Full Length Research Paper
ABSTRACT
The need to accelerate breeding for increased yield and better adaptation to drought is an issue of great concern because of the high demand for food and potential climate change poses further challenges. The study was designed to introgress drought-tolerant possessing genes/quantitative trait loci into popular and farmer-preferred cultivars through marker-assisted backcrossing (MABC) and assess for post-flowering drought tolerance. Sixty-one converted progeny and nine parental lines were evaluated under post-flowering water stress condition. The mean grain yield of genotypes that widely varied (923 to 4585 kg ha-1) was 1991 kg ha-1. Out of the 61 BC2F3, 9.8% were superior in yield ranging from 2831 to 4585 t ha-1, indicating the potential to withstand post-flowering moisture stress. They were also characterized by high chlorophyll content, greater leaf area and greenness at physiological maturity. Relatively high heritability (34.8-74.7%) and genetic gain (1.4-42.7%) were obtained for most agronomic and physiological characters, revealing selection for such characters could be easily attained. Thus, the presence of more green leaves, greater green leaf area and high chlorophyll content both at booting and maturity could contribute to higher photosynthesis and better availability of food reserves for grain-filling and improved yield.
Key words: Drought tolerance, introgression, post-flowering, Sorghum bicolor, water-limited.
INTRODUCTION
Sorghum [Sorghum bicolor (L.) Moench] is the fifth most important cereal crop globally in terms of area coverage and total production after wheat, maize, rice and barley. It has a predominant role in the food and fodder security for millions of rural families in arid and semi-arid regions of the world. Globally, sorghum is cultivated on 43.69 million ha, from which 66 million tons of grain is annually produced; the average productivity is 1.5 t ha-1 (FAO, 2017). In Ethiopia, sorghum is among the most important cereal crops, particularly in areas where rainfall is unreliable and crop failures due to recurrent drought are frequently observed. It plays a significant role for millions of food-insecure people living in such environments. Currently, sorghum is covering a total land area of 1.9 million ha from which 5.2 million tons of grain is annually produced (MoA, 2018; CSA, 2018). The major sorghum producing regions are Oromia, Amhara, and Tigray that contribute 38.8, 35.5 and 13.4% of the area coverage and 40.5, 35.5, and 14.05% of the total production, respectively (CSA, 2018). Despite the multiple importances, the average national yield of the crop has remained very low largely due to drought (Amelework et al., 2015; Mera, 2018; Teshome and Zhang, 2019) and Striga (Ejeta, 2007; Abate et al., 2014).
Drought is a major constraint to sorghum production worldwide, although sorghum by its nature is considered as a highly drought tolerant cereal crop (Kassahun et al., 2010; Sabadin et al., 2012; Reddy et al., 2014; Amelework et al., 2015; Mera, 2018; Teshome and Zhang, 2019). Yield loss due to drought in the tropics alone exceeds 17% and reaches up to 60% in severely affected regions (Ribaut et al., 2002). In Ethiopia, where more than 50% of the total area is drought-prone, insufficient, unevenly distributed, and unpredictable rainfall is usually experienced in drier parts of the country (Amelework et al., 2015; Mera, 2018; Teshome and Zhang, 2019) in which nearly 40% of the population lives (EMA, 1988). It is manifested by either of the delay in onset, dry spell after sowing, and drought during critical crop growth stage such as flowering and grain filling (early withdrawal of rain). Moisture stress during later growth stages (grain filling) is the common phenomenon facing subsistence farmers in the country. It is frequently observed that drought is occurring at more frequent intervals-every two years during recent years. For instance, between 1960 and 1990 there were six drought episodes in the country, but between 1990 and 2014 the episodes increased to nine (USGS, 2017; Mera, 2018) causing as much as complete loss of sorghum and other crops affecting millions of people. This shows drought is becoming very challenging for production and productivity of sorghum and many other crops, possibly due to changing and variable climates. The large loss of sorghum yield is also related to the poor drought tolerance level of the available cultivars/varieties. Hence, control of drought through different options remain an important factor with priority geared towards ensuring food security in Africa as a whole and Ethiopia in particular.
