ABSTRACT
The selection of genotypes based on phenotypic data requires evaluation through different environments in order to accurately measure the genotype x environment (GxE) interaction effect. The purpose of this study was to assess 94 F5:8 recombinant inbred lines (RIL) in terms of their yield performance and other agronomic traits (that is, height, maturity, and lodging); and compare the results to the parental lines (that is, ‘Spencer’ and ‘LS97-1610’) in two different experimental locations (that is, Dowell, IL and Harrisburg, IL) in 2011. One sample t-test was applied in order to test RIL mean yields against mid-parental values. The average yield for RIL was greater than that of the parents in Dowell and in the combined data set. This outcome was dissimilar to that found in Harrisburg where the parent's yield was greater. The broad-sense heritability for each trait was estimated and they ranged from 34.84 to 90.84% for yield and maturity, respectively. Significant correlation coefficients were detected for all pairwise combinations of traits apart from lodging x yield. The results of this study revealed promising broad-sense heritability for yield and other agronomic traits in a RIL population derived from two parental lines whose profiles differ for both yield traits and disease resistance.
Key words: Agronomic traits, Glycine max, yield performance.
The improvement of soybean genotypes for better yield performance across environments demands considerable effort and time. The main factor influencing the selection of genotypes for higher yields is genotype x environment interaction (GxE). This selection of higher-yielding genotypes is based on phenotypic data requiring extensive evaluations across different environments. The main reason for this is the complex inheritance of yield as a quantitative trait (Yuan et al., 2002). GxE interaction is detected when the relative phenotypic performance of a genotype in a given environment does not match that of another environment. This dissimilarity can affect the ranking in genotype performance tests (Cucolotto et al., 2007). Multi-location genotypic evaluation allows us to generate estimates of the interaction component as well as perform further stability and adaptability analysis. In studies aiming to assess the behavior of complex traits (e.g. yield and yield components), the study of RIL populations has shown practical application. These populations can be evaluated in a wide variety of locations and across a range of years due to their high homozygosity level. Furthermore, the function and inheritance of various traits can be studied through the analysis of segregation in RIL populations with distinct backgrounds (Orf et al., 1999).
Another useful tool which would both increase accuracy and save considerable resources, while selecting improved genotypes, is correlation analysis between agronomic traits (Cicek et al., 2006). The appraisal of yield components through the study of different related traits has to be considered in order to select genotypes by the phenotype (Sherrie et al., 2011). Plant height, lodging score, and maturity all play a significant role on yield (Mansur et al., 1996; Orf et al., 1999; Specht et al., 2001; Yuan et al., 2002). Although, some correlation coefficients have been found for these characteristics in soybean, for plant height and yield no consensus has been reached. According to Cicek et al. (2006), we can expect an improvement in yield in taller plants compared to shorter ones. Also, a non-significant correlation between these two traits has been reported (Bobby et al., 2012; Diondra et al., 2008). Height has been negatively correlated with yield and this may be due to a positive correlation between height and lodging (Yuan et al., 2002).
Yield performance is a prevalent issue when combining an advantageous trait (e.g. disease, pest or abiotic stress resistance) with a higher yield. A desirable trait once introduced in a developed cultivar can be beneficial or costly to the plant, depending on environmental conditions faced by the genotype in a given location or year (Walters et al., 2007).
The behavior of different agronomic traits and their association with yield is a matter of interest when crossing parents in order to combine desirable traits (e.g. disease resistance, pest resistance, resistance to abiotic stress, etc.) with higher yields in a single improved cultivar. The objective of this study was to assess yield as well as other agronomic traits of a RIL population derived from the cross between ‘LS-97-1610’ and ‘Spencer’ in two locations (Dowell and Harrisburg) in 2011.
Plant material
The soybean lines used in this experiment were obtained from a population of RIL derived from a cross between LS-97-1610 and ‘Spencer’ (Kenworthy, 1996; Wilcox et al., 1989). Hybridization was performed at Southern Illinois University Carbondale (SIUC) in 2004. The F1 plants were cultivated in greenhouse conditions whereas the F2 plants were grown in the field at the Agronomy Research Center at SIUC. The generation advancements from F3 until F5 were carried out in a winter nursery in Puerto Rico using the single-pod-descent method (Fehr, 1987). The F5:6 RIL were obtained in 2006 by selecting individual plants from the populations. These were later sent to Carbondale, IL for seed increase. In 2011, 94 F5:8 RIL originated from this population along with their parents Spencer and LS-97-1610 were sown in Dowell, IL and Harrisburg, IL. A randomized complete block design with two replications was used. The plots consisted of two rows -3.0 m long and 76 cm apart; plots were spaced 1.22 m from each other. The planting was carried out with eight seeds for each 0.3 m at 1.9 cm depth. The evaluated traits were seed yield, height, maturity and lodging. Maturity was recorded at the R8 stage, when 95% of the pods in a plot had reached the mature pod color (Fehr et al., 1971). At this stage plant height and lodging were also recorded. A method using a scale from 1 to 5 was employed to score lodging, on which 1 means all plants were standing and 5 means all plants were prostrate.
