ABSTRACT
Six extra-early quality protein maize (QPM) inbred lines from IITA were investigated using a partial diallel cross design. The objectives were to assess the hybrids and their parents for their agronomic performance. The six parents along with their hybrids (15) were evaluated using a Randomized Complete Block Design (RCBD) with three replications per site in four locations. General combining ability (GCA) and specific combining ability (SCA) effects as well as mid-parent heterosis were determined. Results of combined analysis of variance (ANOVA) revealed significant environmental effect for all the traits studied. Significant additive effect was observed for only grain yield whilst non-significant GCA and SCA effects were identified for all other traits. The GCA estimate identified parental lines P1, P3 and P5 as the high combiners for grain yield. The GCA estimates identified parental lines P1, P3 and P5 as the best combiners for grain yield. Again, P1 was the most suitable parent for increased cob length, cob diameter, number of rows per cob and reduced anthesis-silking interval; P3 for thousand-grain weight and reduced days to flowering (anthesis and silking days), and P5 for number of kernels per row, and reduced plant height and ear height. Hence, these parents may be used in hybridization programmes as donors of the superior traits indicated. The highest values for SCA and mid-parent heterosis for grain yield were observed in the crosses P1xP4, P5xP6, P1xP5 and P4xP6.
Key words: Quality protein maize (QPM), diallel, combining ability.
Maize is an important staple cereal crop in the world (Michael et al., 1999; Vivek et al., 2007; Moaveni et al., 2011). It is estimated that 94 countries depend on maize for at least 30% of their total daily calories (CIMMYT and IITA, 2011). In sub-Saharan Africa, about 12 countries depend on maize for at least one fifth of their total daily calories intake, and up to 60% for their total daily protein intake (Krivanek and Vivek, 2006). Although, maize plays an important role in global food systems, there is some nutritional deficiency present in the normal maize (NM) varieties. These varieties do not have enhanced protein level and are considered as low quality protein maize. There is paucity of two specific essential amino acids - lysine and tryptophan, which are prerequisite to humandietary protein requirement. The intervention for mitigating protein deficiency from low protein quality maize has led the maize improvement programme of International Institute of Tropical Agriculture (IITA) to develop quality protein maize (QPM) inbred lines through combining ability studies to establish heterotic patterns among inbred populations and to maximize their yields for hybrid development.
Falconer and Mackay (1996) defined heterosis as the difference in performance of hybrid and the mean performance of the two parents. This difference is often called mid-parent heterosis. In effect, heterosis restores reduced vigour associated with inbreeding and leads to higher performance of progenies over the parents. Heterosis has been found to be controlled by dominance complementation, locus-specific over-dominance (Shull, 1908; Crow, 1948) and epistasis effects (Lippman and Zamir, 2006). Combining ability study via diallel crosses is an important tool used by many plant breeders for developing hybrid maize varieties and offers an opportunity in identification and selection of potential inbred lines and parental combinations (Hallauer, 1990).
The method used to analyse crosses, or parents and the crosses on the basis of general combining ability (GCA) and specific combining ability (SCA) concepts is diallel mating design (Griffiths, 1956). Hayman (1954) and Stoskopf et al. (1993) defined “diallel cross” as the set of all possible matings between several genotypes. The estimates from GCA and SCA provide an assessment of relative merits of the individual genotypes in cross combinations to guide in selection and testing schemes. Thus, diallel analysis is among the genetic-statistical approaches developed to assist in selection of parents based on their combining ability and the potential to produce promising segregating populations (Okello et al., 2006). Combining ability for yield and other traits such as disease resistance and high protein concentration play significant role in the identification of appropriate parents for hybrid development. Diallel mating designs have been extensively used in breeding programs for the evaluation of genetic potential of parents that range from inbred lines to wide genetic base varieties (Hallauer and Miranda, 1988; Stoskopf et al., 1993; Bernardo, 2002).
The advent of changes in climatic conditions coupled with unpredictable rainfall pattern and incidence of pest and disease pose threats to crop production especially grains (FAO, 2007). These demand the development of an adapted extra-early maturing QPM hybrid with improved nutritional qualities and high yield potential in Ghana to improve livelihood of farmers. These lines would be a valuable genetic material to enhance extra-early QPM hybrid development in Ghana. The goal of this study was to assess the relative importance of SCA and GCA of six extra-early IITA QPM inbred lines and their single cross hybrids. The specific objectives were to estimate the GCA and SCA effects for grain yield and other agronomic traits and to identify cross combinations expressing high hybrid vigour.
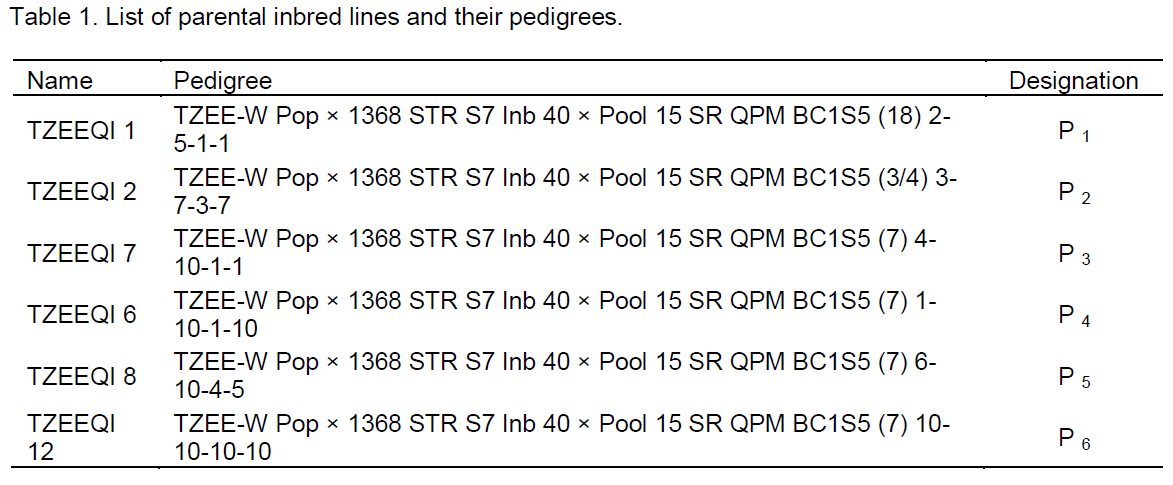
The genetic materials (Table 1) used were made up of six extra-early QPM F6 inbred lines obtained from International Institute of Tropical Agriculture (IITA), Ibadan, Nigeria. These lines were crossed in the major season of the year 2011 in incomplete diallel mating design to form 15 F1 hybrids. F1 single crosses were made by hand-pollination using bulk pollen from each line. The harvested ears were dried and shelled manually. The F1 single cross hybrids and their parents were processed and stored in cold room prior to field evaluation.
In major season of 2012, field evaluation of 15 F1 single crosses and their parents was conducted at Crops Research Institute (CRI) – Fumesua which is located at the forest ecological zone of Ghana with coarse sandy-loam soil. The experiment was replicated in three other out-stations of the Institute. These were Ejura in the forest transition zone with fine coarse sandy-loam soil, Pokuase and Akomadan in the coastal savannah and semi-deciduous forest ecological zones respectively with coarse sandy-loam soil for both locations (Sallah et al., 2004).
The entries were arranged in randomized complete block design (RCBD) with three replications. A plot consisted of two-rows of 5 m long each with planting interval of 75 cm x 40 cm was used. Hills were overplanted and thinned after emergence until a final planting density of approximately 66,000 plants ha-1 was achieved in each trial. Cultural practices such as fertilization, weeding, pest and disease control were accomplished using normal field management practices.
Data collection
Data for days to flowering (anthesis and silking) was taken and anthesis–silking interval (ASI) was calculated as the difference between number of days to silking and anthesis (SD–AD). Plant height (from the ground level to the flag leaf node) and ear height (from the ground level to the node bearing the uppermost ear) were recorded using a graduated measuring pole. Root and stalk lodging (RL and SL) parameters were taken at physiological maturity determined as the percentage of plants leaning at an angle greater than 45° from the vertical and percentage of plants with broken stalks at or below the main ear at maturity respectively. After harvest, data for cob length, cob diameter, number of rows cob-1, number of kernels row-1, thousand grain weight and grain yield plot-1 were taken.
