ABSTRACT
The technique of pollen irradiation for mutation induction technique has been successfully demonstrated in many crop species. Therefore, the mutagenic effects of UV irradiated pollen on cowpea accessions were investigated. Pollen grains of eight cowpea accessions were irradiated with 30,000µWs/cm2 UV for 60, 120, 180, 240, 300 and 360 minutes. Emasculated flowers of each accession were self-pollinated with irradiated pollen to evaluate the effects of pollen mutagenesis on seed setting in the M1 generation. Harvested seeds from M1 plants were advanced to M2 to evaluate the effects of UV radiation on seed germination, plant survival and for mutant selection in the M3 generation. Data were analyzed using descriptive statistics. Pollen irradiation with UV for a short period (60 min) increased seed setting in all the cowpea accessions at M1 except IB-Y-1 where it reduced seed setting by 28.6%. Observed LD50 of UV rays among the cowpea lines ranged from 142.6 to 210.1 minutes. No significant difference was observed in seed germination for all treatments at M2 except irradiation for 120 minutes in IT90K-284-2. A trend similar to seed germination was observed in seedling survival at M2. The three-primary leaf and four-primary leaf mutants selected at M3 generation reverted back to two-primary leaf seedlings at M4. Low mutation frequencies recorded in this study shows that cowpea is considered less amenable to the application of UV irradiation as a practical breeding method.
Key words: Cowpea mutagenesis, pollen irradiation, ultra-violet induced mutant, seed germination, seed setting.
The technique of induced mutation in plant using pollen (male gametophytes) as the starting material was initiated with maize pollen irradiation using X-ray (Stadler, 1939). This was followed by investigations on the procedures and methods for generating and studying induce mutations with particular emphasis on pollen mutagenesis in maize (Neuffer, 1957; Amano and Smith, 1965; Mottinger, 1970) and in barley (Devreux et al., 1972). The use of gamma radiation of pollen for induced mutation in plant breeding was first report in Nicotiana (Pandey, 1975, 1978). Subsequently, the possible application of this procedure for scientific studies and crop improvement has been explored by several authors. Pollen mutagenesis by gamma rays have been demonstrated in Nicotiana (Grant et al., 1980; Pandey, 1980a; 1980b), in maize (Pandey, 1983; Sanford et al., 1984a, b), in rice (Chin and Gordon, 1989a, b), in Arabidopsis (Yang et al., 2004; Naito et al., 2005), in Triticum (Bie et al., 2007), in citrus (Yahata et al., 2010), in winter squash (Kurtar and Balkaya, 2010), in Persian walnut (Grouh et al., 2011), in melon (Godbole and Murthy, 2012) and in cotton (Yue and Zou, 2012). Many other authors have attempted the use of irradiated pollen for egg transformation (Chin and Gorgon, 1989a).
Irradiated pollen can mediate the transfer of limited genetic materials, instead of complete genome from the pollen donor (male parent) to their progenies. This hypothesis was first suggested by Pandey (1975). Thus, in a maternal background, a few paternal characteristics from the pollen parent were apparently incorporated and expressed in the M1 plants and the eggs were said to be “transformed”. He defined “egg transformation” as the transfer of limited intact genes rather than the total pollen genome to the egg cell. He suggested that transformation could be the result of diploid parthenogenesis of the egg induced by irradiated pollen, followed by the incorporation of paternal chromatin during embryogenesis. The biological basis to these assumptions had been explained by several authors. First, the pollen germination and pollen tube growth are highly tolerant to irradiation (Vassileva-Dryanovska, 1966; Gillissen, 1978). In addition to this, despite extensive nuclear aberrations caused by irradiation, fusion of the fragmented paternal genetic materials with the egg nuclei was still possible (Brewbaker and Emery, 1962). Therefore, the use of irradiated pollen for limited gene transfer was considered as a self-contained “DNA injection” system in which the irradiated pollen is both a source of donor DNA fragment as well as a vector for delivering the genetic fragments to the embryo sac (Chin and Gordon, 1989a). The authors proposed this technique, as a natural and rapid means of transferring a few or single genes into plants without resorting to the use of recombinant DNA technology. Moreover, it has been suggested that cross-pollination with irradiated pollen may nevertheless be useful in practical plant breeding by causing a shift in the segregation ratio towards the maternal phenotype in the second (M2) generation (Chin and Gordon, 1989b).
Production of haploids and doubled haploids are important aspect of plant breeding by which homozygous lines are obtained for hybrids generation. The technique of pollen irradiation by gamma rays, UV or X-rays is the most widely used method to induce in situ parthenogenetic haploid plants (Kosmrlj et al., 2013). This technology has been demonstrated and proven to be effective in many crop species elucidated by Kurtar and Balkaya (2010). In addition to these, haploid production by irradiated pollen has also been reported in winter squash (Kurtar and Balkaya, 2010); in citrus (Yahata et al., 2010); in melon (Gonzalo et al., 2011; Godbole and Murthy, 2012); in Persian walnut (Grouh et al., 2011) and in pumpkin (Kosmrlj et al., 2013).Physical mutagens (ionizing and non-ionizing radiation) may be considered as the most suitable option for pollen mutagenesis in some plants for certain reason. For example, in cowpea, chemical mutagens are unsuitable for pollen treatment simply because of the hydrophobic nature of their pollen. Consequently, the pollen grains rupture and lose viability as soon as it get in contact with aqueous medium. Among the radiation mutagens used for pollen treatment, application of UV radiation has been reported by few authors. Pollen mutagenesis by UV irradiation was reported in maize (Gavazzi and Sanguineti, 1983). In legume (faba bean), Haliem et al. (2013) investigated the mutagenic potential of UV on meiotic-pollen mother cells, pollen grains and seed yield.
UV ray is an electromagnetic radiation that does not carry enough energy per quantum to ionize atoms or molecules. It has longer wavelength (100 to 400 nm) with low penetration power into plant tissues when compared with ionizing radiations. According to Mba et al. (2012), UV radiation is classified based on their wavelengths into three forms, ultraviolet A (UVA) 315-400 nm, ultraviolet B (UVB) 280 to 315 nm and ultraviolet C (UVC) 100 to 280 nm. UVC has been implied to be the most energetic and biologically damaging among the three. The mutagenic effect of UV is due to its ability to react with DNA and other biological molecules such as bases in DNA molecules and other aromatic amino acids of proteins. UVB and UVC produce pyrimidine dimers on reacting with DNA, while UVA produces very few of these. Therefore, UVB and UVC are mostly used for mutagenic treatments. The pyrimidine dimers produced form lesions that interfere with transcription and DNA replication, lead to mutations, chromosomal rearrangements and lethality. Moreover, it has been established that exposure of crop plant cells under natural condition of growth and development to UVB resulted in excessive production of free radicals, reactive oxygen species (Agrawal et al., 2009) which can induce structural changes in DNA, such as chromosomal rearrangement, strand breaks, base deletions, pyrimidine dimers, cross-links and base modifications, mutations and their genotoxic effects (Gill and Tuteja, 2010; Haliem et al., 2013).When compared to seed mutagenesis, pollen mutagenesis is of greater advantage. First, when seed is used as the starting material for mutagenesis it always leads to the production of chimeric tissues. This problem can be overcome by starting with pollen because pollen mutagenesis involves mutagenic treatment, usually in the form of irradiation, to the pollen prior to hand pollination, while the female tissue remains free of somatic damage. The M1 plant arising from pollination with mutated pollen is non-chimeric and will be hemizygous for any uniquely induced mutation (Yang et al., 2004). The dominant mutations in this case will be expressed in the M1 while recessive mutations will be expressed in the M2. Because of the absence of chimera, fewer M2 seeds are needed per plant for screening than for an equivalent seed mutagenesis experiment. Further limitations of seed treatment are the possible occurrence of separated female and male germ cell primordial as well as somatic selection against mutant cells during plant development (Gavazzi and Sanguineti, 1983). Mutagenesis of male gametophyte also has another unique advantage, since mutations are directly passed onto the next generation in a hemizygous state and large numbers of pollen grains (haploid nuclei) can be mutated (Yang et al., 2004). However, information on pollen irradiation for mutation induction in cowpea is scarce and there is paucity of report on the application of UV radiation in cowpea pollen mutagenesis. Moreover, there is the need to determine optimal UV radiation dosage for pollen mutagenesis in cowpea for the purpose of widening its genetic base. Therefore, the objective of this study is to evaluate the effects of UV irradiated pollen used for self-pollination on the M1, M2 and M3 generations of eight cowpea accessions.
