ABSTRACT
Allelopathic potential of Citharexylum spinosum L. (Verbenaceae) an exotic tree introduced in Tunisia many years ago was evaluated. Organic extracts using hexane, ethyl acetate and methanol solvents together with aqueous extracts at different concentrations were prepared from different parts of the plant (roots, stems, leaves and flowers). Yields in the 12 organic extracts together with their phenol contents were reported. Leaves methanol extract showed the highest yield and amount in total phenols (6.43%; 617.93±1.12 mg gallic acid equivalent/100 g MS, respectively). All extracts were tested on germination and early growth of two crops: (Lactuca sativa L.) and (Triticum aestivum L.) and two weeds: (Peganum harmala L.) and (Silybum marianum L.). Twelve parameters were established and used to the principal components analysis (PCA) and the hierarchical clusters (HCA) analysis. Three groups of extracts were separated according to their allelopathic potentiality. Almost of organic extracts were totally opposed to seed germination of peganum and thistle.
Key words: Allelopathy, bioherbicides, Citharexylum spinosum L., crops, extracts, polyphenols, weed management.
The overuse of herbicides has provoked increasing incidences of herbicide resistance in weeds (Valverde et al., 2000) and so disappearance of some susceptible species, which affect biodiversity (Itoh, 2004). Moreover, herbicides cause environmental pollution, unsafe agricultural products and human health concerns (Kohli et al., 1998; Xuan et al., 2004, 2005; Khanh et al., 2005). In response to this problem; the adverse effect of herbicides on people and environment, and the interest in environmentally friendly alternatives for weed control have rapidly increased in recent years (Amossé et al., 2013; Dommanget et al., 2014; Kruidhof et al., 2014). This research mainly focused on strategies of Integrated Weed Management System. Among the possible new strategies, agronomic solutions based on the use of plant natural compounds have been suggested (Dudai et al., 1999; Tworkoski, 2002; Campiglia et al., 2007). This approach would mainly rely on the exploitation of allelopathic effects. Allelopathy is defined as “any process that involves secondary metabolites produced by plants, algae, bacteria, and fungi that influence the growth and development of biological systems” (IAS, 1996). Chemicals that impose allelopathic influences are called allelochemicals or allelochemics (Einhelling, 1996).
These chemicals are largely classified as secondary plant metabolites (Rice, 1984). Allelochemicals are present practically in all plant tissues. They may be released from plants into their immediate environment (El-Khawas and Shehata, 2005; Bulut et al., 2006). These chemicals may exert their phytotoxic effect directly or indirectly as they selectively inhibit the growth of other plants, soil microorganisms or both (Lorenzo et al., 2013; Saraf et al., 2014). Originally, classified as waste products, allelochemicals more recently have been investigated extensively by ecologists and pharmacologists, and many complexes biological functions have been discovered (Hadacek, 2002). Now it has been established that allelopathic properties of plants can be exploited successfully as a tool for weed control (Mahajan and Chauhan, 2013). The advantage of utilizing natural compounds in sustainable agriculture patterns such as organic farming depends on their rapid decomposition in the environment (Tworkoski, 2002; Campiglia et al., 2007). Citharexylum spinosum L is one tree among many that produces sufficient biomass with allelopathic extracts that can be exploited for weed control purposes.
Citharexylum spinosum (syn. Citharexylum quadrangular Jacq. and Citharexylum fruticosum L.) (Verbenaceae Family) (Wagner et al., 1999) is native to the Caribbean (Turner and Wasson, 1997) introduced in Tunisia for many years and cultivated along the roadsides and in gardens. This tree possesses medicinal properties and was useful in the treatment of various ailments. A decoction of young twigs was used for children thrush and bark decoction for treating colds (Cordero, 1978; Lachman-White et al., 1992). The leaves were used as a source of an antiallergic and as an alternative in hepatic disorders (Balázs et al., 2006). C. spinosum was used with other plants as anthelmintic (Lans, 2007).
Research information on the allelopathic potential of C. spinosum is relatively paltry. So, the aim of this present study was to determine the allelopathic potential of C. spinosum aqueous and organic extracts on the seed germination and early growth of four target seeds; wheat (Triticum aestivum L.), lettuce (Lactuca sativa L.), harmal (Peganum harmala L.) and milk thistle (Silybum marianum L.).
Plant material
C. spinosum different organs (roots, stems, leaves and flowers) were collected in the garden of the High Institute of Biotechnology of Monastir (latitude 35° 46’ 0’’N, longitude 10° 59’ 0’’ E, coastal region, East of Tunisia, with a sub humid climate). A voucher specimen (CQV 12) was deposited at the Herbarium of the Laboratory of Botanic in the Institute. Roots were cleaned with tap water, and all the plant parts were air-dried in a shaded area at ambient temperature. Dried material was ground into a powder to pass through a 2-mm screen using a Wiley mill (Thomas Scientific, Swedesboro, NJ) and stored at 4°C until use.
