ABSTRACT
Fusarium related root rots have been associated with reduced cowpea productivity in Uganda. Sources of genetic resistance to Fusarium redolens which was found to be the most virulent have been identified but the mode of inheritance of the genes conferring the resistance is unknown. This study aims to investigate how the genes for resistance to F. redolens are inherited in cowpea. Four F. redolens root rot resistant cowpea genotypes were crossed with four intermediately resistant and 2 susceptible cowpea genotypes using North Carolina mating design II. The F1 and the parents were evaluated and data were collected on resistance to seed rot, leaf chlorophyll amount, produced lateral roots, response to plant mortality and root rot severity. Results revealed that additive gene effects were significant for all evaluated traits and non-additive genetic effects were significant in resistance to seed rot and chlorophyll amount. General combining ability (GCA) effects showed that the Asontem genotype was a good combiner for increased lateral roots production and resistance to root rot. Degree of dominance estimates revealed that response to plant mortality, root rots and increased lateral root production traits were recessively inherited while seed rot and amount of leaf chlorophyll were dominantly inherited.
Key words: Vigna unguiculata, Baker’s ratio, combining ability, Fusarium redolens, heritability, Uganda.
Cowpea [Vigna unguiculata (L.) Walp.] which originated in Africa (Tan et al., 2012) is one of the most important grain legume crop grown in sub-Saharan Africa (Badiane et al., 2012). Amongst its important attributes, cowpea can be used in human nutrition where it provides adequate amount and quality of protein and as animal feed (hay) during the dry season in many parts of Africa (Badiane et al., 2012). It has high protein content ranging between 23 and 32% of seed weight rich in lysine and tryptophan, and a considerable amount of vitamins (folic acid and vitamin B) attributes that have led to the crop being referred to as the “poor man’s meat” (Tan et al., 2012). Additionally, cowpea is an important source of income to the resource poor for farmers in Africa (Langyintuo et al., 2003; Timko et al., 2007; Timko and Singh, 2008; Diouf, 2011). Moreover, cowpea is an important rotation and cover crop with nitrogen-fixing ability which makes its valuable when rotated with cereal crops (Timko et al., 2007).
In Uganda, cowpea is intensively grown in the eastern and northern regions with over 90% of the households engaged in commercial production for food and cash income. Although, the expected yield potential of cowpea attained on station is 3,000 kg/ha, yield at farmer’s level averages at 500 kg/ha (Rusoke and Rubaiyaho, 1994). The low productivity has been attributed to several factors, but most importantly is due to prevalence of diseases (Rusoke and Rubaiyaho, 1994; Edema et al., 1997). Among the diseases, Fusarium root rot (Fusarium redolens) has been reported to be devastative to cowpea in Uganda. The disease can result in extremely high infection especially with susceptible cowpea cultivars, when prevailing environmental and host factors are favourable.
Development and use of resistant varieties is the most sustainable and cost effective method in the management of various diseases (Pottorff et al., 2012). Moreover, the information on inheritance of resistance to F. redolens is imperative for future breeding activity to develop resistant cowpea varieties. However, the mode of gene action and the pattern of inheritance of resistance to Fusarium root rots of cowpea in Uganda have not been well understood. Therefore, the use of sources of resistance to introgress resistance into susceptible landraces with desired agronomic traits in breeding programmes is limited. The estimates of combining ability and gene actions are important in identifying parents with superior genes based on the general combining ability and specific combining ability effects with better mean performance. This information could be used as a basis of determining the breeding method and the population to use in order to reach target goal. Therefore, the objective of this study was to determine the mode of inheritance governing resistance to F. redolens in cowpea in Uganda.
Study area
The study was conducted in a screen house at Makerere University Agricultural Research Institute, Kabanyolo (MUARIK). MUARIK is located between 320 37’E, and 00 28’N at 1200 m above sea level in Wakiso district, Central Uganda. The annual rainfall and temperature were 1150 mm and 21.50°C, respectively. The experiment was conducted from May to September, 2016.
Hybridization
Ten cowpea parental lines were selected for this study based on their reaction to root rot caused by F. redolens. The selected resistant cowpea genotypes (males: ASONTEM, IT98KD-288, Dan 1LA and NE 70) were crossed with landraces and cultivars that had intermediate resistance (female: NE 50, NE 6, SECOW 2W and SECOW 3B) and susceptibility (females: KVU 27-1 and WC66) (Table 1) using North Carolina Mating Design II (NCII). The parents were planted in the crossing block in the screen house constituting one row of twenty-six plants (13 hills) at 20 × 100 cm spacing. Five grams of di-ammonium phosphate was used per hill of 2 plants to boost the growth of the plants. At 35 days after planting (DAP), the plants were staked to avoid intertwining with different genotypes. In NCII, every progeny family has half sib relationships through both common male and female. This was accomplished by mating n1 male with n2 female in all possible combinations to give n1n2 progeny families (Nduwumuremyi et al., 2013). Crossing was done to generate 24 F1 family crosses. At maturity, the seeds of each F1 cross were harvested separately.