Statistical analysis
All the descriptive statistics including range, means, standard deviations as well as frequency distributions and the Shapiro-Wilk tests for normality for all traits were calculated using JMP 11.2 (SAS Institute Inc., Cary, NC, USA). All analyses and tests were performed for the RIL population derived from the cross between ‘Spencer’ and ‘LS-97-1610’ at two locations (Dowell and Harrisburg) in 2011 without using parental data, parents’ means and standard deviation were also calculated using JMP 11.2. The comparison of RIL population against Mid-parental values were performed using a one sample t-test. The ANOVA used genotypes, locations and genotypes x locations as main effects. A Dunnett’s comparison procedure was performed to test for significance of transgressive segregations of RIL population against the parents at each location. The Pearson’s correlation matrix was used to determine the correlation between traits. The broad-sense heritability estimates for all traits were calculated using values derived from ANOVA table (PBSTAT 1.2, 2014).
RIL vs Parents
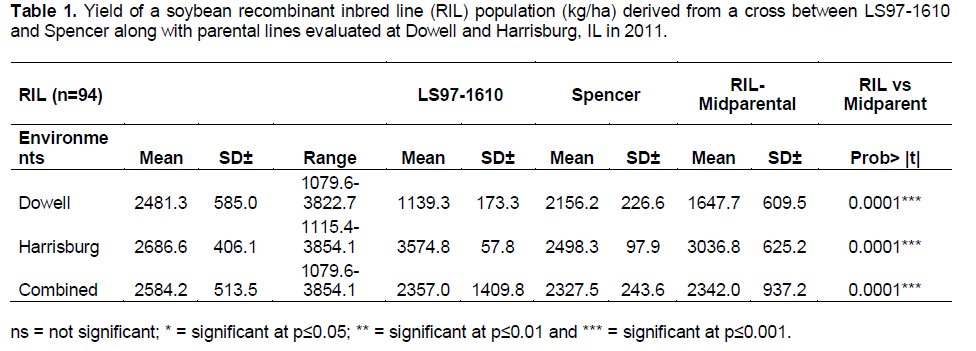
The yield performance of RIL population was compared with the parents. Analysis was performed for each environment and for the combined data set from both locations. The means, standard deviation, and range are presented in Table 1. The RIL yield range varies from 1079 kg.ha-1 to 3822 kg.ha-1 and 1115 kg.ha-1 to 3854 kg.ha-1 for Dowell and Harrisburg, respectively. In order to compare the RIL performance against both parents a one sample t-test was applied. In Dowell and in the combined data set the RIL had highly significant greater yields than their mid-parental values (p≤0.0001). However, in Harrisburg the mid-parental yield was highly significant greater than the RIL (p≤0.0001). Regarding the comparison between parents’ mean yield in each location they were significantly different in Dowell and Harrisburg at p≤0.0371 and p≤0.0055, respectively. However, no significant differences were found in the combined data set for the parents.
Aiming to analyze the RIL’s yield range behavior a transgressive segregation analysis was performed. Significant transgressive segregation was detected for high and low parents (p≤0.05) at all locations and for the combined data set.
Frequency distribution
The RIL frequency distributions for yield at two locations and the combined data set are shown in Figure 1. The yield distribution for Dowell was the only one to approach normality (W=0.986, p>0.05) according to the Shapiro-Wilk test. In Harrisburg and for the combined data set the distributions were found skewed to the left with g1=-0.57 and g1=-0.56, respectively.