Individual analyses of variance (ANOVA) per location or environment and across environments for agronomic traits were carried out using Genstat version 9.2. Genotypes were considered as fixed effects whilst environments and replications were treated as random effects. For each agronomic and morphological trait, an individual ANOVA was conducted to determine the statistical significance for parents and their crosses at each environment and across environments. Combining ability test involving parents and their F1 progenitors were used to assess their performance.
Diallel analysis was conducted using the DIALLEL-SAS program (Zhang and Kang, 1997). Griffiths (1956) linear Model 1 and Method 2 (Table 2) was used for analysis of variance as follows: Xijk = µ + rk + gi + gj + sij + eijk; where Xijk is the observed performance of the cross between ith and jth parents in the kth replication, µ the population mean, rk the replication effect, gi the GCA effect for the ith parent, gj the GCA effect for the jth parent, sij the SCA effect for the cross between ith and jth parents, and eijk is the experimental error for the Xijk observation (Hallauer and Miranda, 1988). Means were compared using the least significant difference (Steel and Torrie, 1980).
The estimates of heterosis over the mid parent heterosis was calculated using Aliu et al. (2009) Mid Parent Heterosis
Where: F
1 is the mean of the F
1 hybrid performance and MP = mid parent value of the particular F1 cross


where P
1 and P
2 are the means of the inbred parents.
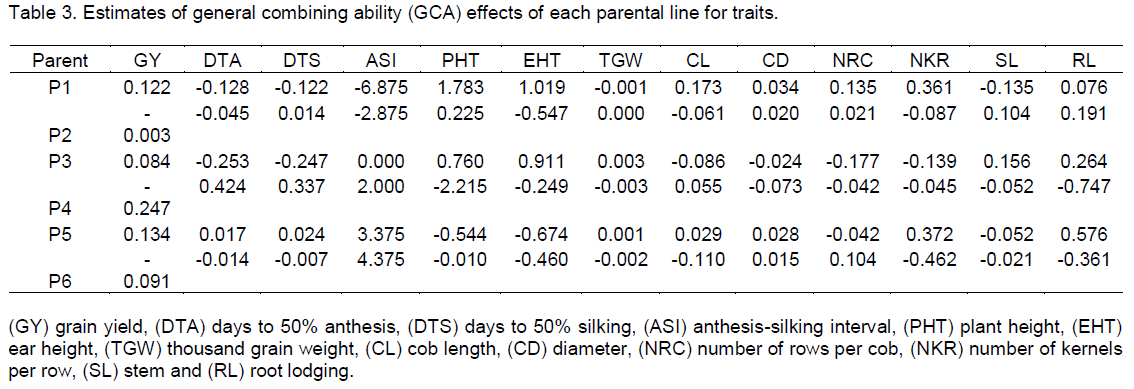
When the genotypic sum of squares was partitioned into general combining ability (GCA) and specific combining ability (SCA), only GCA was found to be significant (p < 0.01) and for only grain yield. Estimates of GCA effects indicated parental performances of the traits across all the locations (Table 3). For days to flowering (anthesis and silking), the highest and lowest GCA values were observed for P4 and P3, respectively. However, P4 had the least GCA values for grain yield, thousand grain weight, cob diameter, plant height and root lodging whilst P3 had the least for stem lodging. P5 had the highest parental GCA value for grain yield, number of kernels per row and root lodging. P1 was the best general combiner for plant height, ear height, cob length, cob diameter and number of rows per cob but exhibited the least and negative value for anthesis-silking interval. P6 had the least GCA effect for cob length and number of kernels per row but had the highest observed GCA value for anthesis-silking interval. The crosses P1×P4 and P5×P6
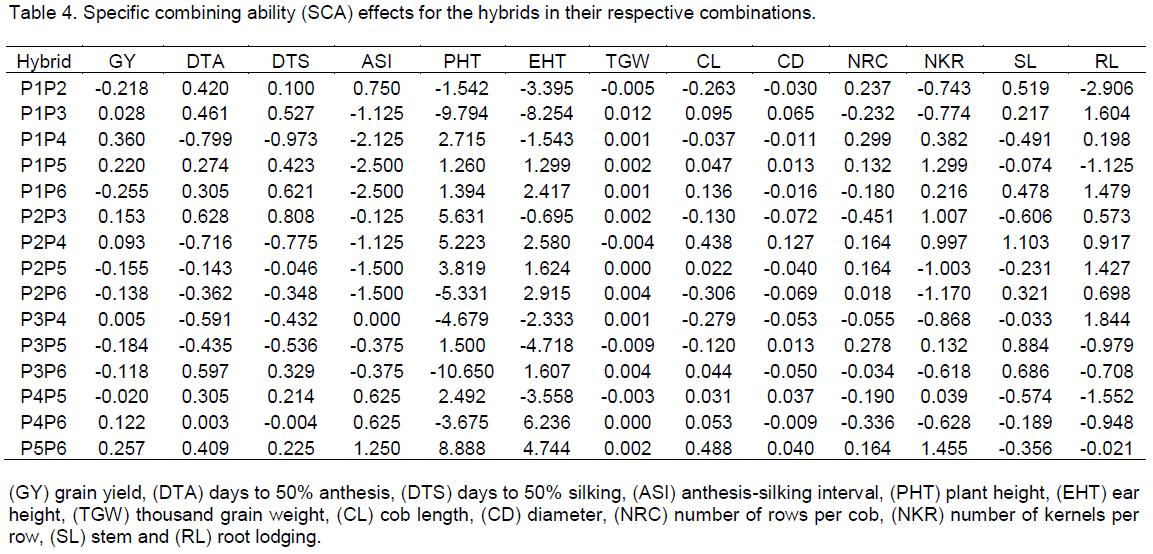
had the highest SCA effect for grain yield but P1xP4 had the highest negative SCA effects for both days to anthesis and silking (Table 4). Similarly, P5×P6 had the highest SCA effects for anthesis-silking interval, plant height, cob length and number of kernels per row. The cross combinations P1×P5 and P1×P6 had the least SCA values for anthesis-silking interval with the latter combination emerging as the least for grain yield. P1×P3 gave high negative SCA effects for both plant and ear heights and high positive value for root lodging. P4 also produced negative SCA effects for stem lodging in all crosses except for P2×P4, which had positive SCA effects for both stem and root lodging. P1×P4 however had a high positive SCA effect for cob length, cob diameter, number of rows per cob and number of kernels per row. Similarly, P5×P6 had positive SCA effects for all the traits estimated with the exception of stem and root lodging.
The mid parent heterosis estimates for the respective 15 hybrid combinations are shown in Table 5. For grain yield, the highest positive mid-parent heterosis was observed in the hybrid P1×P4 followed by P4×P6, P1×P5, P5×P6, P2×P4, P4×P5, P2×P3 and P1×P3 whilst the remaining hybrids had negative estimates. Mid-parent heterosis for grain yield ranged from -15.16 to 26.15% with an average estimate of 0.99% for the 15 hybrids (Table 5). For days to flowering, it ranged between -2.41% and 2.08% for DTA, and -3.24 to 1.94% for DTS with an averages of 0.21% and 0.25% for days to anthesis and silking respectively. The average estimate of mid-parent heterosis for anthesis-silking interval was -6.96% and ranged from -85.19 to 10.55%.
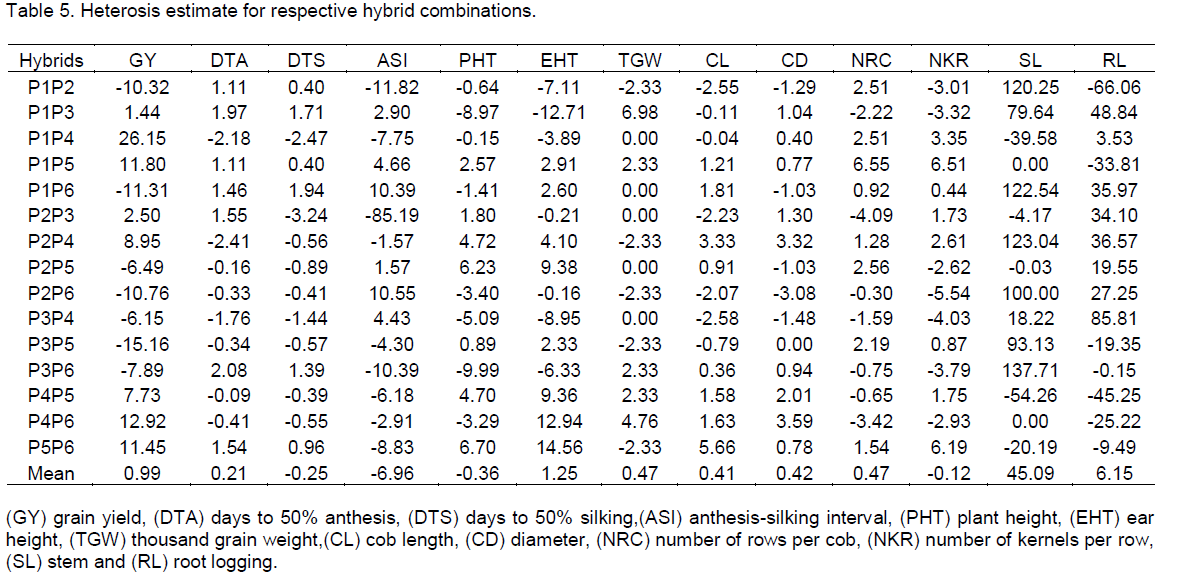
Most of the hybrids had negative mid-parent heterosis for plant height except for P1×P5, P2×P3, P2×P4, P2×P5, P3×P5, P4×P5, and P5xP6. Similarly except for 7 hybrids (P1×P2, P1×P3, P1×P4, P2×P3, P2×P6, P3×P4 and P3×P6). The average estimate of mid-parent heterosis for plant height was -0.36% and ranged from of -9.99 to 6.70%. A range of -12.71 to 14.56% with an average of 1.25% was observed for the ear height (Table 5). For thousand grain weight, the range was -2.33 to 6.98% with 0.47% as the average for 15 hybrids. Cob length and cob diameter had 0.41 and 0.42% for average mid-parent heterosis estimate for 15 hybrids and ranged from -2.58 to 5.66% and -3.08 to 3.59% respectively. The mid-parent value for number of rows per cob also ranged from -4.09 to 6.55% with an average of 0.47%, whilst number of kernels per row had an average of -0.12% and ranged from -5.54 to 6.51%. Stem and root lodging respectively had a mid-parent heterosis range of -54.26 to 137.71% and -66.06 to 85.81% with averages of 45.09 and 6.15%, respectively (Table 5).
The significance of general combining ability (GCA) and specific combining ability (SCA) plays a vital role in developing appropriate breeding approaches. As proposed by Hallauer and Miranda (1988), general and specific combining ability estimates respectively provide relative genetic effects of additive gene and non-additive gene actions (dominance and epistasis). The results indicated highly significant additive gene action for grain yield indicating that further progress can be achieved in these genotypes through recurrent selection methods. This result corroborates the finding of Musila et al. (2010), who also found significant GCA and non-significant SCA effects for grain yield. Baker (1978) and Ojo et al. (2007) suggested that the non-significant differences in SCA estimate permit maximum utilization of GCA in predicting the performance of single cross hybrids. Again, Mhike et al. (2011) suggested possibility of exploring early testing of the genotypes due to the predominance of additive gene to non-additive gene actions. This method becomes more efficient and effective for selecting promising hybrids based on their predictions from GCA effects. This presupposes that, early testing of the selected genotypes from the testcrosses from the studied population can be done for grain yield because of the predominance of GCA variances to SCA variances. The application of early testing becomes necessary since additive gene action is not affected by inbreeding depression. Hence traits that are under control of additive gene action will not suffer from inbreeding.