Evaluation of F1 crosses and parents for resistance to Fusarium root rot in screen house
Sterile sorghum was used as a medium to multiply the inoculum as described by Mugisha (2010). F. redolens was cultured for 3 weeks in 500-ml capacity flasks each containing 200 g of sorghum seeds. Two flasks of mature F. redolens were added in each of the six wooden trays (150 cm× 100 cm× 13 cm) containing thoroughly mixed pre-sterilized soil (3:1, loam: sand) (Mukankusi et al., 2011). The trays were covered with dark polythene for a week to incubate the inoculum in the soil. Three susceptible cowpea genotypes, IT889, KVU 27-1 and WC 66, were then planted in each of the trays for up to 28 days and uprooted. This was repeated 3 times to ensure that the trays had adequate inoculum. The trays were watered 4 days per week (Mugisha, 2010; Ongom et al., 2012). After each cycle, soil was removed from the trays and mixed thoroughly and then redistributed equally to encourage uniform inoculum amounts before the test lines were planted. Planting was done using alpha lattice design (5 blocks × 7 plots) with 6 replications. The seeds of F1 cross and parents were surface sterilized and planted separately in the F. redolens inoculated soil contained in 6 wooden trays. Each plot consisted of a single row of 7 plants representing a particular material (F1 cross or parent). The trays were placed on raised benches in the screen house and watered four times a week (Mugisha, 2010).
Data collection
Seed rot was assessed by counting the number of germinated seeds for all the test populations 6 days after planting (DAP) and expressing the number as a proportion of the total seeds planted (Equation 1). Leaf chlorophyll content was assessed 27 DAP using PhotosynQ; Soil Plant Analysis Development 3 (SPAD 3). On the 28th day, response to plant mortality was assessed by counting the number of dead plants per test population and recorded as a percentage of dead plants (Equation 2). Thereafter, the remaining plants per line (cross or parent) were carefully and separately uprooted and then the below ground parts of the plant (roots and hypocotyls) were washed under running tap water. The percentage of plants per line with lateral roots above or at the ground level was recorded (Equation 3), and root rot severity was assessed by scoring root and hypocotyl damage according to the C1AT 1-9 scale (Abawi and Pastor-Corrales, 1990), where 1=No visible symptoms, 3=Light discoloration either without necrotic lesions or with approximately 10% of the hypocotyl and root tissues covered with lesions, 5=Approximately 25% of the hypocotyl and root tissues covered with lesions, but tissues remain firm with deterioration of the root system, 7=Approximately 50% of the hypocotyl and root tissues covered with lesions combined with considerable softening, rotting, and reduction of root system, 9=Approximately 75% or more of the hypocotyl and root tissues affected with advanced stages of rotting combined with severe reduction in the root system and dead plants.
% Germination = Number of germinated seed / Total planted × 100
(1)
% Dead plants = Counted dead plants / Total emerged plants × 100
(2)
% Lateral roots = Number of plants with lateral roots/
Total scored plants × 100 (3)
Data analysis
Determination of combining ability effects
Variance of the crosses (Equation 4) was analysed using GENSTAT 12th edition (Payne et al., 2009). Female and male parents were considered as fixed factors. General combining ability (GCA) effect was estimated as the difference between the grand mean and the mean of all crosses of a particular parent. High GCA effects indicated predominance of additive genes over the non-additive and vice versa. The specific combining ability (SCA) was estimated as the difference between the predicted mean of a particular cross and its observed mean. High SCA effects meant more none-additive gene effects (where dominance and/or epistasis may be prominent). A two-sided t-test was used to test and determine if individual GCA and SCA effects were significantly different from 0, based on the standard error associated with that effect.
where d/a = degrees of dominance, F1 = cross mean, MP = mean of two parents (P1 + P2)/2.
The results were interpreted as recommended by Kearsey and Pooni (1996), where │d/a│ = 1 indicates complete dominance, 0 < │d/a│< 1 indicates partial dominance, │d/a│ = 0 indicates no dominance, and │d/a│ > 1 indicates over-dominance.