ANOVA and broad-sense heritability
ANOVA for data from yield, height, maturity, and
lodging was performed for the RIL and the results are presented in Table 2. Highly significant differences between genotypes for all agronomic traits were detected. Also, a highly significant effect (p≤0.0001) for the interaction between genotypes and environments for yield, maturity and lodging traits were detected, except for which showed no significant effect at p≤0.05. Thus the RIL had different behaviors for agronomic traits and also showed significant interaction with the analyzed locations. The coefficient of variation (CV) ranged from 13.81% for maturity to 35.50% for lodging whereas yield had a CV of 19.87%. The broad-sense heritability (h2bs) was also calculated for RIL data from agronomic traits and the results are in Table 2. Maturity earned the highest h2bs estimation of 90.83% whereas yield had the lowest h2bs estimation of 34.84%.
Correlation analysis
The Pearson’s r coefficients were estimated for each pair-wise combination of agronomic traits and the results are presented in Table 3. Apart from the combination yield x lodging (r=-0.0685, not significant at p>0.05), all other possible combinations between agronomic traits showed highly significant correlations (p≤0.0001). Analyzing each agronomic trait combination, maturity was positively correlated with lodging, earning the highest coefficient correlation of 0.4313. Lodging showed positive correlation with height which earned an r value of 0.3646. Height was positively correlated with yield (r=0.2454) and with maturity (r=0.1963). The latter trait was positively correlated with a yield (r=0.1579).
This study analyzed the performance of a RIL population derived from a cross between ´LS-97-1610´ and ´Spencer´ regarding some agronomic traits and yield. Considering yield distribution, it approached normality at Dowell. However, it deviated from normality at Harrisburg and for the combined data set (Figure 1). Broad-sense heritability was obtained from the data (h2bs). It scored above 70% for height, maturity and lodging which means it was driven mainly by genetic factor. On the other hand, yield was driven by both genetic and environmental effects given that it scored 38.84%. All the estimated values were higher than those obtained by Panthee et al. (2007) where the heritability estimate varied from 12 to 65% for yield and seed filling period respectively. Therefore, inheritance of the evaluated traits observed in this RIL population from ´LS-97-1610´ x ´Spencer´ can be useful for improvement of soybean genotypes in breeding programs.
Regarding correlation analyses, although none of them have shown strong positive correlation (r>0.5), they were all highly significant except for yield x lodging. Among these estimates we found maturity highly correlated with lodging (r=0.4313) and also lodging correlated with height (r=0.3646). These correlation coefficients confirm the results obtained from Panthee et al. (2007) where they found height being positively correlated with lodging (r=0.58) and lodging significantly correlated with maturity (r=0.17). Height was found being positively correlated with yield (r=0.2454); this estimate is close to the one obtained by Bobby et al. (2012) which was r=0.307 and lower than the value obtained by Cicek et al. (2006) of r=0.58.
Even though the parents’ mean yields were significantly different in both evaluated environments, the mean yields for the combined data set were very similar (Table 1) and no significant difference was detected between them. This might be due to their divergent performance in each environment where ‘LS-97-1610’ was superior to ‘Spencer’ at Dowell whereas at Harrisburg the opposite happened with ‘Spencer’ having greater mean yield than ‘LS-97-1610’. This provides strong evidence that environmental factors played a major role in the phenotype expression of these cultivars.
Indeed, a highly significant effect was detected for genotypes x environments (GxE) interaction (Table 2). Appraisal of genotypes in the presence of GxE interaction can be beneficial since it allows to identify more stable genotypes in the development of new varieties (Hebert et al., 1995).
The mean yield of the RIL population was significantly greater than mid-parental yield at Dowell and the combined data set (Table 1) whereas in Harrisburg mid-parental yield was significantly greater than RIL population mean yield. Furthermore, the transgressive segregation analysis for yield confirmed the RIL population’s behavior with a significant positive transgressive segregation for both parents in Dowell and the combined data set. On the other hand, the lower performance in yield of the RIL population against parents in Harrisburg was supported by a significant negative transgressive segregation for both parents at that location.
This observed behavior validated with the verified heritability estimation for yield (h2bs=34.84) can assist the breeder with substantial information when defining strategies for improving genotypes for higher yields in a plant breeding program.
A major issue when dealing with phenotypic data from RIL populations is the complex inheritance of quantitative traits. A GxE interaction effect can be estimated through evaluation of a RIL population in different environments. A comparative analysis between RIL and their parents allows us to determine a positive and negative transgressive segregation, while at the same time selecting for better yield and other traits. In addition to yield data, other agronomic traits such as height, maturity and lodging enable the breeder to select genotypes from RIL populations. Therefore, the results from this study have revealed promising broad-sense heritability for yield and other agronomic traits for a RIL population derived from SDS resistant and highly susceptible parents. The genotyping of this population for yield components is being carried out through the single nucleotide polymorphism (SNP) method. This will allow the identification of major and minor Quantitative trait loci (QTLs) for yield improvement of RIL populations derived from SDS susceptible and resistant parents.