This assertion reflected in grain yield where the best performing hybrids (P1×P5 and P1×P3) were crosses between three inbred lines (P1, P3 and P5) with the highest GCA estimates for grain yield (0.122, 0.084 and 0.134 t/ha−1, respectively) suggesting that these parents are potentially superior (Woyengo et al., 2001). These parental lines had positive GCA effects for grain yield, indicating the presence of favourable alleles for grain yield. In addition, P1 was a good combiner for reduced days to flowering (both anthesis and silking), anthesis-silking interval, stem lodging and increased number of rows per cob, number of kernels per row, cob length and cob diameter. Consequently, P1 proved to be the best combiner for early maturity and high yields. Similarly, P3 had reduced days to flowering whilst P5 had reduced plant height, ear height and stem lodging suggesting that they have good potentials to be used in maize improvement programmes. Although P4 and P6 were poor combiners for grain yield, both parents exhibited negative GCA effects for plant height, ear height, root and stem lodgings which suggests that these parents can be used for reduced plant height and lodging tolerance improvement. For increased grain yield, it is desirable to make selection based on yield components (Zare et al., 2011). Hence P1 was the suitable genetic resource for cob length, cob diameter and number of rows per cob; P3 for thousand-grain weight, and P5 for number of kernels per row. In similar studies, non-significant GCA effects have been identified for plant height and cob length (Zare et al., 2011). As suggested by Simmonds (1979), GCA effects of parental lines also provide substantive information for selecting outstanding parents to make desirable crosses for advance breeding programmes.
The non-significant SCA effects observed in this study is possibly due to the use of parental lines that are related as proposed by Hill (1983). Similarly, non-significant SCA has been reported for grain yield (Filho et al., 1981; Ojo et al., 2007). To exploit the genetic potentials of these parents, they could be crossed with distantly related inbreds or populations. The SCA estimate gives heterotic response of parental interaction (heterosis) for specific traits (Zare et al., 2011). The high SCA and mid-parent heterosis values for grain yield observed in the following combinations: P1×P4, P5×P6, P1×P5 and P4×P6 suggests that these crosses are suitable for increased grain yield. This was manifested in yield components such as number of kernels per row, number of rows per cob, cob length and diameter where positive SCA and mid-parent heterosis values were observed. The mid parent heterosis for some crosses were negative for days to anthesis and silking indicating earliness in maturity. The maximum negative heterosis for days to flowering recorded for P1×P4, P3×P4, P2×P4, P2×P5, P4×P6 and P4×P5 suggests that the parental lines involved in these crosses may be useful for producing extra-early maturing QPM hybrids. The advent erratic climatic conditions also poses serious threat to existing early maturing varieties there by making them susceptible to biotic and abiotic factors hence the promising hybrid combinations can be useful germplasm to replace them. Earliness in maturity also offers opportunity to utilize minor season cropping where the short rainy periods can efficiently be used for maize cultivation. High negative mid-parent heterosis for plant height was exhibited in the crosses P1×P3, P1×P4, P3xP4 and P3×P6 which also means that the parents could be used as germplasm source for developing short varieties.
The study identified valuable genetic materials which can be exploited for subsequent breeding activities. The GCA estimates identified parental lines P1, P3 and P5 as the best combiners for grain yield. Again, P1 was the most suitable parent for increased cob length, cob diameter, number of rows per cob and reduced anthesis-silking interval; P3 for thousand-grain weight and reduced days to flowering (anthesis and silking days), and P5 for number of kernels per row, and reduced plant height and ear height. Hence, these parents may be used in hybridization programmes as donors of the superior traits indicated. The crosses P1×P5, P5×P6, P1×P3, P2×P3 and P1×P4 were the best performing hybrids as well as for exploiting hybrid vigour. Therefore, they can be further evaluated for possible release for commercial production by farmers.
The authors have not declared any conflict of interests.