Combing ability estimates, variance components, heritability and Baker’s ratio for resistance of cowpea genotypes to F. redolens
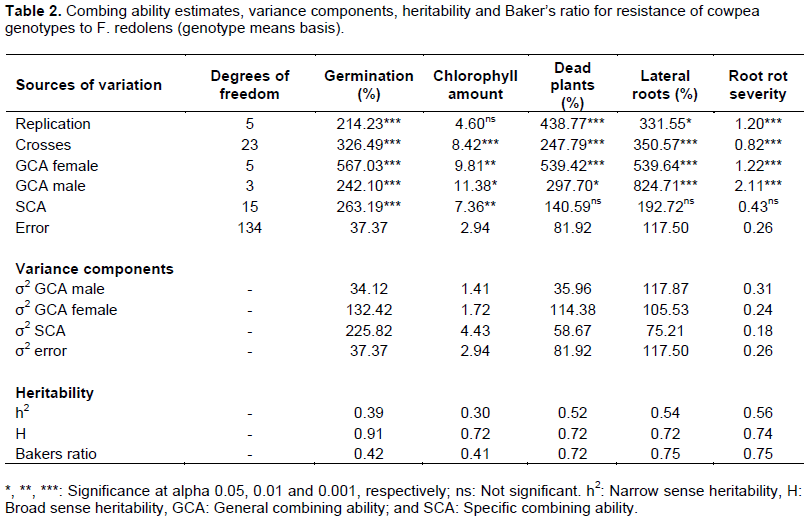
The results of the combining ability analysis are shown in Table 2. The results indicated that the crosses had significantly different effects for all the traits studied (p<0.001). GCA effects of both female and male parents were also significantly different for all the parameters studied. As for the crosses’ SCA effects, significant difference was observed only for percentage of germination and chlorophyll amount (p<0.01). Comparing the relative importance of additive genetic effects over non-additive effects, results showed that parameters, percentage of dead plants, lateral roots and root rot severity had high estimate Baker’s ratio (BR>0.71), while percentage of germination and chlorophyll amount had a relatively moderate Baker’s ratio (0.42 and 0.41, respectively). The estimate of broad sense coefficient of genetic determination was relatively high for all the parameters studied. On the other hand, the estimate of narrow sense coefficient of genetic determination was from relatively low for percentage of germination and chlorophyll amount (0.39 and 0.30, respectively) to moderate for percentage of dead plants and lateral roots and root rot severity (0.51 < h² < 0.57).
General combining ability (GCA) effects for resistance of parental genotypes to F. redolens
The two-sided t-student test (Table 3) showed that the parental lines Dan 1LA, SECOW 2W, and WC 66 had significant negative GCA effects (P<0.01, P< 0.01 and P<0.05, respectively) for germination percentage, while NE 50 and NE 6 had significant positive GCA effects for the same trait. Genotype WC 66 had the only negative and significant (P<0.05) GCA effect for amount of chlorophyll in the leaves. For percentage of dead plants due to F. redolens infection, genotype NE 6 had a negative and significant (P<0.05) GCA effect and WC 66 had a positive significant (P< 0.05) GCA effect. In the percentage of plants with lateral roots, ASONTEM had a significant (P<0.05) positive GCA effect, while NE 70 and WC 66 had negative and significant (P<0.05) GCA effect.
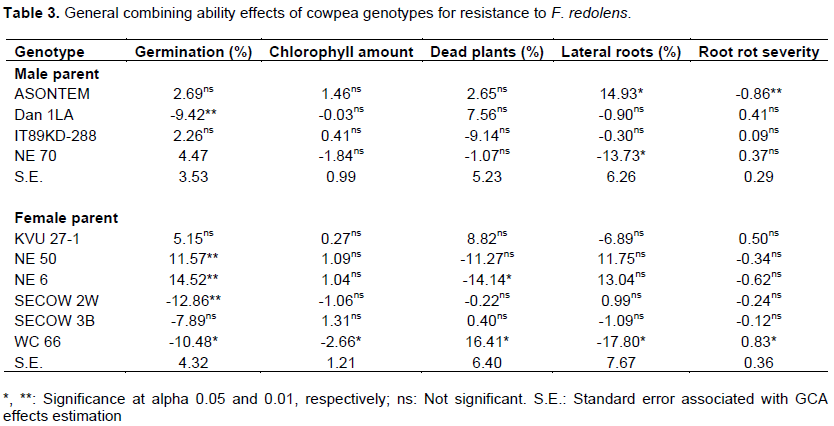
For root rot severity, ASONTEM had a negative and significant GCA effect (P<0.01), while WC 66 had a significant (P<0.05) positive GCA effect. In addition, 3 male parents (Dan 1LA, IT89KD-288 and NE 70) showed positive but non-significant GCA effects for root rot severity, while all the intermediate resistant parents (NE 50, NE 6, SECOW 2W and SECOW 3B) had negative but non-significant GCA effects.
