ABSTRACT
Experimental studies that aimed to determine the effects of crude aqueous resin extracts of the Commiphora swynnertonii on plasma cholesterol levels and weight changes were carried out in rats (Rattus rattus). A total of 24 experimental rats divided into four groups with equal sample size (n=6) were used. Group one (G1) served as negative control that received 0.5ml of distilled water (0 mg/kg) orally. Groups 2 (G2), 3 (G3) and 4 (G4) received 50, 100, and 200 mg/kg body weight orally on daily basis for 21 days respectively. Results revealed a significant decrease (P < 0.05) in the body weight and on cholestrol levels between the treated and the control groups in a dose dependent manner (R2 = 0.89). Commiphora swynnertonii resin lowered cholesterol level by 54, 76 and 79% and weight changes by 18, 31 and 23% for the exposed rats at concentrations of 50, 100 and 200 mg/kg BW respectively. The rats were able to tolerate resin at concentrations lower than 100 mg/kg BW. At higher (>100 mg/kg) doses, few rats showed signs of illness including diarrhoea and finally death. Based on these results, C. swynnertonii has a potential to serve as an anti-cholesterol agent with body weight lowering properties.
Key words: Oltemwai, Cardiovascular diseases, rats, Tanzania, blood chemistry.
Cardiovascular diseases associated with increased levels of blood cholesterol particularly the low density lipoprotein cholesterol are increasingly becoming a worldwide health challenges that lead to human deaths. Such disorders are treated, controlled and prevented using different methods including medicinal plants and herbs (Kochhar et al., 2006). Several Commiphora species have been studied to assess their activities as anti-lipidemic, anti-cholesterolaemic and anti-atherosclerotic, thus reducing serum cholesterol concentrations without causing any detrimental side effects (Adebayo et al., 2006). In various studies C. mukul has been claimed to decrease atherosclerosis and to lower serum cholesterol by 27% and triglycerides by 31% (Singh et al., 1994). Guggulipid, a product from C. mukul, increased the high density lipoprotein cholesterol (HDL) (Singh et al., 1994). It exerts its activity by lowering the level of cholesterol by reducing total cholesterol, low density lipoprotein cholesterol (LDL-c), and very low density lipoprotein (VLDL-c) cholesterol at the same tim elevating the high density lipoprotein cholesterol (HDL-c) (Adebayo et al., 2006). Commiphora mukul contains guggulsterone, a compound that act by antagonizing the effect of the nuclear farnesoid × receptor (F×R) (Huang et al., 2003; Adebayo et al., 2006). This receptor is identified as a bile acid receptor and biological sensor for the regulation of bile acid biosynthesis (Huang et al., 2003). Farnesoid × receptor regulates cholesterol metabolism in two ways: (i) chenodeoxycholic acid (CDCA), a primary bile acid, binds directly to and activates F×R, which then mediates the feedback suppression by bile acids of cholesterol 7 alpha-hydroxylase (CYP7A1), the rate-limiting enzyme in bile acid biosynthesis from cholesterol. (ii) Farnesoid × receptor participates in the activation of intestinal bile acid binding protein (IBABP), which is involved in the enterohepatic circulation of bile acids. Thus F×R constitutes a potential therapeutic target that can be modulated to enhance the removal of cholesterol from the body (Tu et al., 2000). The other mechanism reported by Wang et al. (2004), is through the presence of ketosteroid , an active compound of C. mukul which acts by stimulating the thyroid gland and has also found to reverse the decrease of catecholamine and dopamine –p- decarboxylase activity that is involved with anticholesterolaemia (Wang et al., 2004). This is done by improving the liver’s ability to process, metabolize and excrete cholesterol and improving thyroid function by increasing T3 and T4 conversion (Wang et al., 2004). Ethanolic leaf extract of C. africana and C. myrrha were also shown to exhibit hypolipidaemic activity in experimental rats (Adebayo et al., 2006). Though several studies reported anti-cholesteremic effect of several Commiphora spp, relatively little has been investigated in clinical trials on the effect of C. swynnertonii. Therefore, this study aimed at evaluating the effect of C. swynnertonii on the weight gain and plasma cholesterol levels using rat’s model.
Plant materials
Commiphora swynnertonii plant materials were collected from Simanjiro district in Manyara Region. The plant was identified by a botanist as Commiphora Swynnertonii from the family Burseraceae. A voucher specimen (reference number CK 6489) was prepared and preserved at Tanzania National Herbarium, in Arusha (Kayombo, 2009 Personal communication). Tanzania and transferred to Sokoine University of Agriculture (SUA) for processing and use in the experiments. One hundred grams (100 g) of the resin was brewed in 750 ml distilled water and thereafter allowed to stand for 30 minutes. The mixture was filtered using filter paper No 3 and stored in a clean bottle before administered to rats. Twenty mls aliquots of the decoction were evaporated to dryness using an electric heater at 60 to 70°C. The residues were used to determine the concentrations of C. swynnertonii extracts which were administered to different groups of experimental rats (Edem et al., 2009).