Mutagenesis by pollen irradiation with UV rays
Eight cowpea accessions used in this study were the cultivar, Ife Brown (IB) and its mutant derivatives (IB-Y-1, IB-CR and IB-BPC) and four elite cultivars (IT86D-719, IT86D-1010, IT89KD-374-57 and IT90K-284-2). The cowpea accessions IB, IB-Y-1, IB-CR and IB-BPC, were collected from the Genetics unit of the Department of Crop Protection and Environmental Biology (CPEB), University of Ibadan, Nigeria, while the four elite cultivars were obtained from the Genetic. Resources Centre of the International Institute of Tropical Agriculture (IITA), Ibadan. The cowpea accessions were raised at the rooftop garden of CPEB Department, University of Ibadan. Matured (opened) flowers of each of the cowpea accessions were separately harvested into labeled air-filled transparent nylon bags in the morning (07:00 to 08:00 h). The flowers were stored in the refrigerator at 10°C until further use. Pollen from these flowers was carefully collected from dehisced anthers with the aid of sterile forceps into cell-wells separately. The cell-wells were sealed with paper tape immediately to avoid pollen contaminations. UV irradiation of cowpea pollen was carried out at the Genetics laboratory of CPEB Department. The pollen from each of the cowpea accessions (in cell-wells) were exposed to 30,000µWs/cm2 ultraviolet (UV) radiation for 60, 120, 180, 240, 300 and 360 min. Pollen used for control treatment in all the accessions were harvested into cell-wells but not irradiated. For each radiation treatment, 20 freshly emasculated pre-anthesis flower buds of the original parents were hand-pollinated (selfed) using irradiated pollen grains in the evening time (18:00 to 19:00 h). Each flower pollinated with irradiated pollen was tagged with appropriate label. Data were collected on the number of seed set in each treatment at M1 generation. At maturity, all dry pods in each treatment were harvested into labeled envelopes and their seeds were prepared for advancement to M2 generation.
Determination of Ultra-violet lethal dosage 50% (LD50)
The LD50 was determined by adopting the method described in Olasupo et al. (2016). Percentage seed setting at M1 generation following hand pollination with UV irradiated pollen were calculated for each treatment. The difference in percentage seed setting between each treatment and control was calculated and expressed as percentages of control. A graph of the absorbed dose was plotted against the percentage difference (Dosage Effect Curve) for each accession to show the damage due to mutagenic treatment by UV on cowpea pollen. By inserting the ‘line of best fit’ and reading off the dose corresponding to 50% reduction, the LD50 was obtained or more precisely, calculated using the linear equation: y = mx + c
Evaluation of M2 generation plants following UV mutagenesis
The M2 plants were raised at the rooftop garden of CPEB Department, University of Ibadan. All the pods produced by M1 plants from each treatment were harvested together in an envelope, dried and the seeds were prepared for advancement to M2 generation. The quantity of seeds used to raise the M2 plants was determined by seed setting of M1 plants in each treatment (Table 1). Further screening for mutant phenotypes in the M3 and M4 generations were carried out at the Teaching and Research Farm of the University of Ibadan.
Data collection
In the M1 generation, data were taken on percentage germination and number of surviving plants from each treatment. Data were also collected from 10 M2 plants selected randomly from each treatment on the following parameters: primary leaf area, seedling height at 3 weeks, terminal leaflet area and plant height at 6 weeks. Screening for mutant was carried out by scoring the plants for any change in the phenotype when compared with the parent plants (control treatments). Descriptive statistics was used for data analyses with the aid of Microsoft Excel 2010.
Effect of UV irradiated pollen on the M1 generation
Following cowpea pollen treatment with 30,000 µWs/cm2 UV radiation for 60, 120, 180, 240, 300 and 360 min, mutagenic effect of the treatments as observed in the seed setting at M1 generation, are presented in Figures 1 to 8. As revealed by the radiation dosage effect curves, treatment of fresh pollen grains with 30,000 µWs/cm2 UV rays for up 60 min before pollination increased seed setting in all the cowpea accessions used in this study except IB-Y-1 where it was reduced by 28.6% (Table 2). Percentage increase in seed setting was highest (9.2%) in IB-BPC at the UV treatment for 60 min, followed by IT86D-1010 (8.3%). Further pollen irradiation with 30,000 µWs/cm2 UV above 60 min before pollination reduced seed setting of all cowpea accession used in this study with a highest effect (R2 x100 = 94.8%) recorded in IB-BPC (Figure. 4). There was variation in the observed UV radiation LD50 of pollen among the eight cowpea lines evaluated. The lowest LD50 (142.6 min) and the highest LD50 (210.1 min) were observed in IB-Y-1 and IT90K-284-2 respectively.
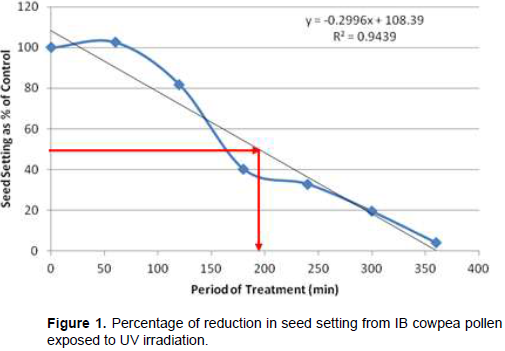
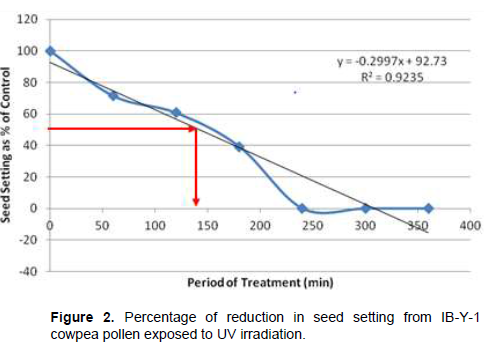
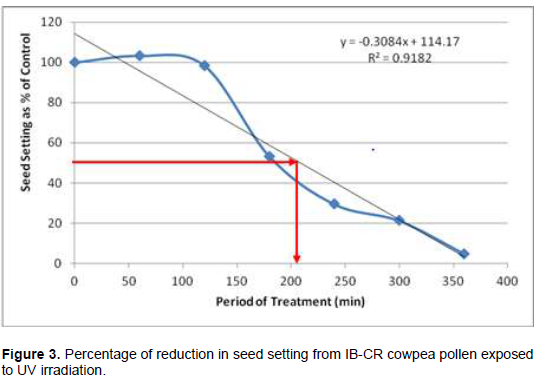
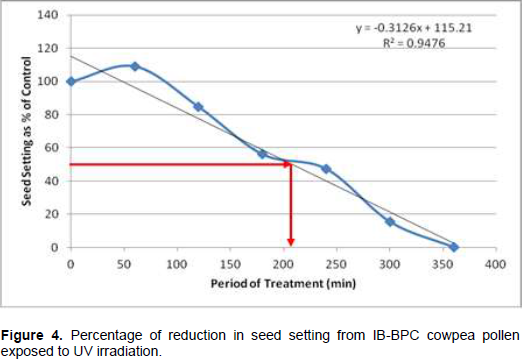

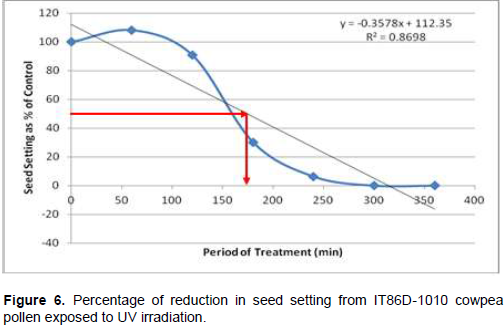
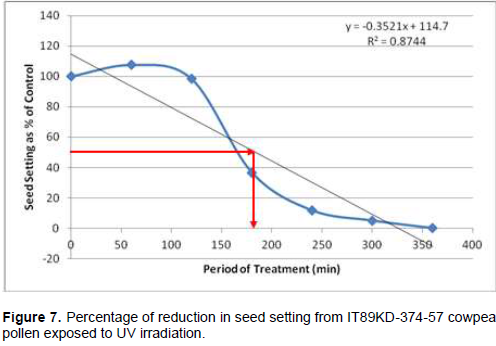


Effects of UV irradiated pollen on cowpea in the M2 generation
Following hand pollination of cowpea with UV irradiated pollen, the results of seed germination and survival of M2 plants are presented in Figures 9 and 10, respectively. Seed germination percentage was based on the number
of seeds which germinated out of the total number of seeds sown (in percentage) for each treatment. Percentage seedling survival was based on the proportion of the seeds planted which became established plants at each treatment level. No significant difference was observed in the percentage seed germination in all treatments across the eight cowpea accessions except radiation treatment 120 min in IT90K-284-2. Similarly, pollination with UV irradiated pollen did not reveal significant difference in the percentage plant survival cowpea plants in all the treatments except in IB-BPC, IT86D-719 and IT86D-1010. Lower percentage seedling survivals were observed at radiation level 240 min in IT86D-1010 and treatment 300 min in IB-BPC and IT86D-719 when compared with control treatments.