The authors have not declared any conflict of interests.
The authors would like to thank the United Soybean Board and the North Central Soybean Research for their support. This study was partially funded by a scholarship from the Brazilian Coordination for the Improvement of Higher Education Personnel (CAPES - Process BEX 11900/13-0).
REFERENCES
|
Bobby R, Bazelle R, Willsheana C, Kantartzi SK, Meksem K, Akond M, Kassem A (2012). Genetic analysis of yield components in the PI438489B by 'Hamilton' recombinant inbred line (RIL) population of soybean [Glycine max (L.) Merr.]. J. Agric. Sci. 4(9):98-105. |
|
|
Cicek MS, Chen P, Saghai Maroof MA, Buss GR (2006). Interrelationships among agronomic and seed quality traits in an interspecific soybean recombinant inbred population. Crop Sci. 46(3):1253-1259.
Crossref |
|
|
Cucolotto M, Pipolo VC, Garbuglio DD, Fonseca Junior NS, Destro D, Kamikoga MK (2007). Genotype x environment interaction in soybean: evaluation through three methodologies. Crop Breeding Appl. Biotechnol. 7:270-277.
Crossref |
|
|
|
Diondra W, Ivey S, Washington E, Woods S, Walker J, Krueger N, Sahnawaz M, Kassem MA (2008). Is there a correlation between plant height and yield in soybean? Reviews Biol. Biotechnol. 7(2):70-76. |
|
|
|
Fehr WR (1987). Principles of cultivar development. v.1. New York, NY. Macmillan Publishing Company. |
|
|
Fehr WR, Caviness CE, Burmood DT, Pennington JS (1971). Stage of development descriptions for soybeans, Glycine max (L.) Merrill. Crop Sci. 11:929-931.
Crossref |
|
|
Hebert Y, Plamion C, Harzic N (1995). Genotype environment interaction for root traits in maize as analysed with factorial regression models. Euphytica 81:85-92.
Crossref |
|
|
Kenworthy WJ (1996). Registration of 'Manokin' soybean. Crop Sci. 36:1079
Crossref |
|
|
Mansur LM, Orf JH, Chase K, Jarvik T, Cregan PB., Lark KG (1996). Genetic mapping of agronomic traits using recombinant inbred lines of soybean. Crop Sci. 36:1327-1336.
Crossref |
|
|
Orf JH, Chase K, Jarvik T, Mansur LM, Cregan PB, Adler FR, Lark KG (1999). Genetics of soybean agronomic traits: I. Comparison of three related recombinant inbred populations. Crop Sci. 39:1642-1651.
Crossref |
|
|
Panthee DR, Pantalone VR, Saxton AM, West DR, Sams CE (2007). Quantitative trait loci for agronomic traits in soybean. Plant Breed. 126:51–57.
Crossref |
|
|
Sherrie I, Khaled O, Washington E, Lage P, Woods S, Kantartzi SK, Meksem K, Lightfoot DA, Kassem MA (2011). Evaluation of several agronomic traits in 'Essex' by 'Forrest' recombinant inbred line population of soybean [Glycine max (L.) Merr.]. Atlas J. Plant Biol. 1(1):13-17.
Crossref |
|
|
Specht JE, Chase K, Macrander M, Graef GL, Chung J, Markwell JP, Germann M, Orf JH, Lark KG (2001). Soybean response to water: A QTL analysis of drought tolerance. Crop Sci. 41:493-509.
Crossref |
|
|
Yuan J, Nijti VN, Meksem K, Iqbal MJ, Triwitayakorn K, Kassem MA, Davis GT, Schmidt ME, Lightfoot DA (2002). Quantitative trait loci in two soybean Recombinant Inbred Line Populations segregating for yield and disease resistance. Crop Sci. 42:271-277.
Crossref |
|
|
Walters D, Heil M (2007). Costs and trade-offs associated with induced resistance. Physiol.Mol. Plant Pathol. 71:3-17.
Crossref |
|
|
Wilcox JR, Roach MT, Abney TS (1989). Registration of 'Spencer' soybean. Crop Sci. 29:830-831.
Crossref |