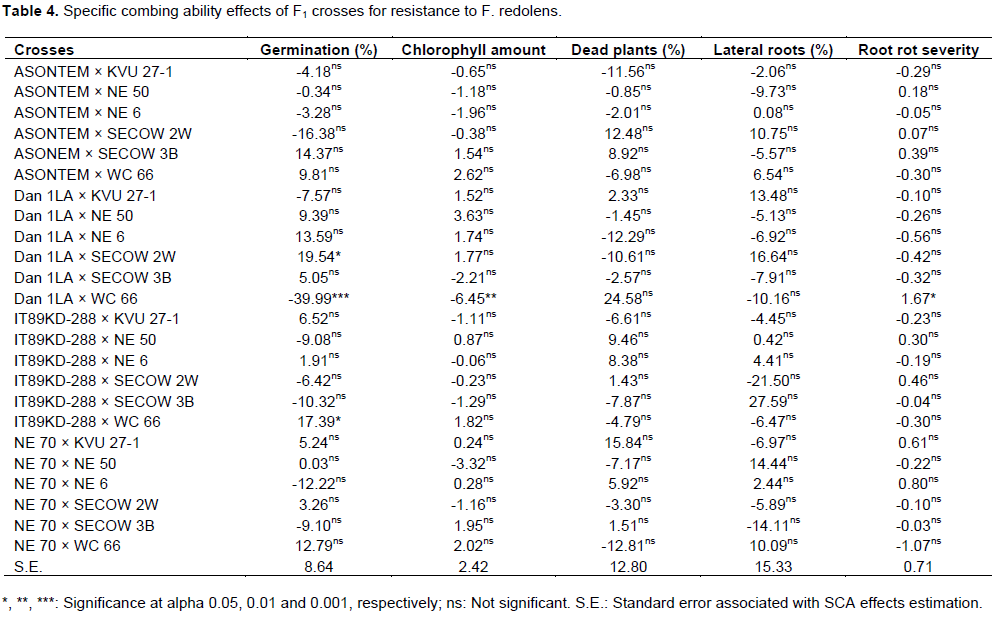
Specific combing ability (SCA) effects of F1 crosses for resistance to F. redolens
Specific combining ability effects are shown in Table 4. The results indicated non-significant SCA effects of all the crosses for percentage of dead plants and percentage of plants with lateral roots. A highly significant (P<0.001) negative SCA effect was recorded in the cross Dan 1LA × WC 66, while Dan 1LA × SECOW 2W and IT89KD-288 × WC66 had positive and significant (P<0.05) SCA effects for percentage of germination. For the amount of chlorophyll in the leaves, the cross Dan 1LA × WC 66 had a negative and significant (P<0.01) SCA effects, while the same cross had a positive and significant (P<0.05) SCA effect for root rot severity. The general observation on root rot severity of F. redolens showed that the crosses NE 70 × WC 66, Dan 1LA × NE 6 and Dan 1LA × SECOW 2W had the most negative but non-significant SCA effects.