Preparation of aqueous and organic extracts
One hundred grams of powder from each dried plant part (roots, stems, leaves and flowers) were separately extracted by soaking in 1 L distilled water at ambient temperature for 24 h (Khanh et al., 2005). The aqueous extracts were filtered through a double layered muslin cloth followed by Whatman no. 1 filter paper and then passed through 0.22 μm micro-filter pore size to remove bacteria. Filtrates were preserved at 4ºC. Each crude aqueous extract at 10% (m/v) was diluted with sterile distilled water to give final concentrations of 1, 2, 3 and 4% (m/v) (Table 1). The 16 extracts were used freshly within a week (Omezzine et al., 2011; El Ayeb et al., 2013).
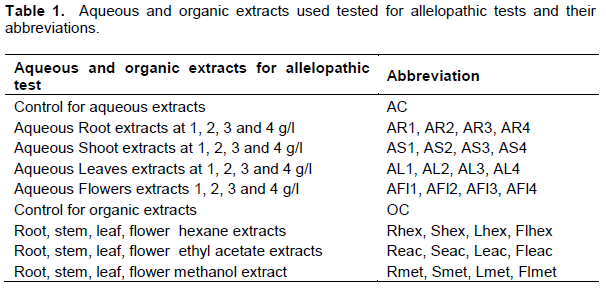
Sequential extraction was carried out in organic solvents with rising polarity: hexane, ethyl acetate and methanol. One hundred grams of powder were immersed in the appropriate solvent for 7 days at room temperature. The 12 organic extracts (Table 1) were evaporated to dryness under reduced pressure in a rotary evaporator at 45°C, to remove the solvent. After determination of the yield the extracts were stored at 4°C until use.
Determination of the total polyphenol content
The content in total polyphenol in each organic extract was measured by spectrophotometric method based on a colorimetric oxidation/reduction reaction. The oxidizing agent used was of the Folin-Ciocalteu’s phenol reagent (Merck) (Singleton and Rossi, 1965; AOAC, 1984). 50 µL of the diluted extract (1 mg/1 mL of methanol) was added to 750 µL of distilled water/Folin-Ciocalteu solution (28:2 v:v). After 3 min, 200 µL of sodium carbonate solution (20% in distilled water) was added and the test tubes were properly shaken before incubating in a boiling water bath for 1 min. The tubes were then allowed to cool in the dark at ambient conditions for 30 min to complete the reaction. For the control sample, 50 µL of methanol was used. The absorbance was measured at 765 nm. Tests were carried out in triplicate. Quantification was obtained by reporting the absorbance in the calibration curve prepared with gallic acid solutions ranging 0.01 to 0.1 mg/ml, results are expressed as mg of gallic acid equivalent (GAE) per gram of extract.
Allelopathic bioassays
Bioassays with aqueous extracts
Four target species; two crops, lettuce and wheat and two weeds; milk thistle and harmal were used to test germination and early growth responses. Lettuce has been used as a test plant because it was too sensitive to chemicals at low concentration (Olofsdotter, 2001). Wheat has been used because it was one of the most important agricultural foods and feed crops worldwide (Högy and Fangmeier, 2008) and milk thistle is a well known competitive weed for crops.
Five millilitres of each diluted aqueous extract was added onto three layers of Whatman no.1 sterilized filter paper, lined on the bottom of a sterile Petri dish (90 mm) and allowed to dry under reduced pressure. All target seeds were surface sterilized by immersing in 0.525 g L-1 of sodium hypochlorite for 5 min, rinsed in sterile deionised water four times and soaked in the last water bath at 22°C for 4 h. Preliminary essays prove that the bleach did not inhibit germination. Thirty swollen seeds of each species were sown in each Petri dish where the filter paper was moistened with 5 mL of sterile distilled water and kept in a growth chamber to germinate in the dark with an average temperature of 23 ± 2°C for 7 days. Distilled water was the control (AC) (Table 1). The experimental design was a randomized complete block replicated three times. A seed was considered germinated when the radical protruded ≥ 2 mm.
Bioassays with organic extracts
The 12 dried organic extracts were dissolved in methanol to compare their phytotoxic effects. Five millilitres of each extract dissolved at 6000 ppm (6 mg mL-1), were added to 3 sheets of filter paper displayed in a Petri dish (90 mm) and evaporated to dryness for 24 h at 24°C. The filter paper was moistened with 5 mL of sterile distilled water and then thirty imbibed seeds from each target species were arranged in each Petri dish and allowed to grow in a growth chamber in the dark at 23 ± 2°C for 7 days. Treatments were arranged in a completely randomized design with three replications. Test conditions were identical to the previous bioassay. Control Petri dishes contained only methanol and distilled water (OC) (Table 1).