In addition to the agronomic moisture conservation methods like tie-ridging, rainwater harvesting and soil-water conservation, breeding for more productive crop cultivars is one of the sound strategies in increasing crop yields in drought-prone environments. This is because better environmental manipulation with moisture-conserving agronomic practices alone may not lead to better yields from inferior genotypes unless they are integrated with crop genotypes that are capable of efficiently exploiting the limited moisture conserved (Singh, 2002). Therefore, the use of resistant/tolerant varieties could be one of the feasible alternatives to further increase its productivity, stabilize production and contribute to food security in areas where drought is a regular feature of most sorghum growing environments. To this end, conventional breeding has been contributing immensely towards genetically insulating sorghum from various abiotic stresses such as drought for the last many decades. Nonetheless, in the current scenario of crop production wherein multiple and new threats have arisen, conventional breeding alone does not seem to be an effective approach because of technical difficulties encountered in making major advances such as long crossing and backcrossing cycles, costs, and influence of genotype by environment interaction (Bartels and Sunkar, 2005; Khera et al., 2013).
Experiences elsewhere show that when modern biotechnological tools are properly applied with the conventional breeding system, it is obvious that the long backcrossing cycles to transfer specific genes of interest would be shortened, gene pyramiding would be simpler and the release of high yielding varieties and their subsequent use as improved seeds would be enhanced and hastened. The conventional sorghum breeding efforts supported by molecular assisted tools have scored remarkable successes in identifying and incorporating genes for tolerance to drought (Subudhi et al., 2000; Tao et al., 2000; Xu et al., 2000a; Haussmann et al., 2002; Sanchez et al., 2002; Kassahun et al., 2010). The development of drought-tolerant varieties has been dominantly focused on two distinct stages: pre-flowering and post-flowering. The best-characterized form of drought tolerance during the later stage of crop growth is the so-called stay-green, which is the ability to resist premature plant senescence (retain green leaf area), resist lodging and fill grain normally (Rosenow et al., 1983). Maintaining the greenness of leaves for a longer period is a principal strategy for increasing crop production, particularly under water-limited conditions (Tao et al., 2000; Xu et al., 2000a; Haussmann et al., 2002; Sanchez et al., 2002; Kassahun et al., 2010; Abdelrahman et al., 2017).
Considerable work has been done on the identification of stay-green genotypes, mapping and identification of quantitative trait loci (QTLs) associated with the trait (Xu et al., 2000a; Haussmann et al., 2002; Sanchez et al., 2002). Therefore, it is advisable to validate, refine and adopt molecular markers already developed elsewhere for drought tolerant to better serve the needs in Ethiopia. On the other hand, local sorghum cultivars are highly preferred by the farming communities mostly for their yield, biomass and other morpho-agronomic attributes despite their susceptibility to terminal moisture stress. To this end, limited works have been made so far to improve the major limitations (such as vulnerability to drought) of these cultivars. Thus, conversion of popular and farmer’s preferred cultivars into their drought tolerant versions through incorporation of the responsible genes employing marker-assisted backcrossing (MABC) seems to be the best strategy in terms of time saving, effectiveness and efficiency. The present study was therefore, conducted to introgress drought tolerant genes/QTLs into popular and farmer preferred cultivars through MABC and assess the stay-green expression and associated agronomic performance.
MATERIALS AND METHODS
Plant materials
The parental sorghum lines used for this backcrossing program were one donor parent “B35” and eight recurrent parents which are released varieties and known farmers’ cultivars (Table 1). The donor parent is known for post-flowering drought tolerant and it has been used as a source of tolerant genes to drought by the national sorghum-breeding program. B35 is a 3-gene dwarf genotype, BC1 derivative of IS12555 accession, a durra from Ethiopian and is known for its stay green behaviour (Rosenow et al., 1983), more specifically a type-A stay-green-delayed onset of leaf senescence (Thomas and Smart, 1993; Thomas and Howarth, 2000). As characterized by several research groups (Crasta et al., 1999; Subudhi et al., 2000; Xu et al., 2000b; Sanchez et al., 2002), it was identified as a source of a number of stay green QTLs involving B35. B35 is also known for a number of other characteristics including early maturing, long in stature, has short compact panicle with copious number of infertile branches; purple genotype with small seeds covered by glumes, dry leaf midrib and relatively low yield potential (Srinivas et al., 2009; Kassahun et al., 2010). The recurrent parents are generally high yielding and biomass under optimum moisture conditions (MoA, 2018) and popular amongst the farmers but susceptible to terminal drought.
Development of backcross progeny
The popular and farmers preferred Ethiopia sorghum pure lines (improved and/or local) were crossed with B35 (with stay-green genes). The crossings were made using hand pollination method to generate F1 progeny and subsequent generations at Melkassa Agricultural Research Center, Ethiopia. Crossing is done by emasculation of selected plant panicles (recurrent parents) and dusting of pollen from identified plants (donor parent). After analysis for the presence of the desired donor parent alleles and recurrent parents’ genome, selected heterozygous F1 plants were backcrossed with respective recurrent parents to generate BC1F1 progeny. Thereafter, the individuals selected based on desired marker(s) were backcrossed to generate BC2F1. After each series of backcrossing, marker-assisted foreground (donor allele) and background (recurrent parent’s recovery potential) selections were made to fix through twice selfing and generated 61 BC2F3. In this study, five QTL (Stg1and Stg2 (on SBI-03), Stg3a and Stg3b (on SBI-02), and Stg4 (on SBI-05) associated with the stay green character was targeted (Xu et al., 2000a; Crasta et al., 1999; Subudhi et al., 2000; Tao et al. 2000; Haussmann et al., 2002; Sanchez et al., 2002).
Evaluation of backcrossed and parental lines for drought tolerance
Field experiment was conducted in Rama Kebele of Mereblekhe district in central zone of Tigray, Ethiopia. The location was selected based on the potential of sorghum growing and availability of irrigation for imposing a managed level of stress. Rama kebele is situated at 14°23’39″ N latitude and 038°48’90″ E longitude. Rama is found at an altitude of 1389 m above sea level, with average minimum and maximum temperatures ranging from 22 to 38°C, respectively, during the study period (December 2018 to May 2019). The district is characterized by eutric cambisols, haplic xerosols, orthic solonchaks, calcic xerosols, chromic cambisols, eutric nitisols, and orthic luvisols soil types in order of their importance. The specific site was characterized by eutric cambisols soil type.
The field trials consisted of 61 BC2F3, one donor parent and eight recurrent parents (Table 2) which were evaluated under well-watered and water-limited conditions arranged in -lattice design with three replications. The limited irrigation (stress) trial was irrigated well during the early growth stages but irrigation was withheld after anthesis. Meanwhile, the well-watered trial was fully-irrigated, so that, essentially, no moisture stress occurred at any stage of the crop development. The trials were planted on the same date and the same field in adjacent blocks. The mean traits obtained from the full-irrigation trial were only used to determine the relative mean trait relative reductions and expressed in percentage. The experimental units were two-rows of 4 m long with 0.15 m plant to plant spacing and 0.75 m row to row spacing. Fertilizer (NPS) was applied at the rate of 100 kg ha-1 at planting and urea at rate of 50 kg ha-1 split two times, half at planting and the remaining half as knee height. All other agronomic management and protection practices were applied uniformly to all plots as recommended.
Data were collected on important morpho-agronomic and physiological parameters on either pre-tagged random sample plants or whole plot basis depending on the trait studied.
Agronomic traits
The important agronomic traits recorded in this study include: plant height (PLHT, in centimeter, the height of the plant from the bottom to the tip of the panicle at maturity), days to flowering (DTF, number of days from emergence to 50% flowering), days to maturity (DTM, number of days from emergence to form a black tip on seed at the junction between seed and plant at the base of the head), biological yield (BM, in kg, sun dried weight of all above ground part from a plot and later converted to kg ha-1) , grain yield (YLD, in kg, the grain yield harvested on hectare basis), panicle length (PL, in centimeters, measured from the bottom to tip of the panicle), panicle width (PW, in centimeters, measured at the middle panicle diameter), panicle weight (PWt, in grams, measured from five heads) and thousand seed weight (TSW, weight of 100 seeds in grams and later converted to thousand seed weight).
Physiological or stay-green characters
The leaf senescence expression of individual introgressed and their parental lines were estimated visually on a scale of 1 to 5 based on the degree of premature leaf and plant death at physiological maturity from five pre-tagged plants, hat is, 1 = very slight senescent, 2 = 25% leaves senescent, 3 = 50% leaves senescent, 4 = 75% leaves senescent, and 5 = 100% or complete senescent as suggested by Wanous et al. (1991). The total chlorophyll contents were measured with a Minolta Chlorophyll Meter SPAD-502 (Konica-Minolta Camera Co., Ltd Tokyo, Japan) at booting (SPADB) and physiological maturity (SPADM). The SPAD readings were taken from the middle of the leaf lamina of the second and fourth leaves from the top on five random pre-tagged sample plants at three places and averaged for analysis (Xu et al., 2000b). The total number of green leaves at booting (NGLB) and maturity (NGLM) were counted and used to determine percent of green leaves retained at maturity (PGLM), obtained as ratio between NGLM to NGLB expressed in percentage (Srinivas et al., 2009). Green leaf area at booting (GLAB in cm2) and maturity (GLAM in cm2) were measured from the length and the width of five green leaves from the top to bottom five pre-tagged plants and the area of each leaf was estimated using a correction factor of 0.70 (Mahalakshmi, 2002; Srinivas et al., 2009) as:
Leaf area = leaf length × leaf width × 0.70
The total green leaf area of each tagged plant was calculated as the sum of all the measured leaves from that particular plant. The upper six leaves were considered for measuring the green leaf area (Haussmann et al., 2002) as the upper leaves are photosynthetically active and directly assimilate mostly to the grain (Joshi et al., 2003). The average percentage green leaf area preserved at maturity (PGLAM) from each plot was calculated by dividing the total green leaf area of each plot at maturity (GLAM) by the total green leaf area of that plot at anthesis (GLAB) (Srinivas et al., 2009). The rate of leaf senescence (RLS in cm2 day−1) was determined as: RLS = [GLAB - GLAM]/number of days taken from booting and maturity (Reddy et al., 2014).
Estimation of relative traits reduction due to water stress
The relative traits reduction (RR) was calculated from traits obtained under full-irrigation (Yp) and water-limited (Ys) conditions as follows:
Data analysis
Data were subjected to statistical analysis using R software version 3.6.1 (R Core Team, 2019). Genotype differences in agronomic and physiological characters were analysed by residual maximum likelihood algorithm (ReML) as suggested by Patterson and Thompson (1971).
Estimation of heritability in broad sense
Broad sense heritability (H2b) was estimated as described by Allard (1960) as follows:
where 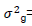 genotypic variance,
genotypic variance, 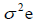 = environmental variance, and r= number of replications.
= environmental variance, and r= number of replications.
Genetic advance from selection
Genetic advance (GA) was calculated with the method suggested (Allard, 1960; Falconer, 1989), assuming the selection intensity of 5%, as:
GA = K× σph×H2b
Where, K= the constant differential (K=2.063 at 5% selection intensity), σph = square root of phenotypic variance and H2b = broad-sense heritability.
The genetic advance as percentage of the mean (GA%) was calculated as described by Johnson et al. (1955) and Falconer (1989) as follow:
x = Grand mean of a character.
RESULTS AND DISCUSSION
Differences among the genotypes were significant (P<0.05) for a number of characters (Table 3). The comparison of the developed progeny with their parents showed superior performances for many agronomic attributes. The overall mean of days to flowering (DTF) was 82 days. It is believed that the difference in DTF was attributed to the genetic background as it was subjected to uniform irrigation until the induction of stress after flowering. The mean plant height of the genotypes was 136.4 cm. Plant height of the converted progeny and recurrent parents ranged from 100.3 to 192.6 cm and 104.1 to 163.8 cm, respectively. This showed that the converted progeny performed well revealing the amalgamation of the targeted genes from their parents. The shortest plant height was recorded from B35 with values of 93.5 cm, as it was expected (Kassahun et al., 2010). Days to maturity (DTM) were 118.7 days. The longest DTM was recorded for the developed progeny than either of the parents. The mean DTM of the backcrossed progeny and recurrent parents varied from 114 to 126.7 and 114.8 to 119.9 and that of B35 was 116.9 days, respectively. About 23% of the converted progeny showed significant delay in days to maturity. This might be due to high vegetative growth and devouring of relatively longer part of their reproductive growth to an end-of-season, which is the behavior of the donor parent ‘stay-green’. The maintenance of grain filling in the last stage of plant maturity has been considered as a key to the success of stay green genotypes (Luche et al., 2015). When looking at the yield components such as panicle length, panicle width, and panicle weight ranged from 14.6 to 27.1 cm, 4 to 7 cm, and 116.7 to 465.4 g, respectively. About 6.6, 9.8, and 13.1% of the backcrossed lines showed good performance for the aforementioned traits, respectively, than their parents (Table 3).
The mean biomass (BM) of the genotypes ranged from 1634 to 11010 kg ha-1. The highest biomass was obtained from the converted progeny indicating the potential of the introgressed progeny and performing well in such environments, as they contain stay-green genes. It is clear that biomass accumulation is a function of water use efficiency by plants (Balota et al., 2008). On the other hand, the main effect of moisture deficit is the reduction of biomass accumulation (Tsuji et al., 2003; Castro-Nava et al., 2012) through drought induced inhibition of leaf and stem elongation, which differs among species (Pelleschi et al., 1997), and a reduction of relative growth and net CO2 assimilation rates (Younis et al., 2000). Therefore, the yield reduction under water deficit is at least partly due to variations in total biomass accumulation (Craufurd and Peacock, 1993) among the genotypes. It was observed that about 27.9% of the developed progeny showed higher biomass ranging from 6128.3 to 11010 kg ha-1 (Table 3). Of the 13 QTLs, backcrossed progeny with stg1+stg2+stg3a+stg4, stg1+stg2+stg3a+stg3b and stg1+stg2+stg4 markers yielded highest biomass. It is also imperative to consider that backcrossed lines with high biomass could be recommended for livestock feed as dual-purpose, but also affected by drought episodes.The mean grain yield of the genotypes (923 to 4585 kg ha-1) was 1991 kg ha-1. The result showed that the highest yield was obtained from BC2F3_ETSC_16258 (4585 kg ha-1) followed by five backcrossed progeny namely BC2F3_ETSC_16216 (3538 kg ha-1), BC2F3_ETSC_16257 (3107 kg ha-1), BC2F3_ETSC_16213 (2924 kg ha-1), BC2F3_ETSC_16248 (2886 kg ha-1), and BC2F3_ETSC_16141 (2831 kg ha-1). Of the 13 cumulative QTLs, the highest yield was obtained from progeny with QTL stg1+stg2+stg3a+stg4 (2924 kg ha-1) and stg1+stg3a+stg3b+stg4 (2549 kg ha-1). Similarly, thousand seeds weight (TSW) of the genotypes ranged from 20.8 to 41.7 g. The results showed that nearly 36.1% of the converted progeny showed superior thousand seed weight ranging from 33.8 to 41.7 g. It may imply that converted progeny may have better structural and functional fitness to apt well on the water use efficiency, water extraction, growth, and good seed-sink interaction during grain filling period under water deficit environments than the seed parents. This confirmed that the developed progeny had the target genes responsible for the stay-green trait from the donor parent and yield potential from their respective recurrent parents (through subsequent backcrossing). Although there was no single genotype showed consistent superiority for grain yield and stay-green characters, 9.8% of the developed progeny showed superior performances for many attributes and hence, needs further evaluation for potential release.
On the other hand, about 67.2% of progeny showed significant lower yield due to terminal drought depending on the genetic background with yield ranging from 923 to 1987 kg ha-1. Reports showed that reduction in yield under water stress usually resulted from reduction in starch accumulation during grain development (Barnabás et al., 2008) and grain number (van Oosterom and Hammer, 2008) which differs among genotypes. The yield reduction of the developed progeny could be attributed to the expression of QTLs likely affected by the genetic background (epistasis interaction) and incomplete conversion of the generated progeny (BC2F3), that is, 87.5%.
Differences among genotypes were significant (P<0.05) for all stay green characters (Table 3). The comparison of backcrossed progeny with their parents, revealed the existence of superior performance for many stay-green characters. In order to determine if the introgression of the B35 stay-green markers into the recurrent parents background also affected chlorophyll content, two SPAD measures were made at booting (SPADB) and maturity (SPADM). In this context, the highest SPADB values were observed for B35 indicating high chlorophyll concentration index (Kassahun et al., 2010; Reddy et al., 2014). The mean of SPAD values at booting was 48.6. At booting/flowering stage, almost all genotypes showed a good leaf health and chlorophyll concentration index (40-60) showing a good indicator of the transfer of energy to the reaction center of the photosystems (Mullan and Mullan, 2012). The highest SPADB was recorded from B35 followed by those progeny containing stg1+stg3a+stg3b and stg2+stg3b+stg4 QTLs. The highest mean SPADM were registered from BC2F3_ETSC_16258 (43.5), BC2F3_ETSC_16225 (39), BC2F3_ETSC_16253 (36.9) and BC2F3_ETSC_16139 (35.6), Wediaker (40) and B35 (34.6) indicating some of the progeny maintained better chlorophyll content until physiological maturity and are comparable with B35. This showed that the introgression of responsible genes enhanced the relative amount of total chlorophyll present in plant leaves for better structural and functional activities under water-limited conditions. B35 showed better records of SPADM than recurrent parents, did as expected (Xu et al., 2000b; Kassahun et al., 2010; Reddy et al., 2014) followed by progeny with stg1+stg3a+stg3b+stg4 QTLs. The mean percent of green leaves at maturity (PGLM) was 38.8%. The highest mean PGLM was observed for BC2F3_ETSC_16139 (64.01%) followed by 11 converted progeny with values ranging from 50 to 60.6% and that of B35 (48%). This indicated that the presence of more green leaves at maturity contributed to higher photosynthesis and better availability of food reserves for grain filling (Kassahun et al., 2010; Vadez et al., 2011; Jordan et al., 2012).
The mean green leaf area at booting (GLAB) was 1520.2 in cm2 plant-1. The highest GLAB was measured from line BC2F3_ETSC_16229 (2070.1 cm2 plant-1) followed by seven converted progeny with values ranging from 1814.8 to 2043 cm2 plant-1. Equally, the green leaf area at maturity (GLAM) was high for the converted progeny. The highest GLAM was measured for BC2F3_ETSC_16223 (1407.2 cm2 plant-1) followed by four progeny (1283.2-1325.3 cm2 plant-1). In the same manner, the percent of green leaf area preserved at maturity (PGLAM) ranged from 51 to 96.3% for converted progeny, 67.4 to 78.98% for recurrent parents, and that of B35 was 80.8%. The highest PGLAM was obtained consistently from 15 converted progeny indicating their potential in maintaining high green leaf area in the entire season. This study was found in agreement with previous reports that green leaf area at physiological maturity has proved to be an excellent indicator of stay green, and has successfully been used to select drought resistant sorghums (Rosenow et al., 1983; Henzell et al., 1992; Borrell et al., 2014). This is in harmony with Kassahun et al. (2010) and Pask and Pietragalla (2012).
The score of leaf senescence (LS) of the genotypes ranged from lowest (stay-green) 1.85 to 4.6 (leaf drying). Among the developed progeny, 34.4% exhibit delayed/reduced LS with values ranged from 1.85 to 3. The converted progeny with stg1+stg2+stg3a+stg4, stg1+stg2+stg3a+stg3b and stg1+stg3a+stg3b+stg4 QTLs showed relatively delayed LS. The donor parent (B35) had LS score of 2.25. The mean rate of leaf senescence (RLS) was 1.1 cm2 day-1. Among the converted progeny, 15 had lower RLS ranged from 0.35 to 0.85 cm2 day-1 and comparable with B35. In most cases, those genotypes with stay-green (LS) trait also had lower RLS.
The results showed that there were six progeny that exhibit good stay-green characters, which can be recommended for further evaluation as potentially released, particularly in environments in which available water during grain filling is not adequate to support potential transpiration. It is a fact that LS is associated with the balance between hormones such as cytokinins and ethylene, and the over expression or suppression of these hormones show changes in the timing of senescence, accelerating and retarding the process (Buchanan-Wollaston et al., 2003; Gregersen et al., 2013). Genotypes with stay-green characteristics have been found to contain higher cytokinin levels (Reguera et al., 2013; Ambler et al., 1987); more stem sugars (Duncan et al., 1981; Dahlberg, 1992; Borrell et al., 1999, 2000b; Zwack and Rashotte, 2013) and more nitrogen possibly associated with a higher transpiration efficiency (Borrell and Hammer, 2000; Borrell et al., 2001; Mahalakshmi and Bidinger, 2002) than senescent genotypes. In addition, drought increases the C/N ratio and this C/N imbalance is associated with various senescence-related symptoms, including decreases in photosystem II efficiency and chlorophyll content, along with up-regulation of senescence-related genes (Reguera et al., 2013; Chen et al., 2015).
Furthermore, the stay-green phenotype may be achieved via the modification of root architecture (nodal root angel) (Mace et al., 2012), canopy development (Borrell et al., 2000a), or both. Mace et al. (2012) reported that nodal root angle in sorghum influences vertical and horizontal root distribution in the soil profile and is thus relevant to drought adaptation. The same report also indicates colocation of the QTLs between nodal root angle and the stay-green drought response in sorghum. Generally, characters such as GLAM, LS, and subsequent RLS are important factors determining greater green leaf area during grain-filling (Van Oosterom et al., 1996; Borrell et al., 2000a; Mahalakshmi and Bidinger, 2002). It is believed that stay-green plants photosynthesize for a longer period (Hörtensteiner, 2006; Tian et al., 2013; Borrell et al., 2014; Abdelrahman et al., 2017) though C-N transition point is delayed, or the transition occurs on time but subsequent yellowing and N remobilization run slowly (Yoo et al., 2007; Thomas and Ougham, 2014).
Relative trait mean performance reduction due to water-stress
Drought stress affects all phenological growth stages, reduces the normal growth and development periods, dry matter production and final yield. The relative reduction was determined on population basis, implying that the parents (recurrent and donor) and developed introgressed lines (whole and 10% selected based on the high yield). All the traits considered in this study were affected by terminal water-stress, although at different magnitude. In general, the mean relative traits performance reduction ranged from 5.7 to 38.9%. The mean relative reduction of plant height, days to maturity, panicle length, panicle width, panicle weight, biomass, thousand seed weight, and grain yield were 13.6, 5.7, 6.9, 16.7, 34.1, 43.3, 14.7, and 38.9%, respectively (Table 4). The relative reduction of grain yield ranged from 34.8 to 43.2% with a mean of 38.9%. The highest relative reductions in grain yield were recorded for the overall developed progeny (43.2%) followed by the recurrent parent (40%) depending on the genetic background and QTLs/genes expression. The relative reduction for the 10% selected converted progeny was intermediate (34.8%), indicating the presence of some promising lines that better tolerate the effects of water- stress as compared to the parents. In general, panicle weight, dry biomass weight and grain yield was amidst the severely affected by the terminal drought or stress.
Estimates of broad sense heritability
The majority of characters showed medium to moderately high H2b (40-74.67%) except days to flowering and maturity (Table 5). As a bench mark, heritability values greater than 80% were grouped as very high, values from 60-79% were moderately high, values from 40-59% were medium and values less than 40% were low (Johnson et al., 1955; Singh, 2002). Accordingly, most of the characters were categorized as medium or moderately high H2b. The characters having very high heritability (≥80%) indicated that the relative small contribution of the environmental factors to the phenotype and selection for such characters could be effective. Conversely, a trait with low broad-sense heritability (below 40%) indicated that selection could be difficult or virtually impractical due to the environment, concealing genotypic effects (Vinodhana et al., 2009; Keneni, 2012).
Genetic advance from selection
It is obvious that heritability in conjunction with genetic advance has a greater role to play in determining the effectiveness of selection of a character. In this study, genetic advance were high for plant height (23.86%), panicle length (13.87%), panicle width (34.11%), biomass (44.1%), yield (42.53%), SPADM (14.69%), GLAB (13.58%), and LS (22.6%) as described by Johnson et al. (1955). Thus, characters with both high H2b and GA indicate selection based on these traits could be effective (Table 5).
CONCLUSION
The results demonstrated that stay-green QTL from the donor parent have been successfully introgressed into the recurrent parents and are expressed in the developed backcrossed lines. This was exemplified by the presence of more green leaves, greater green leaf area, and high chlorophyll content especially at physiological maturity and consequently enhanced grain yield. This shows the potential of MABC in building up the existing cultivars profile in enhancing drought tolerance, which might have limited success with only phenotypic selection.
CONFLICT OF INTERESTS
The authors have not declared any conflict of interests.
ACKNOWLEDGMENTS
The authors greatly appreciate the support by the Ethiopian Institute Agricultural Research (EIAR), Melkassa Agricultural Research Center (MARC), Addis Ababa University and Agricultural Growth Program-II (AGP-II).
REFERENCES
|
Abate M, Mekbib F, Hussien T, Bayu W, Reda F (2014). Assessment of genetic diversity in sorghum (Sorghum bicolor (L.) Moench) for reactions to Striga hermonthica (Del.) Benth. Australian Journal of Crop Science 8(8):1248-1256. |
|
|
Abdelrahman M, El-Sayed M, Jogaiah S, Burritt DJ, Tran LSP (2017). The "Stay-Green" trait and phytohormone signalling networks in plants under heat stress. Plant Cell Reports 36(7):1009-1025. |
|
|
Allard RW (1960). Principles of Plant Breeding. John Willey and sons, Inc. New York |
|
|
Amelework B, Shimelis H, Tongoona P, Laing M (2015). Physiological mechanisms of drought tolerance in sorghum, genetic basis and breeding methods: a review. African Journal of Agricultural Research 10 (31):3029-3040. |
|
|
Balota M, Payne WA, Rooney W, Rosenow D (2008). Gas exchange and transpiration ratio in sorghum. Crop Science 48(6):2361-2371. |
|
|
Barnabás B, Jäger K, Fehér A (2008). The effect of drought and heat stress on reproductive processes in cereals. Plant, cell and environment 31(1):11-38. |
|
|
Bartels D, Sunkar R (2005). Drought and salt tolerance in plants. Critical Reviews in Plant Sciences 24(1):23-58. |
|
|
Borrell AK, Bidinger FR, Sunitha K (1999). Stay-green associated with yield in recombinant inbred sorghum lines varying in rate of leaf senescence. International Sorghum and Millets Newsletter 40:31-34. |
|
|
Borrell, A.K. and Hammer, G.L. (2000).Nitrogen dynamics and the physiological basis of stay-green in sorghum. Crop Science 40(5):12951307. |
|
|
Borrell AK, Hammer GL, Douglas ACL (2000a). Does maintaining green leaf area in sorghum improve yield under drought? I. Leaf growth and senescence. Crop Science 40(4):1026-1037. |
|
|
Borrell AK, Hammer GL, Henzel RG (2000b). Does maintaining green leaf area in sorghum improve yield under drought? II. Dry matter production and yield. Crop Science 40(4):1037-1048. |
|
|
Borrell AK, Hammer GL, van Oosterom E (2001). Stay-green: a consequence of the balance between supply and demand for nitrogen during grain filling? Annals of Applied Biology 138:91-95. |
|
|
Borrell KA, van Oosterom JE, Mullet EJ, George-Jaeggli B, Jordan RD, Klein EP, Hammer LG (2014). Stay-green alleles individually enhance grain yield in sorghum under drought by modifying canopy development and water uptake patterns. New Phytologist 203(3):817-830. |
|
|
Castro-Nava S, Ortiz-Cereceres J, Mendoza-Castillo MDC, Huerta AJ (2012). Biomass production and grain yield of three sorghum lines differing in drought resistance. Phyton (Buenos Aires) 81:149-156. |
|
|
Chen Y, Cothren JT, Chen DH, Ibrahim AMH, Lombardini L (2015). Ethylene inhibiting compound 1-MCP delays leaf senescence in cotton plants under abiotic stress conditions. Journal of Integrative Agriculture 14(7):1321-1331. |
|
|
Crasta OR, Xu WW, Rosenow DT, Mullet J, Nguyen HT (1999).Mapping of post-flowering drought resistance traits in grain sorghum: association between QTLs influencing premature senescence and maturity. Molecular Genetics and Genomics 262(3):579-588. |
|
|
Craufurd PQ, Peacock JM (1993). Effect of heat and drought stress on sorghum (Sorghum bicolor). II. Grain yield. Experimental Agriculture 29(1):77-86. |
|
|
Central Statistical Agency (CSA) (2018). Area and production of major crops (private peasant holdings). Statistical bulletin 586, Addis Ababa, Ethiopia, pp. 1-10. |
|
|
Dahlberg, JA (1992).Variation of 14C assimilate export and partitioning in reduced progressive senescent and senescent sorghums [S.bicolor(L) Moench] and the potential use of anatomical features as a genetic marker for variation in sorghum. Doctoral dissertation, Texas A &M University. |
|
|
Ejeta G (2007). Striga resistance in sorghum: exploitation of the intricate host-parasite biology. Crop Science 47:216-227. |
|
|
Falconer DS (1989). Introduction to Quantitative Genetics. 3rd edition Longman, London, England. |
|
|
Food and Agriculture Organization (FAO) (2017). Food and Agriculture Organization of the United Nations, Rome, Italy. |
|
|
Gregersen PL, Culetic A, Boschian L, Krupinska K (2013). Plant senescence and crop productivity. Plant Molecular Biology 82:603-622. |
|
|
Haussmann B, Mahalakshmi V, Reddy B, Seetharama N, Hash C, