint stress-induced gastric mucosal lesions
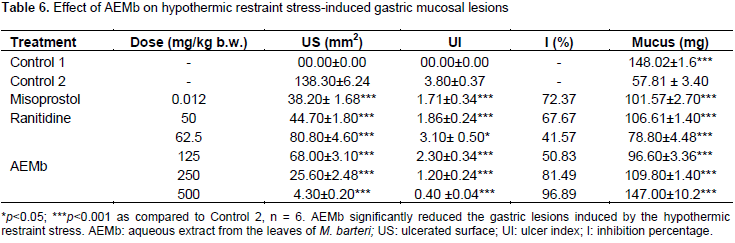
In the absence of the extract and stress caused by cold, no lesion of the gastric mucous membrane was observed. However, when rats were stressed and did not receive any treatment (Figure 1D), the ulcer area and ulcer index were 138.3 ± 6.24 mm2 and 3.80 ± 0.37, respectively (Table 6). The mucus produced in rats of Control 2 group (57.81 ± 3.4 mg) significantly (p<0.001) decreased when compared with Control 1 (148.02 ± 1.6 mg). Oral administration of AEMb at doses ranging from 62.5 to 500 mg/kg b.w., significantly (p < 0.001) inhibited intraluminal bleeding and ulcer formation induced by hypothermic restraint stress. This significant reduction (p<0.001) of the ulcerated surface, fluctuating between 41.57 and 96.89% of inhibition was accompanied by a significant increased (p<0.001) secretion of mucus. The amount of mucus was augmented from 78.8 ± 4.48 to 147 ± 10.2 mg in rats treated with AEMb at doses ranging from 62.5 to 500 mg/kg b.w., respectively (Table 6) as compared to that of Control 2 rats. Pretreatment of the animals with standard drugs (misoprostol and ranitidine) induced 72.37 (misoprostol) and 67.67% (ranitidine) inhibition corresponding to 38.2 ± 1.68 mm2 (misoprostol) and 44.7 ± 1.8 mm2 (ranitidine) ulcer area and 1.71 ± 0.34 (Misoprostol), 1.86 ± 0.24 (ranitidine) ulcer index, respectively. The ulcer index was significantly (p < 0.001) impaired by AEMb (62.5 to 500 mg/kg b.w.) from 3.1 ± 0.5 to 0.4 ± 0.04.
The acute toxicity study performed using the limit test, showed that the LD50 of the aqueous extract of M. barteri (AEMb) is higher than 5000 mg/kg b.w. According to OECD Protocol 420 (2001), AEMb can be classified in the "non-classified category" of products considered to be nontoxic in the Globally Harmonized System (GHS) of classification and labelling of chemicals. These results are similar to those of ¶Asiedu-Gyekye et al. (2014) and ¶Oussou et al. (2016) who¶ showed respectively that the LD50 of the aqueous extracts of the leaves of M. oleifera and the ethyl acetate fraction of Lophira lanceolata were higher than 5000 mg/kg b.w. ¶No signs of toxicity and mortality were recorded during the experimentation with this dose of AEMb.¶¶¶ Moreover, the animals treated with AEMb showed normal weight gain. This weight gain of the rats may be due to a stimulation effect of AEMb on the central nervous system centers managing appetite. As a consequence, animals’ observations showed an increase in the daily food consumption. This can explain the use of AEMb in traditional medicine as aperitif (Oliver-Bever, 1986). ¶It is well-known that the loss of weight was generally due to high lipolysis. In this study, it is noted that the animals gained weight when administered AEMb. The same conclusion was also drawn by Afolayan et al. ¶(2016) with the extract of Monsonia angustifolia. The results of phytochemical screening carried out in order to identify major metabolites in AEMb revealed that the extract contained polyphenols, tannins, flavonoids, coumarines, saponins, sterols and polyterpenes. Quinones were not detected in the extract. According to Awaad et al. (2013), phytoconstituents extracted from medicinal plants possess antiulcerogenic activity and act by various mechanisms.