Statistical analysis
Percentage of germinated seeds was recorded and the root and shoot lengths were measured for all seedlings in each Petri dish on day 7 after placing the seeds on the medium. The data were transformed to percent of control for analysis. Data from the experiments were transformed using arcsin-square root (arcsine √x) to conform with assumptions of normality for analysis of variance (ANOVA) using SPSS 12.0, for Windows program. The significance of the differences between means was determined at P < 0.05 using Duncans’s multiple range tests. We evaluated whether the type of extract (or group of extracts) was useful in reflecting its phytoxic effect on the germination and the early growth of each target seed species. The data obtained for all parameters in accordance with all extracts tested were subjected to Principal Components Analysis (PCA) and Hierarchical Cluster Analysis (HCA) using SPSS 12.0 software (SPSS Inc. Chacago, IL, USA).
Yields and total polyphenol contents in the organic extracts
Yields in the 12 organic extracts together with their polyphenol contents were reported in Table 2. Leaves have the highest yield with the three organic solvents; hexane, ethyl acetate, and methanol (0.91, 1.31 and 6.43%, respectively) followed by flowers then stems and finally roots. On the other hand, methanol gave the highest yield in all the plant parts analyzed. According to Folin Ciocalteau test, the different extracts from the different organs contained phenols. Leaf, flower, root and stem methanol extracts showed the highest amount with 617.93±1.12, 346.85±2.38, 134.95±0.69 and 94.77±0.78 mg GAE/g extract. For all the other extracts contents in total phenol were low and varied between 3.72±0.02 and 32.51±1.21 mg GAE/g.
Allelopathic activity of aqueous and organic extracts
To evaluate the allelopathic effects of organic and aqueous C. spinosum extracts, data recorded were subjected to Principal Component Analysis (PCA) and Hierarchical Cluster Analysis (HCA). The results indicated that, extracts have varying degree of inhibitory or stimulatory effect on germination and seedling growth. Effects of organic and aqueous extracts on germination and early growth of the 4 target seeds tested were reported in Table 3.
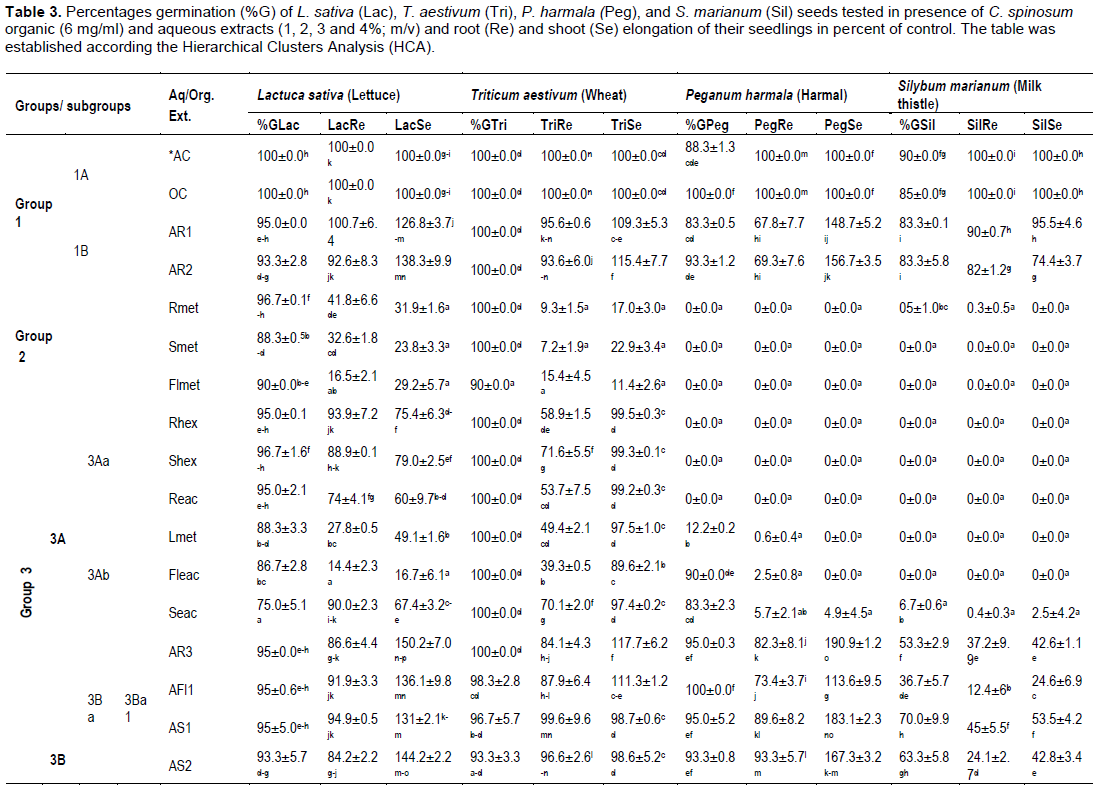
The HCA (data not given) based on the Euclidean distance between groups indicated three groups of extracts; Groups 1, 2 and 3, identified by the parameters with which they correlate. Data were reported in Table 3 according to the HCA analysis. The 3 groups were separated according to their allelopathic activity. Those groups clearly stand out forming separate groups and responses for tested parameters were different from one group to another. The group 3 was divided into 2 subgroups 3A and 3B, 3A was divided into subgroups 3Aa, 3Ab, 3B was subdivided into subgroups 3Ba and 3Bb. The later (3Bb) still divided into subgroups 3Bb1 and 3Bb2.