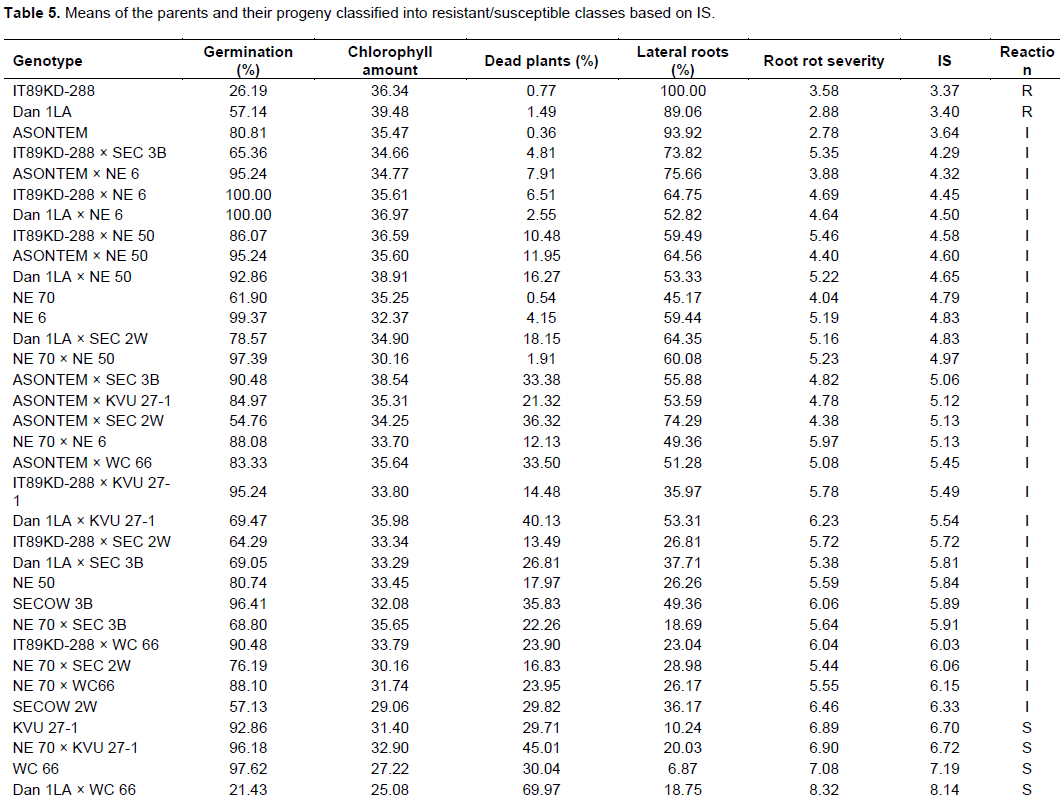
Mean performance of 10 parental genotypes and 24 F1s in response to F. redolens infection
Genotypic mean performance (Table 5) indicated that genotype NE 70 had higher root rot severity scores and index of susceptibility (IS) mean than was expected since it was selected as a resistant parent. However, 3 resistant parents showed desirable performance with all having an IS score below 3.65. On average, none of the progeny families performed better than their resistant parents in the specific crosses, but were noticed to lean more towards the susceptible parents. In fact, some crosses like Dan 1LA × WC 66, NE 70 × KVU 27-1, NE 70 × SEC 3B and NE 70 × NE 6 had a greater value of IS than their susceptible parents.
Degree of dominance of F1 crosses for resistance to F. redolens
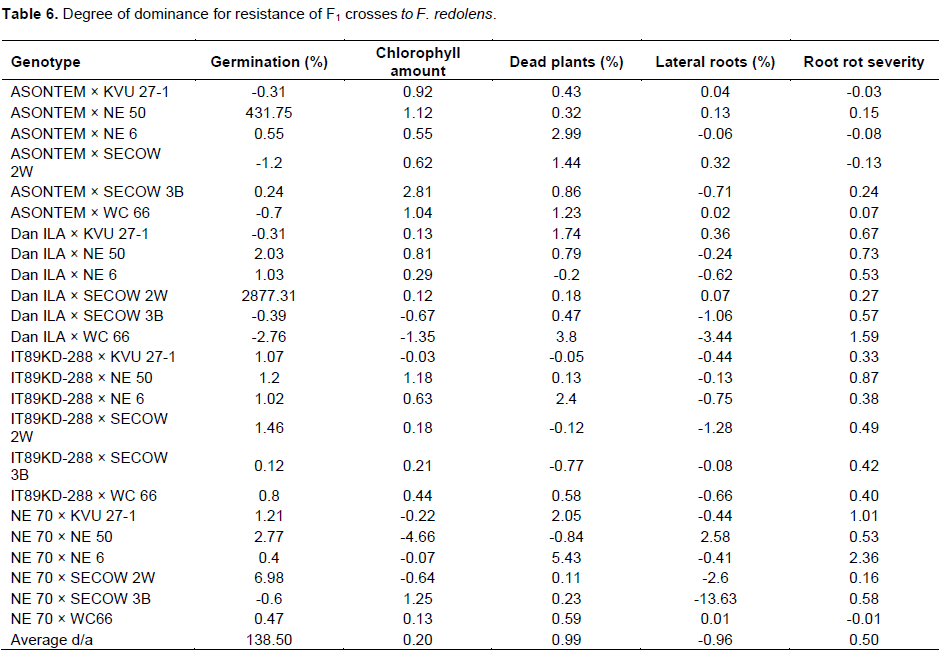
Degree of dominance (d/a) results of the various traits considered in the study are presented in Table 6. Results from germination percentage had 7 crosses with d/a<0. The cross Dan 1LA × WC 66 had the most negative d/a (-2.76), while the cross Dan 1LA × SECOW 2W had the most positive d/a (2877.31). Considering the amount of chlorophyll in the leaves, 7 crosses had d/a<0 with the cross NE 70 × NE 50 being the most negative (d/a= -4.66), while 4 crosses had d/a>1 with the cross ASONTEM × SECOW 3B having the most positive d/a (2.81). The percentage of dead plants indicated that 19 crosses had d/a>1 with the cross NE 70 × NE 6 being the most positive d/a (5.43). In regards to percentage of plants with lateral roots, it was revealed that 16 crosses had d/a<1 with the cross NE 70 × SECOW 3B having the most negative d/a (-13.63), while the cross NE 70 × NE 50 had d/a>1. Results of root rot severity revealed that 20 F1 crosses had d/a>0. For this parameter, it was observed that the cross NE 70 × NE 6 had the most positive d/a (2.36). Moreover, 3 out of the 4 negative d/a for root rot severity were observed in the crosses where ASONTEM was the male parent. Averagely, the d/a for germination percentage was greater than 1, while for percentage of plants with lateral roots was less than 0, but greater than -1. The average d/a for percentage of dead plants, amount of chlorophyll in the leaves and root rot severity was greater than 0, but less than 1.