REFERENCES
|
Aliu S, Fetahus S, Kaciu S, Salillari A (2009). Combining ability study for Yield Kernel per ear of maize (Zea mays L.) hybrid. 44th Craotian and 4th International Symposium on Agriculture 476-480. |
|
|
|
Baker RL (1978). Issues in diallel analysis. Crop Sci. J. 18: 533–536. |
|
|
|
Bernardo R (2002). Breeding for quantitative traits in plants. |
|
|
|
Crow JF (1948). Alternative hypotheses of hybrid vigor. J. Genet. 33: 477–487. |
|
|
|
Falconer DS, Mackay TFC (1996). Introduction to quantitative genetics. 4th edition. Longman, Essex, England. |
|
|
|
FAO (2007). Global change impacts on agriculture, forestry and soils: The programme of the global change and terrestrial ecosystems core project of IGBP. Department of Plant Sciences, University of Oxford, UK. |
|
|
|
Filho VN, Elto GEG, Vianna RT, Môro JR (1981). General and specific combining ability for yield in a diallel cross among 18 maize populations (Zea mays L.) Revista Brasileira De Genetica 4: 571•577. |
|
|
|
Griffiths B (1956). Concept of general and specific combining ability in relation to diallel crossing systems. Austr. J. Biol. Sci. 9: 463–493. |
|
|
|
Hallauer AR (1990). Methods used in developing maize inbreds. Maydica 35: 1-16. |
|
|
|
Hallauer AR, Miranda JB (1988). Quantitative genetics in Maize breeding. Iowa State University Press, Ames. |
|
|
|
Hayman BI (1954). The theory and analysis of diallel crosses. Genetics 39: 789-809. |
|
|
|
Hill JRR (1983). Heterosis in population crosses of Alfalfa. Crop Sci. J. 23: 48–50. |
|
|
|
Krivanek A, Vivek B (2006). Science behind breeding QPM. CIMMYT, Mexico. |
|
|
|
Lippman ZB, Zamir D (2006) Heterosis: Revisiting the magic. Trends in Genetics 23: 2 www.sciencedirect.com, (Accessed on 4-11-2011) |
|
|
|
Mhike X, Lungu DM, Vivek B (2011). Combining ability studies amongst AREX and CIMMYT maize (Zea mays L.) inbred lines under stress and non-stress conditions. Afr. J. Agric. Res. 6(8): 1952-1957. |
|
|
|
Michael LM, Rosert T, Dankyi AA (1999). Adoption and impacts of improved Maize production technology: A case study of the Ghana Grains Development Project. CIMMYT Economics Program Paper 9. |
|
|
|
Moaveni P, Lotfi M, Farahani, Maroufi K (2011). Effect of spraying TiO2 nano particles on some of physiological and chemical parameters in maize (Zea mays Lo), Int. J. Biosci. 1(4): 63-67 |
|
|
Musila RN, Diallo AO, Makumbi D, Njoroge K (2010). Combining ability of early-maturing quality protein maize inbred lines adapted to Eastern Africa. Field Crops Res. 119: 231–237.
Crossref |
|
|
|
Ojo GOS, Adedzewa DK, Bello LL (2007). Combining ability estimates and heterosis for grain yield and yield components in maize (Zea mays L.). J. Sustain. Dev. Agnananthe Environ. 3:49-57. |
|
|
|
Okello DK, Manna R, Imanywoha J, Pixley K, Edema R (2006). Agronomic performance and breeding potential of selected inbred lines for improvement of protein Quality Adopted Ugandan maize Germplasm. Afr. Crop Sci.J.14(1): 37-47. |
|
|
Sallah PYK, Abdulai MS, Obeng-Antwi K (2004). Genotypes and environment interactions in three maturity groups of maize cultivars. Afr. Crop Sci. J. 12(2): 95 -104.
Crossref |
|
|
Shull GH (1908). The composition of field of maize. Am. Breeders Assoc. Rep. 4: 296–301.
Crossref |
|
|
|
Simmonds NW (1979). Principles of Crop Improvement. Longman, London. |
|
|
|
Steel RGD, Torrie JH (1980). Principles and Procedures of Statistics, A Biometrical Approach. 2nd Edition. McGraw Hill Inc., New York, Toronto, London. |
|
|
|
Stoskopf NC, Tomes DT, Christie BR (1993). Plant Breeding: Theory and Practice. Westview Press, Oxford, UK. |
|
|
|
Vivek B, Krivanek PRN, Twumasi-Afriyie S, Diallo A (2007). Breeding Quality Protein Maize (QPM): Protocols for developing QPM cultivars, CIMMYT. Mexico. |
|
|
|
Woyengo VW, Odongo OM, Ajanga SI (2001). Combining ability analysis of 72 maize inbred lines and development of Topcross hybrids resistant to follar diseases. Master Science Thesis submitted University of Nairobi, Kenya. |
|
|
|
Zare M, Choukan R, Heravan EM, Bihamta RM, Ordookhani K (2011). Gene action of some agronomic traits in corn (Zea mays L.) using diallel cross analysis. Afr. J. Agric. Sci. 6(3): 693-703. |
|
|
Zhang Y, Kang MS (1997). DIALLEL-SAS: a SAS program for Griffiths's diallel analyses. Agron. J. 89: 76–182.
Crossref |