Group 1 and subgroups 1A and 1B
The group 1 represented by controls (AC, OC) and root aqueous extracts at 1 and 2% (AR1 and AR2) correlated with all parameters related to germination and seedlings growth. We note however that the germination percentage of harmal seeds was between 88.3 to 100%, for milk thistle it was equal to 90 and 85%, in AC and OC, respectively. In contact with AR1 and AR2 extracts, the germination percentages of target seeds were slightly reduced compared to control (93.3-95, 100, 83.3-93 and 83.3, respectively for lettuce, wheat, harmal and milk thistle seeds). Root elongation was also close to control for lettuce (92.6 to 100.7% of control), wheat (93.6 to 95.6% of control), and milk thistle (82 to 90% of control). Root elongation of harmal was more reduced (67.8 to 69.3% of control). Those extracts highly correlate with the elongation of lettuce, wheat and harmal shoot seedlings and so stimulate their development in percentages exceeding the control (126.8-138.3, 109.3-115.4 and 148.7-156.7, respectively). Nevertheless development of milk thistle was slightly less than the control (74.4-95.5% of control).
Group 2 (Rmet, Smet, Flmet)
The group 2 consisting of the three methanol extracts from roots (Rmet), stems (Smet) and from flowers (Flmet), was totally opposed to the germination of harmal and milk thistle seeds and so there is no seedling development. On the other side, those extracts correlated with the germination of wheat seeds (90-100%) and least for the germination of lettuce seeds (88.3-96.7%) and with shoot and root lettuce seedlings elongation. The development of these seedlings (16.5-41.8% of control for root, 23.8-31.9% of control for shoot), was more important than that of wheat (7.2-15.4 and 11.4-22.9% of control).
Group 3Aa (Rhex, Reac, Shex)
Ethyl acetate and hexane root extracts and hexane stem extract were totally opposed to seed germination of milk thistle and harmal (0%) but correlated with wheat and lettuce seeds germination (95-96.7 and 100%, respectively). The development of lettuce and wheat root seedlings was moderately reduced (93.9-74.0% of control, and 53.7-71.6% of control, respectively). Unlike the shoot of wheat seedlings was more elongated than this of lettuce ones (99.2-99.5% of control and 60-79% of control, respectively).
Groupe 3Ab (Lmet, Fleac, Seac)
Germination of lettuce and wheat seeds were weakly inhibited (75-88.3%) in contact with those extracts, which were almost totally opposed to germination of milk thistle seeds (0-6.7%). Germination of harmal seeds was correlated with Fleac and Seac extracts and reached 90 and 83.3%, respectively, but was weakly correlated with Lmet extract (12.2%), solely all harmal seedlings grow slightly. The development of lettuce seedlings was highly reduced in Lmet and Fleac extracts (14.4-27.8% of control for roots; 16.7-49.1% of control for shoots) compared to those of wheat (39.3-49.4% of control for roots 89.6-97.5% of control for shoots). Seedlings of the two target plants (lettuce and wheat) were less sensitive to Seac extract.
Group 3B
All extracts (17) from this group correlate with germination percentages of lettuce, wheat and harmal
seeds and with the development of their seedlings. We note also that several extracts stimulate the development of shoots; percentages of shoot elongation exceed that of the control. On the other side, the percentages germination of thistle seeds and the development of seedlings were low or equal to zero, with the exception for extracts for the subgroup 3Ba1.
Subgroups 3Ba1 and 3Ba2 (AR3,4, AFl1, AS1-3)
When grown in contact with those extracts, germination percentages for lettuce, wheat, and harmal seeds reached 88.3-95.0, 92.2-100 and 83.3-100, respectively. The development of root seedlings was important (75.9-94.9, 78.6-99.6 and 66.7-93.3% of control, respectively). The development of shoot seedlings was higher than that of control and harmal shoot was more stimulated (113.6-208.2% of control), than this for lettuce (131-163.9% of control). The shoot of wheat seedlings was stimulated by AR3 and AR4 and by AFl1 (from 111.3 to 117.7% of control). Although, AR4 extract was highly correlated with shoot elongation of harmal seedlings which reached 208.2% of control.
Extracts from subgroups 3Ba1 and 3Ba2 were less correlated with thistle seed germination and with their seedling development. In presence of AR3, AFl1, AS1-3 (3Ba1 subgroup), milk thistle seed percentage germination was between 36.7 and 70% and root and shoot elongation varied from 12.4 to 45.0% of control and from 24.6 to 53.5% of control, respectively. Germination of this target seeds was completely inhibited by AR4, the only representative of 3Ba2 subgroup.
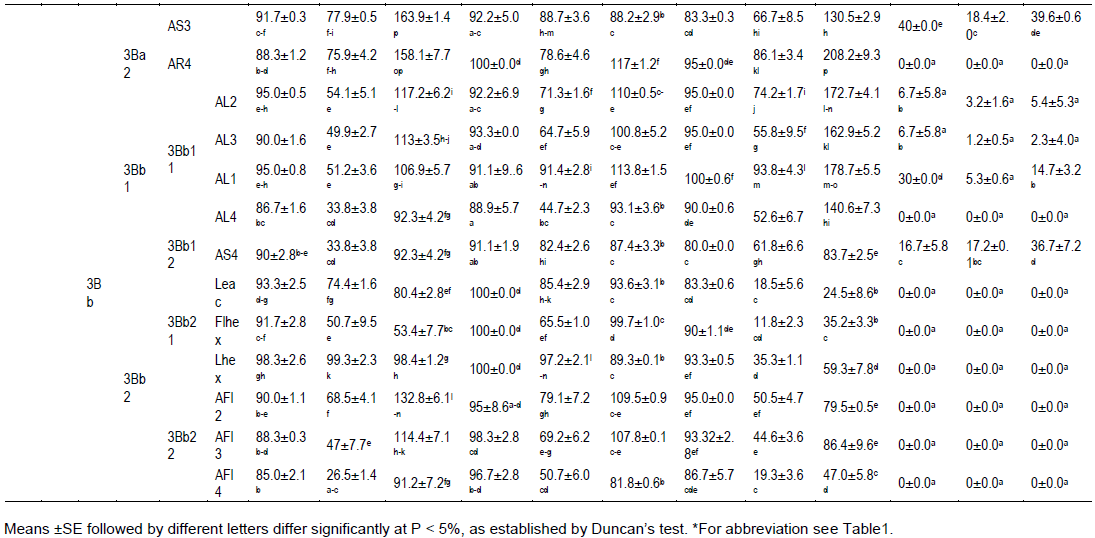
Subgroup 3Bb1 and 3Bb2
Group 3Bb, was shared in 3Bb1 and 3Bb2, and all extracts reduced the development of lettuce, wheat and harmal more than those from 3Ba group. The subgroup 3Bb1 consisted of extracts AL1-4, AS4 which correlated with lettuce, wheat and harmal seeds germination (86.7-95%, 88.9-93.3 and 80-100%) but less with milk thistle seeds germination (6.7-30%). More, AL4 inhibited milk thistle seed germination. Extracts from 3Bb1 subgroup were moderately correlated with the development of wheat root and harmal seedlings (44.7-91.4% of control; 52.6-93.8% of control, respectively) and less with that of lettuce (33.8-54.1% of control). Contrary to the results obtained for wheat and harmal, a low correlation was reported between those extracts and the root elongation of milk thistle seedlings (1.2-17.2% of control).
In presence of AL1-3 extracts from 3Bb11 subgroup, the development of lettuce, wheat and mainly harmal shoots was enhanced (106.9-117.2, 100.8-113.8 and 162.9-178.7% of control). Milk thistle seedling shoot elongation was reduced in the presence of those extracts (2.3-14.7% of control). In AL4 the shoot elongation of harmal was also highly stimulated (140.6% of control). AS4, the only representative of subgroup 3Bb12, stand out of the group 3Bb1 by its moderate effect on milk thistle seedling development (17.2-36.7%).
All extracts from the subgroup 3Bb2 (Leac, Flhex, Lhex, AFl2, AFl3 and AFl4) inhibited the germination of milk thistle seeds. Nevertheless, percentages of germination of lettuce and wheat seeds were close to the effect reported in presence of extracts from subgroup 3Bb1. When seeds where in contact with Leac, Flhex, Lhex (3Bb21 group extracts), the development of roots (50.7-99.3 and 65.5-97.2% of control, respectively) and of shoots (53.4-98.4 and 89.3-99.7% of control) of lettuce and wheat seedlings was important but less than the control. The development of harmal seedlings was less important (for the root 11.8-35.3% of control; for the shoot 24.5-59.3% of control). When grow in contact with AFl2-4% (subgroup 3Bb22), elongation of lettuce and wheat root seedlings varied from 26.5 to 68.8% and from 50.7 to 79.1% of control, respectively. Nevertheless, the development of their respective shoot was important (91.2-132.8, 81.8-109.5% of control) and was higher than the control. On contrary, the development of the harmal seedlings was reduced (for the root 19.3-50.5% of control, for the shoot 47-86.4% of control).
Our findings were supported by previous reports that demonstrated the allelopathic effects of many other trees such as Melia azedarach (Hong et al., 2003, 2004), Azadirachta indica (Neem) (Al-Charchafchi et al., 2007; Ashrafi et al., 2008 ; Abdus Salam and Kato-Noguchi, 2010), Sesbania sesban (Mubarak et al., 2009), Acacia cyanophylla (El Ayeb et al., 2013), and more recently for six tree from South Africa (Sunmonu and Staden, 2014). For C. spinosum, to the best of our knowledge, findings that indicate about its allelopathic effects are not available. Hence, it was for the first time that we have systematically evaluated and demonstrated the allelopathic inhibitory effects of C. spinosum on seed germination and early growth of milk thistle and harmal.
Phenol compounds are well known as potential phytotoxins (Seal et al., 2004). In our study we demonstrate that the inhibition of seed germination and the reduction of seedling elongation were not only related to the content in polyphenols of the extract (Table 2) but probably with the presence in all plant parts of one or more of phenol compound responsible for the activity (Inderjit, 1996; Khan and Siddique, 2012). In fact, we demonstrate that extracts containing low quantities in total phenol show a great inhibitor power and that the toxic metabolites are distributed in all plant parts in various concentrations (Harborne, 1977).
The effect of C. spinosum extracts varied with the kind of organ, concentration and target species. Roots of the different target species appeared to be more sensitive to C. spinosum extracts than shoots, presumably because of their more ultimate contact with the treated filter paper (Ahn and Chung, 2000). Similar results were reported with other crops by Maharjan et al., 2007, Ashrafi et al. (2008) and Wakjira et al., (2009). Abdus Salam and Kato-Noguchi (2010) reported that the extracts of allelopathic plants had more inhibitory effect on root growth than onshoot growth because the root is the first organ to absorb allelochemicals from the environment. Germination inhibition would be attributed to those allelochemicals (Bulut et al., 2006). Furthermore, the permeability of allelochemicals to root tissues was reported to be greater than that to shoot tissues (Nishida et al., 2005) due to the direct contact between the root and phytotoxic compounds present in extract. Those compounds might inhibit or reduced rate of cell division (Wang et al., 2002; Qin et al., 2006) which is highly active at meristematic tissue of the growing root tip.
In all organic extracts germination of lettuce and wheat seeds was not or weakly reduced and germination percentages were proximate to control in the majority of cases (75.0-96.7 and 88.9-100%, respectively). Harmal seeds germination was strongly reduced (12.2%) or totally inhibited by all methanol extracts from stems, roots, leaves and flowers, hexane extract from roots and stems and ethyl acetate extract from roots. However, the two weeds: harmal and milk thistle were more sensitive than lettuce and wheat and the flowers extracts were the most toxic and milk thistle was more susceptible to extracts, than harmal. Organic extracts were more toxic than aqueous ones. Those later extracts, at high concentrations and all organic extracts reduced strongly or inhibited totally germination of milk thistle seeds. Contrariwise, the aqueous extracts at 1-3% (from leaves, stems and roots) stimulated elongation of lettuce, wheat, and harmal shoot seedlings in percentages exceeding control.
The present study demonstrated that aqueous and organic extracts of C. spinosum possess allelopathic potential and contain inhibitory substances. Allelopathic substances present in C. spinosum under favourable conditions should release into the environment and likely act synergistically to affect the growth of weed plants. These results suggest that C. spinosum could be one of the useful natural resources for developing bioherbicides for weed management and crude extracts of this tree could be a cost effective way for crops protection against weeds. Further research in order to know the growth inhibitory substances from C. spinosum organs are underway.
The authors have not declared any conflict of interest.
REFERENCES
|
Abdus SM, Kato-Noguchi H (2010). Evaluation of allelopathic potential of neem (Azadirachta indica A. Juss) against seed germination and seedling growth of different test plant species. Int. J. Agric. Sustain. 2(2):20-25.
|
|
|
|
Ahn JK, Chung IM (2000). Allelopathic potential of rice hulls on germination and seedling growth of Barnyard grass. Agron. J. 92(6):1162-1167.
Crossref
|
|
|
|
|
Amossé C, Jeuffroy MH, Celette F, Christophe D (2013). Relay-intercropped forage legumes help to control weeds in organic grain production. Eur. J. Agron. 49:158-167.
Crossref
|
|
|
|
|
Al Charchafchi FMR, Al-Nabhani I, Al-Kharousi H, Al-Quraini E, Al-Hanai A (2007). Effect of aqueous extract of Azadirachta indica (Neem) leaves on germination and seedling growth of Vigna radiate (L.). Pak. J. Biol. Sci. 2:3885-3889.
|
|
|
|
|
AOAC (1984). Official methods of analysis of the association of official analytical chemists. 14nd ed. Arlington, Virginia, USA.
|
|
|
|
|
Ashrafi ZY, Rahnavard A, Sadeghi S, Alizade HM., Mashhadi HR (2008). Study of the allelopathic potential of extracts of Azadirachta Indica (Neem). J. Biol. Sci. 3:57-61.