Sample size determination for experiment animals
The sample size for the experimental animals was determined according to Kirkwood and Sterne (2003).
Experimental design
Twenty four white albino rats (Rattus rattus) of seven months old of both sexes weighing 125.7 to 180 g were used in this study. The rats were obtained from the small animal unit at Sokoine University of Agriculture Faculty of Veterinary Medicine. Once in the experimental house, all rats were assessed for signs of diseases. They were caged and maintained on grower mash and drinking water ad libitum. The rats were left for three weeks to acclimatize with experimental environment. Following acclimatization, they were weighed, tagged and randomly assigned into four experimental groups of six rats each.
Treatment allocation
The animals were randomly assigned into four groups of six rats (n=6) each. All rats were housed in well-ventilated cages. Groups; G2 - G4 rats were given different doses of aqueous resin extract orally for 21 days consecutive days. G1 remained as negative control that received distilled water only. Blood samples were collected for evaluation of haematological and biochemical parameters.
Preparation of plasma and analysis
At baseline, the body weights of the rats were recorded. About 3 mls of blood samples for plasma preparation were collected from the tail artery using sterile syringes and blood samples were stored in EDTA sterile vacutainer tubes. The blood samples were thereafter centrifuged at 1300 × g for 5 minutes using a bench top centrifuge model to obtain the plasma. The plasma was stored in a refrigerator for analysis of biochemical parameters.
Cholesterol analysis
Total plasma cholesterol level was determined according to Erba Mannheim protocol (Trinders, 1969; Erba, 2010). All analyses on plasma were completed within 24 h after blood collection as recommended (Goji et al., 2009).
Statistical analysis
The data obtained were compiled, coded and analysed using Microsoft excel statistical package (2007) and SAS (Statistical Analysis System) program (Version 8.3) for WindowR. Results from experimental Tests for differences between the means were done and compared by Duncan’s Multiple Range Test (DMRTS) at (p < 0.05).
Signs of toxicity
Animals used in the study in the negative control group were apparently in good health condition, as they remained alert, consumed food (growers mash) and water freely and exhibited normal weight increase over time. Depression and diarrhoea were observed in all groups treated with resin extract. The symptoms of toxicity observed with extract administration were dose dependent. Three to seven days after administration of extract all rats in the various groups were very weak. Signs observed before death included loss of appetite, diarrhoea, blindness and coma.
Mortality rate
Mortality was observed in one rat from G3 and two rats from G4 by day eight following an oral administration of resin extract. The rats treated in G2 and G3, were depressed and less active compared to the rats in the other groups during the day.
Changes in body weight
Animals in control group (G1) maintained their weight gain throughout the experimental period (Table 1). The body weights of rats receiving the oral dose of C. swynnertonii extract decreased significantly in a time and dose dependent manner. Rats receiving 50 mg/kg (G2) body weight lost about 16.5% of their average body weight during the first week, 15.7% during the 2nd and 18.2% during the 3rd week. Rats receiving 100 mg (G3) of C. swynnertonii extract the weight losses were 24.0% (week 1), 30.0% (week 2) and 31.0% (week 3). Likewise, for rats receiving 200 mg of C. swynnertonii extract, weight losses were 23.0% (week 1), 21.5% (week 2) and 23.0% (week 3). Overall, animals receiving the C. swynnertonii extract lost weight in the range 18 to 31% (Figures 1 and 2).
Changes in the levels of plasma cholesterol
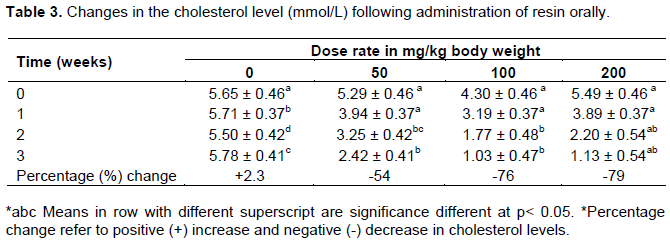
The plasma cholesterol levels of rats before and after administration of C. swynnertonii extract at three different doses are shown in Table 2. There was a significant (P < 0.05) decrease in plasma cholesterol levels in all rats in the treated groups in a dose dependent manner (R2-0.89: P=0.03). The body weight for rats in G2, decreased significantly (P < 0.05) from week 2 of treatment with resin extract. The plasma cholesterol levels for rats in G2, decreased by 54 % while in G3 and G4, the decrease was by 76 and 79 % respectively. There was a significant positive correlation between weight and cholesterol at different concentrations of C. swynnertonii (Figure 3). The correlations were r = 0.432 and P=0.035 for control group, and for exposed groups, the values were r = 0.432 and P=0.009; r = 0.712 and P=0.000 and r = 0.487 and P =0.282; for G1, G2 and G3 treated rats, respectively.