The high significant difference observed among the crosses’ performance was indicative of high Genetic diversity among the parental lines and their progenies. Thus selection can be made among these genotypes for genetic improvement of the parameters studied. Besides, there was significant difference observed for GCA mean of squares of both male and female parents for all the traits suggesting that the resistance of cowpea to F. redolens is mainly controlled by additive genetic effects. Since the male parent was expected to pass resistance and female parent to pass susceptibility, this would mean that both resistance and susceptibility genes were passed on to the progeny. In addition to the GCA effects, SCA mean of squares were also significant for percentage of germination and amount of chlorophyll in the leaves implying that these two traits were controlled by both additive and non-additive genetic effects. In contrast, SCA mean of squares were not significant for percentage of dead plants, percentage of plants with lateral roots and root rot severity. These implied that the non-additive genetic effects had minor influence on these traits and additive gene action provided a larger contribution in the crosses than the non-additive gene action. This was further confirmed by the relatively high values of Baker’s ratio (BR >0.71) that were observed for these traits, suggesting that the performance of the progeny could be accurately predicted based on the parental GCA effects as reported by Baker (1978) and Bernardo (2002). As far as percentage of germination and chlorophyll amount are concerned, the non-additive genetic effects were predominant over the additive, hence poor predictability of the progeny’s performance.
The estimated moderate h² indicated that about 50% of the total phenotypic variation observed for percentage of dead plants, % of plants with lateral roots and root rot severity, was due to additive genes effects. These results suggested that selection at early generation would be fairly effective for improving F. redolens resistance in cowpea as outlined by Baker (1978) and Piepho and Möhring (2007).
Earlier studies have proven that lateral roots are very essential to the complexity of resistance to root rots (Snapp et al., 2003). However, in order to target this trait for improvement of resistance to F. redolens through breeding, it is essential to consider parents that exhibit high frequency of lateral roots. For percentage of germination and amount of chlorophyll, h2 was low, suggesting that additive genes had a small contribution to the overall phenotypic variation; hence, selection would be more appropriate at advanced generation. Further observations revealed that genotypes which developed cracks on the testa were highly vulnerable to seed rot. Accordingly, Souza and Marcos-Filho (2001), seed coat traits (e.g. permeability) which determines the ability of the seed to resist fungal rots are influenced by both genetic and environmental effects. Earlier study by Ismail et al. (2000) into stay green traits led to the conclusion that the ability of cowpea to retain chlorophyll (delayed leaf senescence) in stressed condition is highly correlated to its resistance to pathogenic Fusarium spp. These results are consistent with their findings where the materials (parents/crosses) that showed moderate to high resistance retained high chlorophyll amount throughout the test period.
The parents NE 50 and NE 6 showed positive and desirable significant GCA effects for high germination and therefore were associated with resistance to seed rot caused by F. redolens showing that they are good combiners for improvement of this trait. On the contrary, Dan 1LA, SECOW 2W, SECOW 3B and WC 66 had negative GCA effects for low germination and therefore were not associated with resistance to seed rot caused by F. redolens implying that they are poor combiners for improvement of this trait. Likewise, WC 66 significantly contributed to reduced leaf chlorophyll level, reduced lateral roots but increased mortality of plants and increased root rot severity in its progenies due to F. redolens infection. The genotype NE 70 with a negative and significant GCA effect contributed to the reduction of susceptibility of cowpea plant mortality in its progenies thus making it a good combiner for this trait. However, NE 70 had a negative and significant GCA effect for percentage of plants with lateral roots implying that its crosses would have reduced lateral roots thus become vulnerable to F. redolens infection. On the other hand, ASONTEM had significant positive GCA for lateral roots and negative for root rot severity making it a good combiner for improving these two traits. The other male parents had positive but non-significant GCA effects for root rot severity. This indicated that there is a possibility of these parents passing susceptibility to root rot to their progenies. Contrastingly, all the intermediate resistant parents had negative GCA effects for root rot severity which implied they were good combiners and would pass resistance to root rots to their progenies.