Spectra and frequencies of mutations in the M3 generation
Based on the observed phenotypic changes, only three mutant spectra were selected across the treatments in
the M3 plant generation (Table 3). Yellow albino mutant seedlings which died after a week of germination were observed in IB-Y-1 at 60, 120 and 180min treatment levels. A four-primary leaf mutant (Figure 12) was observed in IT90K-284-2 at 360 min, while three-primary leaf mutants (Figure 11) were observed in IT86D-719, IT86D-1010 and IT90K284-2. The three-primary leaf and four-primary leaf mutants produced the normal trifoliate secondary leaves. However, these mutants were not stable, but reverted back to normal (two-primary leaf plants). The three-primary leaf mutant reverted to normal plant when advanced from M3 to M4 generation, while the four-primary leaf mutant reverted back to three-primary leaf in the M4 and finally to normal (two-primary leaf) plant in the M5 generation. The mutation frequencies in the M3 generation were very low with no mutant phenotype observed in most treatments. Highest mutation frequency (0.02%) was observed in IT90K-284-2 at 60 min period of UV radiation treatment. This result suggests that UV irradiation of pollen has low efficiency for mutation induction in cowpea.


Treatment of fresh pollen grains with 30,000 µWs/cm2 UV rays for up 60 min before pollination appeared to enhance seed setting in cowpea, while exposure of pollen to the radiation for longer period (>60 min) has inhibitory effect on seed setting. Similar observation was made by Haliem et al. (2013) that UVB irradiation of pollen for 60 min had a positive effect on seeds yielded following radiation treatment from their molecular analysis. The result of radiation dosage effect curves shows that seed setting in cowpea following pollination with UV irradiated pollen appeared to be dose dependent. Plant cells and nucleic acids are damaged when exposed to high level of UV radiation which may cause the pollen grains to lose viability. Britt (1995), Ravanat et al. (2001) and Lagoda (2012) reported that UV radiation has deleterious effects on cellular DNA which may be either mutagenic or toxic and the induced damage can lead to cell death due to photochemical damage. The variability observed in LD50 could be as a result of genotypic variation among the cowpea accessions.
However, the results obtained from percentage seed germination and seedling survival at M2 generation showed that pollination with UV irradiated pollen at various treatment levels did not produce significant effects in cowpea. This suggests there appeared to be reversions of most UV induced mutagenic changes in the
plant genetic materials from M1 to M2 generation. The null effect of UV radiation on seed germination and seedling survival in the M2 generation could also be attributed to the repairs of some induced damages to the DNA by certain biochemical mechanisms present in plants. This is consistent with observed reversion of the three-primary leaf and four-primary leaf seedling mutants when advanced from M3 to M4 and from M4 to M5 generation respectively. Since plants are unique in the obligatory nature of their exposure to UV, Britt (1995) hypothesized therefore that they may have evolved particularly efficient mechanisms for the elimination of UV-induced DNA damages and mutations. In addition to this, it has been reported that the production of reactive oxygen species in plant tissues as a response to ionizing and nonionizing radiations is controlled by the very efficient enzymatic and non-enzymatic antioxidant defense systems which serve to keep down the levels of free radicals, permitting them to perform useful biological functions without too much damage and act as a cooperative network employing a series of redox reactions (Gill and Tuteja, 2010; Karuppanapandian et al., 2011). Leguminous plants, especially faba bean had been reported to have high antioxidant activity due to the fact that they contained phenolic and flavonoid compounds (Aly and El-Beltagi, 2010; Chaieb et al., 2011; Ismael et al., 2012; Haliem et al., 2013). This may be responsible in part, for the low mutagenic effects of UV irradiation observed in this study.
Low frequencies of mutations recorded by phenotyping in this study revealed that cowpea plant is considered less amenable to the application of UV irradiated pollen as a practical breeding method. This may not be unexpected since UV radiation has low penetration power and less mutagenic potential in plants when compared to the ionizing radiations. The weak mutagenic effect of UV, even at higher doses has been suggested to be the result of the occurrence of a dark repair system in plant cells (Britt, 1995; Gavazzi and Sanguineti, 1983). Although the results obtained in this study corroborates the findings of Chin and Gordon (1989b) who concluded that the method of pollination with irradiated pollen has not been promising and may not be a useful technique of mutation breeding in rice. However, recent study of the mutagenic potential of UVB irradiation on meiotic-pollen mother cells, pollen grains and seed yield of faba beans revealed wide range of mutagenic action on the frequency and type of chromosomal anomalies, fertility of pollen grains and seed yield productivity based on irradiation dosages, while SDS-PAGE and RPAD-PCR analyses of seeds yielded from irradiated seedlings from UV-B dose for 60 minutes recorded bio-positive effects (Haliem et al., 2013). Therefore, cytogenetic and molecular analysis of the M2 and M3 cowpea populations would reveal detail information on the mutagenic potency of UV irradiation of cowpea pollen.
Pollen mutagenesis by UV radiations has not been able to produce any stable cowpea mutant in this study. The method of pollination with UV irradiated pollen has not been promising and this suggests that it may not be an efficient technique for cowpea mutation breeding. However, there is the need to further investigate the mutagenic effect of pollination with irradiated pollen in cowpea using other physical mutagens.
The authors have not declared any conflict of interests.
REFERENCES
|
Agrawal SB, Singh S, Agrawal M (2009). Ultraviolet-B induced changes in gene expression and antioxidants in plants. Adv. Bot. Res. 52:47-86.
Crossref
|
|
|
|
Aly AA, El-Beltagi HES (2010). Influence of ionizing irradiation on the antioxidant enzymes of Vicia faba L. Grasas Y Aceites. 61(3):288-294.
Crossref
|
|
|
|
|
Amano E, Smith HH (1965). Mutations induced by ethylmethanesulfonate in maize. Mutat. Res. 2:344-351.
Crossref
|
|
|
|
|
Bie T, Cao Y, Chen P (2007). Mass production of intergeneric chromosomal translocations through pollen irradiation of Triticum durum-Haynaldia villosa Amphiloid. J. Integr. Plant Biol. 49(11):1619-1626
Crossref
|
|
|
|
|
Brewbaker JL, Emery GC (1962). Pollen Radiobotany. Rad. Bot. 1:101-154.
Crossref
|
|
|
|
|
Britt AB (1995). Repair of DNA damage induced by ultraviolet radiation. Plant Physiol.108:891-896.
Crossref
|
|
|
|
|
Chaieb N, González JL, López-Mesas M, Bouslama M, Valiente M (2011). Polyphenols content and antioxidant capacity of thirteen faba bean (Vicia faba L.) genotypes cultivated in Tunisia. Food Res. Int. 44(4):970-977.
Crossref
|
|
|
|
|
Chin S, Gordon GH (1989a). Pollination with irradiated pollen in rice – Oryza sativa L.I. First (M1) generation. Heredity 63:163-170.
Crossref
|
|
|
|
|
Chin S, Gordon GH (1989b). Pollination with irradiated pollen in rice– Oryza sativa L.II. Second (M2) generation. Heredity 63:171-179.
Crossref
|
|
|
|
|
Devreux M, Donini B, Scarascia-Mugnozza GT (1972). Genetic effects of gametophyte irradiation in barley III. Frequecies and types of mutations induced. Rad. Bot. 12:87-98.
Crossref
|
|
|
|
|
Gavazzi G, Sanguineti MC (1983). Pollen mutagenesis in maize. In Mulcahy DL, Ottaviano E (Eds.) Pollen: Biology and implication for plant breeding, (pp 352-357). Elsevi Biomedical, New York.
|
|
|
|
|
Gill SS, Tuteja N (2010). Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 48(12):909-930.
Crossref
|
|
|
|
|
Gillissen LJW (1978). Post-X-irradiation effects on pollen germination in-vivo. Env. Expt. Bot. 18:81-86.
Crossref
|
|
|
|
|
Godbole M, Murthy HN (2012). Parthenogenetic haploid plants using gamma irradiated pollen in snapmelon (Cucumis melo var. momordica). Plant Cell Tissue Organ Cult.109:167-170.
Crossref
|
|
|
|
|
Gonzalo MJ, Claveria E, Monforte AJ, Dolcet-Sanjuan R (2011). Parthenogenic haploids in melon: Generation and molecular characterization of a doubled haploid line population. J. Am. Soc. Hortic. Sci. 136:145-154.
|
|
|
|
|
Grant JE, Pandey KK, Williams EG (1980). Pollen nuclei after ionizing irradiation for egg transformation in Nicotiana. NZ. J. Bot. 18:339-341.
Crossref
|
|
|
|
|
Grouh MSH, Vahdati K, Lotfi M, Hassani D, Biranvand NP (2011). Production of haploids in Persian walnut through parthenogenesis induced by gamma-irradiated pollen. J. Am. Soc. Hortic. Sci. 136:198-204.
|
|
|
|
|
Haliem EA, Abdullah H, AL-Huqail AA (2013). Oxidative Damage and Mutagenic Potency of Fast Neutron and UV-B Radiation in Pollen Mother Cells and Seed Yield of Vicia faba L. BioMed Res. Int. 2013: Article ID 824656, 12p.
|
|
|
|
|
Ismael DS, Timoracka M, Vollmannova A (2012). Influence of variety, locality and soil contamination on total polyphenol content and antioxidant activity of faba bean grains. J. Microbiol. Biotechnol. Food Sci. 2012(1):931-941.
|
|
|
|
|
Karuppanapandian T, Moon J, Kim C, Manoharan K, Kim W (2011). Reactive oxygen species in plants: their generation, signal transduction, and scavenging mechanisms. Aust. J. Crop Sci. 5(6):709-725.
|
|
|
|
|
Kosmrlj K, Murovec J, Bohanec B (2013). Haploid induction in hull-less seed pumpkin through parthenogenesis induced by X-ray-irradiated pollen. J. Am. Soc. Hortic. Sci. 138(4):310-316.
|
|
|
|
|
Kurtar ES, Balkaya A (2010). Production of in vitro haploid plants from in situ induced haploid embryos in winter squash (Cucurbita maxima Duchesne ex Lam.) via irradiated pollen. Plant Cell Tissue Organ Cult. 102:267-277.
Crossref
|
|
|
|
|
Lagoda PJI (2012). Effects of radiation on living cells and plants. In Shu QY, Forster BP, Nakagawa H (Eds.), Plant Mutation Breeding and Biotechnology, Joint FAO/IAEA Division of nuclear Techniques in Food and Agriculture International Atomic Energy Agency, Vienna, Austria. pp. 123-134.
Crossref
|
|
|
|
|
Mba C, Afza R, Shu QY (2012). Mutagenic Radiations: X-Rays, Ionizing Particles and Ultraviolet. In Shu QY, Forster BP, Nakagawa H (Eds.), Plant Mutation Breeding and Biotechnology, Joint FAO/IAEA Division of nuclear Techniques in Food and Agriculture International Atomic Energy Agency, Vienna, Austria. pp. 83-90.
Crossref
|
|
|
|
|
Mottinger JP (1970). The effects of X-rays on the bronze and shrunken loci in maize. Genetica 64:259-271.
|
|
|
|
|
Naito K, Kusaba M, Shikazono N, Takano T, Tanaka A, Tanisaka T, Nishimura M (2005). Transmissible and nontransmissible mutations induced by irradiating Arabidopsis thaliana pollen with gamma rays and carbon ions. Genetica 169:881-889.
Crossref
|
|
|
|
|
Neuffer MG (1957). Additional evidence on the effect of x-ray and ultraviolet radiation on mutation in maize. Genetica 42(3):273-282.
|
|
|
|
|
Olasupo FO, Ilori CO, Forster BP, Bado S (2016). Mutagenic effects of gamma radiation on eight accessions of cowpea (Vigna unguiculata [L] Walp.). Am. J. Plant Sci. 7:339-351.
Crossref
|
|
|
|
|
Pandey KK (1975). Sexual transfer of specific genes without gametic fusion. Nature 256:310-313.
Crossref
|
|
|
|
|
Pandey KK (1978). Gametic gene transfer in Nicotiana by means of irradiated pollen. Genetica 49:53-69.
Crossref
|
|
|
|
|
Pandey KK (1980a). Further evidence for egg transformation in Nicotiana. Heredity 45:15-29.
Crossref
|
|
|
|
|
Pandey KK (1980b). Parthenogenetic diploidy and egg transformation induced by irradiated pollen in Nicotiana. NZ J. Bot. 18:203-207.
Crossref
|
|
|
|
|
Pandey KK (1983). Evidence for gene transfer by the use of sub-lethal irradiated pollen in Zea mays and theory of occurrence by chromosome repair through somatic recombination and gene conversion. Mol. Gen. Genet. 191:358-365.
Crossref
|
|
|
|
|
Ravanat J, Douki T, Cadet, J (2001). Direct and indirect effects of UV radiation on DNA and its components. J. Photochem. Photobiol. B: Biol. 63:88-102.
Crossref
|
|
|
|
|
Sanford JC, Chyi YS, Reisch BI (1984a). An attempt to induce "egg transformation" in Lycopersicon esculentum Mill. Using irradiated pollen. Theor. Appl. Genet. 67:553-558.
Crossref
|
|
|
|
|
Sanford JC, Chyi YS. Reisch BI (1984b). Attempted "egg transformation" in Zea mays L. using irradiated pollen. Theor. Appl. Genet. 68:269-275.
Crossref
|
|
|
|
|
Stadler LJ (1939). Genetic studies with ultra-violet radiation. In: Punnrtt, R.C. ed. Proceedings of the seventh international genetics congress. Cambridge, UK: Cambridge University Press. pp. 269-276.
|
|
|
|
|
Vassileva-Dryanovska OA (1966). Fertilization with irradiated pollen in Tradescantia. Rad. Bot. 6:469-479.
Crossref
|
|
|
|
|
Yahata M, Yasuda K, Nagasawa K, Harusaki S, Komatsu H, Kunitake H (2010). Production of haploid plant of Banpeiyu' pummel [Citrus maxima (Burm.) Merr.] by pollination with soft x-rayirradiated pollen. J. Jap. Soc. Hortic. Sci. 79:239-245.
Crossref
|
|
|
|
|
Yang C, Mulligan BJ, Wilson ZA (2004). Molecular genetic analysis of pollen irradiation mutagenesis in Arabidopsis. New Physiol. 164:279-288.
Crossref
|
|
|
|
|
Yue J, Zou J (2012). Study of radiation effects on upland cotton (Gossypium hirsutum L.) pollen grain irradiated by 60Co- ray. J. Agric. Sci. 4(7):85-94.
Crossref
|
|