Polyphenols compounds protect the gastrointestinal mucosa from lesions produced by various experimental ulcer models and against different necrotic agents. Polyphenols have anti-histaminic properties, thus, decreases histamine levels. They prevent the release of histamine from gastric mast cells and inhibit the gastric H+/K+ proton pump thereby diminishing acid gastric secretion. On the other hand, they possess cytoprotective effects, which increase the mucosal blood flow, stimulate the synthesis of muco-bicarbonate in the gastric mucosa and raise prostaglandin levels (Sakat and Juveka, 2009). Flavonoids are known to possess cytoprotective and antisecretory properties. They stimulate prostaglandin, bicarbonate, mucus secretion and prevent degrading effects of reactive oxidants in gastro-intestinal system (Dashputre and Naikwade, 2011). Flavonoids have also been reported to offer protection in ulcer development by increasing capillary resistance and improving microcirculation (Sabiha et al., 2011). It is acknowledged that tannins protect the outermost layer of mucosa and make it less permeable and more resistant to chemicals and mechanical injury or irritation and thus prevent ulcer development (Sabiha et al., 2011). Saponins induce mucus production which protect the gastric mucosal membrane against the acid effects and inhibit selectively the production of PGF2 (Agwu and Okunji, 1986).
Phytochemical contents of AEMb found in this investigation are similar to those of Adesegun et al. (2007), who revealed the presence of saponins and phenolic compounds in the methanolic extract of the leaves of M. barteri. Some compounds such as benzamide, undecane, nerolidol, known to have antimicrobial potential were isolated by ¶Ogundajo et al. (2017) who showed 50 phytoconstituents from the methanol fractions of Macaranga barteri leaves. Anti-ulcerogenic effects of AEMb were investigated on HCl/ethanol, ibuprofen, pylorus ligation and cold restraint stress-induced gastric lesions in rats. These results pointed out the prevention of ulcerations by pre-administration of the aqueous extract of M. barteri at 62.5, ¶125, 250 and 500 mg/kg b.w. ¶This extract exerted cytoprotective and anti-secretory effects against gastric mucosal lesions¶¶. Thus, this extract possesses an antiulcerogenic potential. Oral administration of HCl/ethanol to rats induced a very great ulcer area.¶ Indeed, it has been demonstrated that ¶ethanol causes gastric secretion disorders, deteriorations of the permeability, exhaustion of gastric mucus and production of free radicals (Salim, 1990). ¶The lesions and necrosis of the epithelial surface cells of the stomach mucosal membrane, lead to their erosion and congestion inducing cellular necrosis (Oates and Kakkinen, 1988).¶ This explains the great ulcer area observed in Control 2 rats. In AEMb pretreated animals, reduction of ulcer areas and a significant increase in mucus secretion were observed. ¶This gastric mucosal membrane protection resulted from the cytoprotective and anti-secretory effect of this extract which could be due to an increase in mucus secretion that protects the gastric mucosal membrane from the corrosive effects of ethanol.
¶These results are similar to those shown with Terminalia superba, Piptadeniastrum africanum and Ziziphus abyssinica by Kouakou et al. (2013), Ateufack et al. (2015) and Yau et al. 2017 respectively.¶ Indeed, these authors revealed that these medicinal plant extracts reduced remarkably ulcers provoked by necrosing agents in experimental rat models by protecting the gastric mucosal membrane¶. Nonsteroidal anti-inflammatory drugs (NSAIDs) such as ibuprofen induce gastric ulceration by inhibition of various mechanisms like cyclo-oxygenase 2 (COX-2), the biosynthesis of cytoprotective prostaglandins, mucus, bicarbonate and blood flow (Takeeda et al., 2004). ¶The prostaglandin deficiency is responsible for ulcerations and the small quantity of mucus observed in the control rats as compared to those which received the various treatments. Ibuprofen causes a direct irritation effect by increasing H+ ion transport and free radical formation (Scheiman et al., 1996). ¶The protective effect in the various groups treated with AEMb in the ibuprofen-induced ulcer model could be due to an increase in the mucus secretion. ¶The absence of lesion in AEMb pretreated rats corroborates the anti-inflammatory effect of the extract. ¶This effect can probably be attributed to the presence of phytochemical compounds in this extract with endogenous prostaglandins-like effect. ¶Bairy et al. ¶(2001) and Deepanjana et al. ¶(2011) demonstrated similar effects respectively with the alcoholic extract of Tinospora cordifolia and the aqueous leaves extract of Moringa oleifera.
Hypothermic restraint stress was used to evaluate the effect of AEMb on gastric ulcer parameters. ¶Indeed, stress reduces cellular proliferation rate, promotes an increase in the gastric secretion and supports prostaglandin synthesis inhibition and involves deteriorations of nitric oxide circulation and the mucus layer that covers the gastric epithelium (Bjarnason et al.¶, 2007). ¶Consequently, an impairment of bicarbonate level of the mucosal membrane, gastric motility and blood circulation leads to the development of lesions on gastric mucosal membrane (Overmier and Murison, 2000). ¶The destruction of the gastro-intestinal mucosal membrane observed in Control 2 rats is probably due to these factors. AEMb¶ pretreated rats and subjected to a hypothermic constraint presented an important gastric mucosal membrane protection. ¶These results indicate that the active compounds present in the extract could control the production or the action of the mediators responsible for the acid excess or the increase of the production of mucus and bicarbonate of the mucous membrane (Oates and Hakkinen, 1988). ¶Similar suggestions were made by Kumar et al. (2013), Ghader et al. (2015) and Amang et al., 2017¶ working on Careya arborea, Glycyrrhiza glabra and Eremomatax speciose. Ulcers induced by pylorus ligation are due to the digestion of the gastric mucosal membrane and the rupture of the gastric mucosal barrier.¶ Generally, ulcer occurs because of the overproduction of acid by the stomach or the reduction in the mechanisms of protection of the gastric mucosal membrane (Sairam et al., 2002; Bharath et al., 2014).
¶In pylorus ligation, ulcers are developed by the accumulation of acid overproduction leading to auto digestion of gastric mucosa. ¶AEMb rats pretreatment dose-dependently induced a significant reduction in gastric acid volume, free acidity, ulcer area and an increase in pH and mucus production. According to Hiruma-Lima et al. ¶(2006), mucus is a significant protection factor of the gastric mucosal membrane.¶ Mucus is presented as a transparent gel formed by water and glycoproteins which cover the gastro-intestinal mucosal membrane and protect it against irritating agents. ¶¶Although, the ulcer prevention mechanism of this extract is not clearly defined, flavonoids by their capacity of free radicals scavenging (Borrelli and Izzo, 2000) and saponins by their capacity to produce mucus could protect the gastric mucosal membrane against the acid effects which inhibit selectively the PGE2 as hypothesized by Agwu and Okunji (1986) in their studies on Pyrenacantha staudii leaf extracts.¶ ¶These results are similar to those obtained with a hydro-ethanol 70% extract of Terminalia superba (Goze et al., 2013), the ethanolic leaves extract of Terminalia catappa (Bharath et al., 2014) and the ethanolic extract of Cardiospermum halicacabum (Sheeba et al.¶, 2016).
The results of this study suggest that the aqueous extract of the leaves of M. barteri is safe for use and could protect the gastric mucosa against HCl/ethanol, ibuprofen, pylorus ligation and cold restraint stress-induced gastric injury. This cytoprotective action was accompanied by significant increases in gastric mucus production. The various chemical groups contained in this extract could justify the use of the plant by traditional healers. Further work is envisaged to evaluate the healing effect of M. barteri extract as well as its possible long-term toxicity, to isolate, purify and characterize the active constituent(s) and elucidate the exact mechanism of action of AEMb.
The authors have not declared any conflict of interests.
The authors are thankful to all laboratory staff of Physiology, Pharmacology and Pharmacopoeia of UFR-SN, Nangui Abrogoua University, Côte d’Ivoire, for their encouragement during these investigations.
REFERENCES
|
Adesegun SA, Elechi NA, Coker HAB (2007). Antioxidant power of Macaranga barteri leaf. Am. J. Food Technol. 2(6):543-549.
Crossref
|
|
|
|
Afolayan AJ, Wintola OA, Fouche G (2016). Acute and subacute toxicological evaluation of the aerial extract of Monsonia angustifolia E. Mey. Ex. A. Rich in Wistar Rats. Evid-Based Complement. Altern. Med. 2016:4952485.
|
|
|
|
|
Agwu CN, Okunji CO (1986). Gastrointestinal studies of Pyrenacantha staudii leaf extracts. J. Ethnopharmacol. 15:45-55.
Crossref
|
|
|
|
|
Amang AP, Mezui C, Tchokomeni GS, Zondengoumba EN, Enonchong GE, Tan PV (2017). Prophylactic and healing activities of the leaves aqueous extract of Eremomastax speciosa on gastric ulcers in rats. J. Adv. Biol. Biotechnol. 12(3):1-13.
Crossref
|
|
|
|
|
Asiedu-Gyekye IJ, Frimpong-Manso S, Awortwe C, Antwi DA, Nyarko AK (2014). Micro-and macroelemental composition and safety evaluation of the nutraceutical Moringa oleifera leaves. J. Toxicol. 2014:1-13.
Crossref
|
|
|
|
|
Ateufack G, Mokam ECD, Mbiantcha M, Feudjio RBD, Nana D., Kamanyi A (2015). Gastroprotective and ulcer healing effects of Piptadeniastrum africanum on experimentally induced gastric ulcers in rats. BMC Complement. Altern. Med. 15:214-223.
Crossref
|
|
|
|
|
Awaad AS, El-Meligy RM, Soliman GA (2013). Natural products in treatment of ulcerative colitis and peptic ulcer. J. Saudi Chem. Soc. 17:101-124.
Crossref
|
|
|
|
|
Bairy KL, Roopa K, Malini S, Rao CM (2001). Protective effect of Tinospora cordifolia on experimentally induced gastric ulcers in rats. J. Nat. Remedies 2(1):49-53.
|
|
|
|
|
Bekro Y, Bekro J, Boua BB, Tra Bi F, Ehilé EE (2007). Etude ethnobotanique et screening phytochimique de Caesalpinia benthamiana (Baill.) Herend et Zarrucchi (Caesalpiniaceae). Sci. Nature 4(2):217-225.
|
|
|
|
|
Bharath KG, Divya K, Sravanthi G, Rajeshwar G, Umadevi V, Niranjan GK (2014). Antiulcer activity of ethanolic extract of Terminalia catappa leaves against gastric ulcers by pyrolic ligation induced model in rats. Int. J. Pharm. Sci. Drug Res. 6(1):38-40.
|
|
|
|
|
Bjarnason I, Scarpignato C, Takeuchi K, Rainsford KD (2007). Determinants of the short-term gastric damage caused by NSAIDs in man. Alimentation Pharmacol. Thermodyn. 26:95-106.
Crossref
|
|
|
|
|
Borrelli F, Izzo AA (2000). The plant kingdom as source of antiulcer remedies. Phytother. Res. 14:581-591.
Crossref
|
|
|
|
|
Bouquet A, Debray IM (1974). Plantes médicinales de côte d'Ivoire. Travaux et documents de L'O.R.S.T.0.M. No 32, Paris, France 231p.
|
|
|
|
|
Brown LM (2000). Helicobacter pylori: epidemiology and routes of transmission. Epidemiol. Rev. 22(2):283-297.
Crossref
|
|
|
|
|
Burkill HM (1985). Entry for Lasiurus hirsutus (Forssk) Boiss. Family Poaceae) in the useful plants of west tropical Africa, 2.
|
|
|
|
|
Chiu PJS, Gerhart C, Brown AD, Barnett A (1984). Effects of a gastric antisecretorycytoprotector2-methyl-8–(phenylmethoxy) imidazo (1,2a) -pyridine-3-acetonitrine (Sch28080) on cyteamine, reserpine and stress ulcers in rats. Gastroenterology 34:783-786.
|
|
|
|
|
Dashputre NL, Naikwade NS (2011). Evaluation of anti-ulcer activity of methanolic extract of Abutilon indicum Linn leaves in experimental rats. Int. J. Pharm. Sci. Drug Res. 3(2):97-100.
|
|
|
|
|
Deepanjana D, Devasrita D, Tatiyana M, Anup K, Bairy KL (2011). Protective effects of Moringa oleiferaon experimentally induced gastric ulcers in rats. Res. J. Pharm. Biol. Chem. Sci. 2(2):50-55.
|
|
|
|
|
Gbadamosi IT, Erinoso SM (2015). A review of twenty ethnobotanicals used in the management of breast cancer in Abeokuta, Ogun State, Nigeria. Afr. J. Pharm. Pharmacol. 10(27):546-564.
Crossref
|
|
|
|
|
Ghader JA, Vahid N, Ehsan A, Mostafa M, Hadi K (2015). Antiulcer Properties of Glycyrrhiza glabra L. extract on experimental models of gastric ulcer in mice. Iranian J. Pharm. Res. 14(4):1163-1170.
|
|
|
|
|
Goze NB, Kouakou KL, Bléyéré NM, Amonkan KA, Konan BA, Abo KJ-C, Yapo AP, Ehilé EE (2013). Anti-ulcerogenic effects of a hydroethanol 70% extract from stem bark of Terminalia superba engl. et diels (combretaceae) in rats and phytochemical screening. Int. J. Sci. Innov. Discov. 3(5):539-550.
|
|
|
|
|
Gupta MB, Nath R, Gupta GP, Bhargava KP (1985). A study of the antiulcer activity of diazepan and other tranquillose datives in albinos' rats. Clin. Exp. Pharmcol. Physiol. 12:61-63.
Crossref
|
|
|
|
|
Hara N, Okabe S (1985). Effects of Gefanate on acute lesions in rats. Folia Pharmacol. 85:443-448.
Crossref
|
|
|
|
|
Harold K, Grant DM, Mitchel J (2007). Principles of medical pharmacology. Seventh Ed. Elsevier Canada Ltd. pp. 557-559.
|
|
|
|
|
Hiruma-Lima CA, Akiko C, Calvo TR, Rodrigues CM, Andrade FDP, Vilegas W, Brito ARMS (2006). Antiulcerogenic activity of Alchornea castaneaefolia effects on somatostatin, gastrin and prostaglandin. J. Ethnopharmacol. 10(4):215-224.
Crossref
|
|
|
|
|
Kadjo K, Ouattara B, Sanogo S, Diallo AD, Adom AH, Yangni-Angate Y, Ouattara D, Niamkey EK, Beda YB (1999). Aspects épidémiologiques des ulcères gastro duodenaux. Méd. d'Afrique Noire 46(2):99-102.
|
|
|
|
|
Keïta BJ (1990). Ulcères gastro-duodénaux en chirurgie «B» Hôpital du point «G». Thèse de médecine, N° 18, Bamako, Mali, 69p.
|
|
|
|
|
Kouakou KL, Goze NB, Bleyere NM, Konan BA, Amonkan KA, Abo KJ-C, Yapo AP, Ehile EE (2013). Acute toxicity and anti-ulcerogenic activity of an aqueous extract from the stem bark of Terminalia superba Engl. and Diels (Combretaceae). World J. Pharm. Sci. 1(4):117-129.
|
|
|
|
|
Kumar K, Kenganora M, Satish K, Rajendran M (2013). Anti-ulcer activity of ethanol extract of the stem bark of Careya arborea Roxb. Int. Curr. Pharm. J. 2(3):78-82.
Crossref
|
|
|
|
|
Kunchandy J, Khanna S, Kulkarni SK (1985). The histamine H3 receptor: a target for new drugs. Arch. Int. Pharmacodyn. Ther. 275:123-126.
|
|
|
|
|
Oates PJ, Kakkinen JP (1988). Studies on the mechanism of ethanol induced gastric damage in rats. Gastroenterology 94:10-21.
Crossref
|
|
|
|
|
Organization for Economic Cooperation and Development (OECD) (2001). Guideline for testing of chemicals. Guideline 420: Acute Oral Toxicity-Fixed Dose Procedure.
View. Site consulté le 15/08/2016
|
|
|
|
|
Oliver-Bever B (1986). Medicinal plants in tropical West Africa. Cambridge University Press, Cambridge London, United Kingdom pp 240-245.
Crossref
|
|
|
|
|
Operations Management Suite (OMS) (2002). Promotion du rôle de la médecine traditionnelle dans le système de santé : stratégie de la région africaine. AFR/RC50/9, pp. 12-15.
|
|
|
|
|
Oussou NJ-B, Asiedu-Gyekye IJ, Yapo AF, N'guessan BB, Amoateng P, Kouakou KL, Asante IK, Ehile EE (2016). In-vitro scavenging activity and acute toxicity study of methanol leaves extract and fractions of Lophira lanceolata Tiegh. Ex Keay (Ochnaceae) in rats. International Journal of Phytomedicine 8(3):411-421.
Crossref
|
|
|
|
|
Overmier JB, Murison R (2000). Anxiety and helplessness in the face of stress predisposes, precipitates, and sustains gastric ulceration. Behavioural Brain Research10:161-174.
Crossref
|
|
|
|
|
Parmar NS, Desai JK (1993). A review of current methodology for the evaluation of gastric and duodenal antiulcer agents. Indian Journal of Pharmacoly 25:120-135.
|
|
|
|
|
Robert A, Nezamis JE, Lancaster C, Davis JP, Field SO, Hanchar AJ (1983). Mild irritants prevent gastric necrosis through 'adaptive cytoprotection' mediated by prostaglandins. American Journal of Physiology-Gastrointestinal and Liver 245:113-121.
Crossref
|
|
|
|
|
Sabiha S, Mohd AA, Asif M, Akhtar M (2011). Role of phenolic compounds in peptic ulcer: An overview. Journal of pharmacy and bioallied sciences 3(3):361-367.
Crossref
|
|
|
|
|
Sairam K, Rao CV, Dora BM, Agrawal VK, Goel RK (2002). Antiulcerogenic activity of methanolic extract of Emblica officinalis. Journal of Ethnopharmacology 82(1):1-9
Crossref
|
|
|
|
|
Sakat SS, Juvekar RA (2009). Antiulcer activity of methanol extract of Erythrina indica Lam. leaves in experimental animals. Pharmacognosy Research 1:396-401.
|
|
|
|
|
Salim AS (1990). Removing oxygen-derived free radicals stimulates healing of ethanol induced erosive gastritis in the rat. Digestion 47:24-28.
Crossref
|
|
|
|
|
Scheiman JM, Dubois RN, Giardiello FM (1996). NSAIDs, Eicosonoids, and the gastroenteric tract. Philadelphia Saunder 25:279-289.
|
|
|
|
|
Shay H, Komarov SA, Fels SS, Meranze D, Gruenstein M, Siplet H (1945). A simple method for the uniform production of gastric ulceration in the rat. Gastroenterology 5:43-61.
|
|
|
|
|
Sheeba MS, Sheena P, Greeshma T, Gayathri LT, Asha VV (2016). Comparative evaluation of the efficacy of Cardiospermum halicacabum Linn. on Indomethacin, Pylorus ligation and Helicobacter pylori induced gastric ulcer in rats. Annals of Phytomedicine 1: 63-72.
|
|
|
|
|
Soberg T, Hofstad B, Sandvik L, Johansen M, Lygren I (2010). Risk factors for peptic ulcer bleeding. Tidsskr Nor Laegeforen 130:1135-1139.
|
|
|
|
|
Soro KG, Mahassadi KA, Koffi GM, Kissi YH, Coulibaly A, Assohoun T, Ehua AM, Seu GS, Afum-Adjei AA, Ehua SF (2016). Postoperative morbidity and mortality of perforated peptic ulcer: retrospective Cohort study of risk factors among black africans in Côte d'Ivoire. Gastroenterology Research and Practice ID 2640730.
|
|
|
|
|
Takeeda M, Hayashi Y, Yamato M, Murakami M, Takeuchi K (2004). Roles of endogenous prostaglandins and cyclooxygenase isozymes in mucosal defense of inflamed rat stomach. Journal of Physiology and Pharmacology 55:193-205.
|
|
|
|
|
Yau S, Abdulazeez MA, Anigo KM, Garba A (2017). Gastro-protective effect of Ziziphus abyssinica root extracts in ethanol-induced acute ulcer in Wistar rats. Journal of Acute Disease 6(2):62-65.
Crossref
|
|
|
|
|
Yeomans ND, Hawkey CJ, Brailsford W, Næsdal J (2009). Gastroduodenal toxicity of low-dose acetylsalicylic acid: a comparison with non-steroidal anti-inflammatory drugs. Current Medical Research Opinion 25:2785-2793.
Crossref
|
|