The cross Dan 1LA × WC 66 with undesirable SCA effects for all the traits was a poor combination in all the traits. These undesirable observed SCA effects observed indicated that the cross’s performance was below what could be predicted from the GCA effects of the parents (Bernardo, 2002; Falconer and Mackay, 1996). Contrary to this, IT89KD-2288 × WC 66 showed a positively significant SCA effect for percentage of germination making it a good combination as the progeny had 17.39% better germination than the expected germination. Moreover, several crosses showed negative but non-significant SCA effects for root rot severity which indicated they could be good combiners.
The results of parental means saw 2 of the parents (ASONTEM and NE 70) performing lower than had been recorded in previous study. This could be attributed to higher levels of inoculum in the soil that might have affected their response to the pathogen especially in relation to lateral roots production. Moreover, the progenies were observed to perform poorer than the resistant parents. The results from the d/a indicated an average over dominance for high germination percentage (d/a>1) and an average partial dominance for high amount of chlorophyll in the leaves (0<d/a<1), high percentage of dead plants (0<d/a<1), low percentage of plants with lateral roots (-1<d/a<0) and high root rot severity (susceptibility) (0<d/a<1) in accordance with the description provided by Kearsey and Pooni (1996) and Falconer and Mackay (1996). This implies that for the parameters considered in this study, the favourable alleles contributing to high germination percentage and high amount of chlorophyll in the leaves were dominantly inherited while the alleles of low percentage of dead plants, high percentage of plants with lateral roots and low root rot severity (resistance) were recessively inherited as observed by the values of average d/a. The high frequency of crosses showing desirable over dominance for germination percentage, suggests that there was great possibility for improvement through selection methods by targeting those crosses that showed better performance than the better parent (Rieseberg et al., 1999). In contrast, the traits with recessive favourable alleles require extensive testing at a segregating generation to select progenies with desirable phenotypes. The cross Dan 1LA × WC66 showed over dominance towards susceptibility to F. redolens, thus provided poor combination when all the traits are considered. Furthermore, the combination of Dan 1LA and WC 66 led to poor seed development that was coupled with cracks on the testa and these paved ways to early infection leading to low germination and high mortality of plants at early stages of growth. Souza and Marcos-Filho (2001) emphasized on the importance of seed coat in protecting the seed from infection, a factor that was confirmed in this study.
Dead plants percentage, lateral roots percentage, and root rot severity were found to be majorly conditioned by additive genetic effects, while both additive and non-additive gene effects were involved in the inheritance of genes leading to increased germination and amount of leaf chlorophyll. Early-generation selection could be effective for percentage of dead plants, percentage lateral roots and root rot severity, while selection at advanced would be preferable for percentage germination and amount of leaf chlorophyll. Genotypes, NE 50 and NE 6 were the best combiners for percentage germination, while ASONTEM was the best combiner for percentage lateral roots and root rot severity as observed from GCA effects. These parents with desirable GCA effects for particular traits should be incorporated into breeding programmes and used for improving the other genotypes so as to achieve better resistance to F. redolens.
Response to plant mortality, lateral roots production and resistance root rot were found to be recessively inherited, while percentage germination and level of chlorophyll in the leaves were dominantly inherited. Moreover, the cross Dan 1LA × WC 66 had the worst performance across all the traits with net over-dominance towards susceptible parent being recorded and significantly undesirable SCA effects for percentage germination, amount of leaf chlorophyll and root rot severity. Contrary to this, the cross Dan 1LA × SECOW 2W and IT89KD-288 × WC 66 had significant positive SCA effects in percentage of germination showing that they are good combination for this trait.
The authors have not declared any conflict of interests.
REFERENCES
|
Abawi GS, Pastor-Corrales MA (1990). Root rots of beans in Latin
|
|
|
|
America and Africa: Diagnosis, research methodologies, and management strategies. CIAT publication No. 35.
|
|
|
|
Badiane FA, Gowda BS, Cissé N, Diouf D, Sadio O, Timko MP (2012). Genetic relationship of cowpea (Vigna unguiculata) varieties from Senegal based on SSR markers. Genet. Mol. Res. 11(1):292-304.