The present study has demonstrated the effect of aqueous crude resin extracts from C. swynnertonii on plasma total cholesterol in rats. Results revealed that rats in a negative control group were clinically healthy and their body weights increased significantly all along the study period. Diarrhoea and deaths observed in few treated rats implies that prolong administration of resin extract could be lethal and toxic to the rats. Similar observations were reported in previous studies (Ruitang, 2007) in which extracts from C. mukul lead to gastrointestinal discomfort such as loose faeces, mild nausea, and hiccup. The reduced weight gain in a dose dependent manner could be due to reduced feed consumption since the animals were depressed, inactive and with lost appetite. Studies done by Bakari et al. (2012a,b) and Scott, (2005) reported the association between the reduction in weight and the plasma cholesterol and glucose levels through stimulation of thyroid hormone (T3 and T4) function thus interfering with basal metabolic rate leading to loss of body weight (Scott, 2005). Thyroid hormones (T3), stimulates the production of RNA polymerase I and II and, therefore, increases the rate of protein synthesis, potentiates the effects of the β-adrenergic receptors on the metabolism of glucose (Guyton and John, 2006). Therefore, it increases the rate of glycogen breakdown and glucose synthesis in gluconeogenesis. Also stimulates the breakdown of cholesterol and increases the number of LDL receptors, thereby increasing the rate of lipolysis (Guyton and John, 2006).
In the current study, administration of resin from C. swynnertonii significantly lowered blood cholesterol. This finding is significant in managing conditions such as coronary heart and atherosclerosis. This was also demonstrated by Helal et al. (2005 and 2006); Ojha et al. (2008); Goji et al. (2009) whereby the application of extracts from C. myrrha, C. africana and C. mukul using rats lowered the blood cholesterol and glucose while maintaining the myocardial membrane integrity thus preventing myocardial impairment. The effect of C. swynnertonii may also base on its ability to bind bile acids in the intestinal lumen and to interrupt the entero-hepatic circulation of bile acids, leading to increased excretion of steroids (Singh et al., 1994). Depletion of the hepatic sterol pool may cause compensatory increases in cholesterol biosynthesis, which may cause increased catabolism of LDL particles from plasma (Singh et al., 1994). Commiphora mukul contains guggulsterone, a compound which act by antagonizing the effect of the nuclear farnesoid X receptor (FXR) (Huang et al., 2003; Adebayo et al., 2006). This receptor is identified as a bile acid receptor and biological sensor for the regulation of bile acid biosynthesis (Huang et al., 2003). Thus, according to Tu et al. (2000), FXR constitutes a potential therapeutic target that can be modulated to enhance the removal of cholesterol from the body. Another possible mechanism is through the presence of ketosteroid, an active compound of C. mukul which acts by stimulating the thyroid gland and has also found to increase the activity of catecholamine and dopamine –p- decarboxylase that are involved in lowering plasma cholesterol (Wang et al., 2004). Some secondary plant metabolites such as coumarin, flavonoid, terpenoid, arginine and glutamic acids have been shown to confer cholesterol lowering effects in various experimental animal models (Akah and Okafor, 1992; Marles and Farnsworth, 1995). The significant anticholesterol observed in the current study can therefore be explained by the fact that C. swynnertonii contain remarkable amounts of saponins terpenoids and flavanoids. Saponins are glycoside components often referred to as “natural detergent” because of their foamy nature (Edeoga et al., 2005) and are reported to posses health benefits such as cholesterol lowering activity (Edeoga et al., 2005). The observed reduction in body weight of resin-treated rats is well connected with the observed levels of plasma cholesterol. High carbohydrate (glucose) and cholesterol intake are known to increase body fats, hence increased body weight and eventually obesity (Scott, 2005).
In conclusion, this study has demonstrated that rats were able to tolerate oral administration of C. swynnertonii resin at doses lower than 100 mg/kg bodyweight. Administration of higher doses had negative effects thus causing diarrhoea and genera body weakness in rats. The observed anti-cholesteremic and body weight lowering effect warrants further studies on potentials of this plant.
The authors have not declared any conflict of interests.
REFERENCES
|
Adebayo AH, Aliyu R, Gatsing D, Garba IH (2006). The Effects of Ethanolic Leaf Extract of Commiphora africana (Burseraceae) on Lipid Profile in Rats. Int. J. Pharm. 2:618-622.
Crossref
|
|
|
|
Akah PA, Okafor CL (1992). Blood sugar lowering effect of Veronia amygdalina (Del) in an experimental rabbit model. Phytother. Res. 6:171-173.
Crossref
|
|
|
|
|
Bakari GG, Max RA, Mdegela RH, Phiri ECJ, Mtambo MMA (2012a). Antiviral activity of crude extracts from Commiphora swynnertonii (Burrt) against Newcastle disease virus in ovo. Tropic. Anim. Health Prod. 44(7):1389-93.
Crossref
|
|
|
|
|
Bakari GG, Max RA, Mdegela RH, Phiri ECJ, Mtambo MMA (2012b). Effect of crude extracts from Commiphora swynnertonii (Burrt) against selected microbes of animal health importance. J. Med. Plants Res. 6(9):1795-1799.
Crossref
|
|
|
|
|
Edem DO, Usoh IF (2009). Biochemical changes in wistar rats on oral doses of mistletoe (Loranthus micranthus). Am. J. Pharma. Toxicol. 4(3):94-97.
Crossref
|
|
|
|
|
Edeoga HO, Okwu DE, Mbaebi BO (2005). Phytochemical constituents of some Nigerian plants. Afr. J. Biotech. 4(7):685-688.
Crossref
|
|
|
|
|
Goji ADT, Dikko AAU, Bakari AG, Mohammed A, Tanko Y (2009). Evaluation of the Effect of Aqueous-ethanolic Stem Bark Extract of Commiphora africana on Blood Glucose Levels of Alloxan Induced Diabetic Wistar Rats. Asian J. Med. Sci. 1:18-21.
|
|
|
|
|
Guyton AC, John E (2006). Textbook of Medical Physiology (11th ed.). Philadelphia: Elsevier Inc. ISBN 0-7216-0240.
|
|
|
|
|
Helal EGE, Mahmoud A, El-Badawy EE, Kahwash AA (2006). Effect of Commiphora myrrha extract on some physiological parameters and histological changes in diabetic albino rats. Egyptian J. Hosp. Med. 148-162.
|
|
|
|
|
Huang CJ, Zhao L, Lew A, Yu JL, Sahoo J, Meinke S, Royo PT, Pelaez I, Wright F (2003). Guggulsterone is an FXR antagonist in co activator association assays but acts to enhance transcription of bile salt export pump. J. Biol. Chem. 1-3.
|
|
|
|
|
Kirkwood BR, Sterne JAC. (2003). Essential Medical Statistics. Blackwell, Cornwall, United Kingdom. 146p.
|
|
|
|
|
Marles RJ, Farnsworth NR (1995). Antidiabetic plants and their active constituents. Phytomedicine 2:137-187.
Crossref
|
|
|
|
|
Ojha SK, Nandave M, Arora S, Mehra RD, Joshi S, Naran R, Arya DS, (2008). Effect of Commiphora mukul extract on cardiac dysfunction and ventricular function in isoproterenol-induced myocardial infarction. Indian J. Exp. Biol. 46:646-652.
|
|
|
|
|
Ruitang D (2007). Therapeutic effects of guggul and its constituent guggulsterone: cardiovascular benefits. J. Cardiovasc. Drug Rev. 25(4):375-390.
|
|
|
|
|
Scott AM (2005). Gum Guggul and Some of Its Steroidal Constituents. Review of Toxicological Literature Prepared for National Toxicology Program (NTP) and National Institute of Environmental Health Sciences (NIEHS). National Institutes of Health U.S Department of Health and Human Services. Research Triangle Park, North Carolina. pp. 18-21.
|
|
|
|
|
Trinders P (1969). Determination of glucose in blood using glucose oxidase with an alternative oxygen acceptor. Ann. Clin. Biochem. 6:24.
Crossref
|
|
|
|
|
Tu H, Okamoto AY, Shan B (2000). FXR a bile acid receptor and biological sensor. Trends Cardiovasc. Med. 10(1):30-5.
Crossref
|
|
|
|
|
Wang XJ, Greiberger G, Ledinski G, Kager B, Paigenand GJ (2004). The hypolipidemic natural product, Commiphora mukul and its component guggulsterone inhibit oxidative modification of LDL. Atherosclerosis 172:239-249.
Crossref
|
|