Crossref
|
|
|
|
|
Balázs B, Tóth G, Duddeck H, Soliman HSM (2006). Iridoid and lignan glycosides from Citharexylum spinosum L. Nat. Prod. Res. 20:201-5.
Crossref
|
|
|
|
|
Bulut Y, Kordalt S, Atabeyoglu O (2006). The allelopathic effect of Pictacia leaf extracts and pure essential oil components on Pelargonium ringo deep scarlet F1 hybrid seed germination. J. Appl. Sci. 6(9):2040-2042.
Crossref
|
|
|
|
|
Campiglia E, Cavalieri A, Mancinelli R, Caporali F (2007). Use of essential oils of cinnamon (Cinnamomum zeylanicum L.), lavender (Lavandula spp.) and peppermint (Mentha x piperita L.) for weed control. Ital. J. Agron. 2:171-175.
Crossref
|
|
|
|
|
Cordero AB (1978). Manual de medicina domestica: plantas medicinales Dominicanas. Editora Taller, Santo Domingo, Dominican Republic.
|
|
|
|
|
Dommanget F, Evette A, Spiegelberger T, Gallet C, Pacé M, Imbert M, Navas ML (2014). Differential allelopathic effects of Japanese knotweed on willow and cottonwood cuttings used in riverbank restoration techniques. J. Environ. Manag. 132:71-78.
Crossref
|
|
|
|
|
Dudai N, Poljakoff-Mayber A, Mayber AM, Putievsky E, Lerner HR (1999). Essential oils as allelochemicals and their potential use as bioherbicides. J. Chem. Ecol. 25:1079-1089.
Crossref
|
|
|
|
|
El Ayeb A, Ben Jannet H, Harzallah-Skhiri F (2013). Effects of Acacia cyanophylla Lindl. extracts on seed germination and seedling growth of four crop and weed plants. Turkish J. Biol. 37:305-314.
|
|
|
|
|
El-Khawas SA, Shehata MM (2005). The allelopathic potentialities of Acacia nilotica and Eucalyptus rostrata on monocot (Zea mays L.) and dicot (Phaseolus vulgaris L.). Biotechnology. 4(1):23-34.
Crossref
|
|
|
|
|
Einhelling FA (1996). Interactions involving allelopathy in cropping systems. Agron. J. 88:886-893.
Crossref
|
|
|
|
|
Hadacek F (2002). Secondary metabolites as plant traits: Current assessment and future perspectives. Cr. Rev. Plant Sci. 21:273-322.
Crossref
|
|
|
|
|
Harborne JB (1977). Introduction to ecological biochemistry. New York, Academic Press,
|
|
|
|
|
Högy P, Fangmeier A (2008). Effect of elevated atmospheric CO2 on grain quality of wheat. J. Cereal Sci. 48:580-591.
Crossref
|
|
|
|
|
Hong NH, Xuan TD, Eiji T, Hiroyuki T, Mitsuhiro M, Khanh TD (2003). Screening for allelopathic potential of higher plants from Southeast Asia. Crop Prot. 22:829-836.
Crossref
|
|
|
|
|
Hong NH, Xuan TD, Tsuzuki E, Terao H., Matsuo M, Khanh TD (2004). Weed control of four higher plant species in paddy rice fields in Southeast Asia. J. Agron. Crop Sci. 190:59-64.
Crossref
|
|
|
|
|
IAS (1996). International Allelopathy Society. In: September 1996, First World Congress on Allelopathy: A Science for the Future. Constitution and Bylaws, Cádiz, Spain.
|
|
|
|
|
Inderjit (1996). Plant phenolics in allelopathy. Bot. Rev. 62:186-202.
|
|
|
|
|
Itoh K (2004). Importance of biodiversity of aquatic plants in agroecosystem for rice production, In: Schaal, B.A., Chiang, T.Y., Chou, C.H. (Eds.), Plant Evolutionary Genetics and the Biology of Weeds. Endemic Species Research Institute, Chi-Chi, Taiwan, pp. 245-266.
|
|
|
|
|
Khanh TD, Hong NH., Xuan TD, Chung IM (2005). Paddy weed control by medicinal and leguminous plants from Southeast Asia. Crop Prot. 24:421-431.
Crossref
|
|
|
|
|
Khan MR, Siddique F (2012). Antioxidant effects of Citharexylum spinosum in CCl4 induced nephrotoxicity in rat. Exp. Toxic. Pathol. 64:349-355.
Crossref
|
|
|
|
|
Kohli RK, Batish D, Singh HP (1998). Allelopathy and its implication in agroecosystem. J. Crop Product. 1:169-202.
Crossref
|
|
|
|
|
Kruidhof HM, Dam NM van, Ritz C, Lotz LAP, Kropff MJ, Bastiaans L (2014). Mechanical wounding under ï¬eld conditions: A potential tool to increase the allelopathic inhibitory effect of cover crops on weeds? Eur. J. Agron. 52:229-236
Crossref
|
|
|
|
|
Lachman-White DA, Adams CD, Trotz Ulric OD (1992). A guide to the medicinal plants of coastal Guyana. London: Commonwealth Science Council, London.
|
|
|
|
|
Lans C (2007). Comparison of plants used for skin and stomach problems in Trinidad and Tobago with Asian ethnomedicine. J. Ethnobiol. Ethnomed. 3(3):1-12.
|
|
|
|
|
Lorenzo P, Pereira CS, Rodríguez-Echeverrí S (2013). Differential impact on soil microbes of allelopathic compounds released by the invasive Acacia dealbata Link. Soil Biol. Biochem. 57:156-163.
Crossref
|
|
|
|
|
Maharjan S, Shrestha BB, Jha PK (2007). Allelopathic effects of aqueous extract of leaves of Parthenium hysterophorus L. on seed germination and seedling growth of some cultivated and wild herbaceous species. Scientific World. 5(5):33-39.
|
|
|
|
|
Mahajan G, Chauhan BS (2013). The role of cultivars in managing weeds in dry-seeded rice production systems. Crop Prot. 49:52-57.
Crossref
|
|
|
|
|
Mubarak AR, Daldoum DMA, Sayed AM (2009). Note on the influence of leaf extracts of nine trees on seed germination, radicle and hypocotyl elongation of maize and sorghum. Int. J. Agric. Biol. 11(3):340-342.
|
|
|
|
|
Nishida N, Tamotsu S, Nagata N, Saito C, Sakai A (2005). Allelopathic effects of volatile monoterpenoids produced by Salvia leucophylla: Inhibition of cell proliferation and DNA synthesis in the root apical meristem of Brassica campestris seedlings. J. Chem. Ecol. 31:1187-1203.
Crossref
|
|
|
|
|
Olofsdotter M (2001). Rice-A step toward use of allelopathy. Agron. J. 93:3-8.
Crossref
|
|
|
|
|
Omezzine F, Ladhari A, Rinez A, Haoula R (2011). Potent herbicidal activity of Inula crithmoïdes L. Sci. Hortic. 130:853-861.
Crossref
|
|
|
|
|
Qin B, Perry LG, Broeckling CD, Du J, Stermitz FR, Paschke MW, Vivanco JM (2006). Phytotoxic allelochemicals from roots and root exudates of leafy spurge (Euphorbia esula L.). Plant Signal. Behav. 1(6):323-327.
Crossref
|
|
|
|
|
Rice EL (1984). Allelopathy, 2nd ed. USA, New York, Academic Press.
|
|
|
|
|
Saraf M, Pandya U, Thakkar A (2014). Role of allelochemicals in plant growth promoting rhizobacteria for biocontrol of phytopathogens. Microbiol. Res. 169:18-29.
Crossref
|
|
|
|
|
Seal AN, Haig T, Pratley JE (2004). Evaluation of putative allochemicals in rice root exudates for their role in the suppression of arrowhead root growth. J. Chem. Ecol. 30:1663-1678.
Crossref
|
|
|
|
|
Singleton VL, Rossi JA (1965). Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 16:144-158.
|
|
|
|
|
Sunmonu TO, Staden JV (2014). Phytotoxicity evaluation of six fast-growing tree species in South Africa. S. Afr. J. Bot. 90:101-106.
Crossref
|
|
|
|
|
Turner RJ Jr, Wasson E (1997). Botanica. NSW Australia, Mynah Publishing.
|
|
|
|
|
Tworkoski T (2002). Herbicide effects of essential oils. Weed Sci. 50:425-431.
Crossref
|
|
|
|
|
Valverde BE, Riches CR, Caseley JC (2000). Prevention and management of herbicide resistant weeds in rice. Grafos, Cartago, Costa Rica. pp. 25-30.
|
|
|
|
|
Xuan TD, Tsuzuki E, Tawata S, Khanh TD (2004). Method to determine allelopathic potential of crop plants for weed control. Allelopathy J. 13:149-164.
|
|
|
|
|
Xuan TD, Shinkichi T, Khan TD, Chung MI (2005). Biological control of weeds and plant pathogens in paddy rice by exploiting plant allelopathy: an overview. Crop Prot. 24:197-206.
Crossref
|
|
|
|
|
Wagner WL, Herbst DR, Sohmer SH (1999). Manual of the flowering plants of Hawai'i. 2 vols. Bishop Museum Special Publication 83, Honolulu, HI, University of Hawai'i and Bishop Museum Press.
|
|
|
|
|
Wang LY, Wang NL, Yao XS, Miyata S, Kitanaka S (2002). Diterpenes from the roots of Euphorbia kansui and their in vitro effects on the cell division of Xenopus. J. Nat. Prod. 65(9):1246-51.
Crossref
|
|
|
|
|
Wakjira M, Berecha G, Tulu S (2009). Allelopathic effects of an invasive alien weed Parthenium hysterophorus L. compost on lettuce germination and growth. Afr. J. Agric. Res. 4(11):1325-1330.
|
|