Crossref
|
|
|
|
Baker RJ (1978). Issues in diallel analysis. Crop Sci. 18(4):533-536.
Crossref
|
|
|
|
Bernardo R (2002). Breeding for quantitative traits in plants (No. 576.5 B523). Stemma Press.
|
|
|
|
Diouf D (2011). Recent advances in cowpea [Vigna unguiculata (L.) Walp.] "omics" research for genetic improvement. Afr. J. Biotechnol. 10(15):2803-2810.
Crossref
|
|
|
|
Edema R, Adipala E, Florini DA (1997). Influence of season and cropping system on occurrence of cowpea diseases in Uganda. Plant Dis. 81(5):465-468.
Crossref
|
|
|
|
Falconer DS, Mackay TFC (1996). Introduction to Quantitative Genetics. Harlow, Essex, UK: Longmans Green.
|
|
|
|
Fehr WR (1987). Principles of cultivar development. Volume 1. Theory and technique. Macmillan publishing company.
|
|
|
|
Ismail AM, Hall AE, Ehlers JD (2000). Delayed-leaf-senescence and heat-tolerance traits mainly are independently expressed in cowpea. Crop Sci. 40(4):1049-1055.
Crossref
|
|
|
|
Kearsey MJ, Pooni HS (1998). The genetical analysis of quantitative traits. Stanley Thornes (Publishers) Ltd.
|
|
|
|
Langyintuo AS, Lowenberg-DeBoer J, Faye M, Lambert D, Ibro G, Moussa B, Kergna A, Kushwaha S, Musa S, Ntoukam G (2003). Cowpea supply and demand in West and Central Africa. Field Crops Res. 82(2):215-231.
Crossref
|
|
|
|
Mugisha CM (2010). Improving resistance to Fusarium root rot [Fusarium solani (Mart.) Sacc. f. sp. phaseoli (Burkholder) WC Snyder & HN Hans] in common bean (Phaseolus vulgaris L.). Doctoral dissertation University of KwaZulu- Natal.
|
|
|
|
Mukankusi CM, Melis RJ, Derera J, Buruchara RA, Mark D (2011). A screening technique for resistance to Fusarium root rot of common bean. Afr. J. Plant Sci. 5(3):152-161.
|
|
|
|
Nduwumuremyi A, Tongoona P, Habimana S (2013). Mating designs: helpful tool for quantitative plant breeding analysis. J. Plant Breed. Genet. 1(3):117-129.
|
|
|
|
Payne RW, Murray DA, Harding SA, Baird DB, Soutar DM (2009). GenStat for windows. Hemel Hempstead, VSN International. 11.
|
|
|
|
Piepho HP, Möhring J (2007). Computing heritability and selection response from unbalanced plant breeding trials. Genetics 177(3):1881-1888.
Crossref
|
|
|
|
Pottorff M, Wanamaker S, Ma YQ, Ehlers JD, Roberts PA, Close TJ (2012). Genetic and Physical Mapping of Candidate Genes for Resistance to Fusarium oxysporum f.sp. tracheiphilum Race 3 in Cowpea [Vigna unguiculata (L.) Walp]. PLoS ONE 7(7):e41600.
Crossref
|
|
|
|
Rieseberg LH, Archer MA, Wayne RK (1999). Transgressive segregation, adaptation and speciation. Heredity 83(4):363-372.
Crossref
|
|
|
|
Rusoke DG, Rubaihayo PR (1994). The influence of some crop protection management practices on yield stability of cowpeas. Afr. Crop Sci. J. 2(1):43-48.
|
|
|
|
Snapp S, Kirk W, Román-Avilés B, Kelly J (2003). Root traits play a role in integrated management of Fusarium root rot in snap beans. HortScience 38(2):187-191.
|
|
|
|
Souza FH, Marcos-Filho J (2001). The seed coat as a modulator of seed-environment relationships in Fabaceae. Braz. J. Bot. 24(4):365-375.
Crossref
|
|
|
|
Tan H, Tie M, Luo Q, Zhu Y, Lai J, Li H (2012). A review of molecular makers applied in Cowpea (Vigna unguiculata L. Walp.) Breeding. Life Sci. 6(11):1190-1199.
|
|
|
|
Timko MP, Ehlers JD, Roberts PA (2007). Cowpea in: Pulses, sugar and tuber crops, pp. 49-67.
Crossref
|
|
|
|
Timko MP, Singh BB (2008). Cowpea, A Multifunctional Legume. in: Genomics of Tropical Crop Plants (Moore PH and Ming R, eds.). Springer Science+Business Media, New York, pp. 227-258.
|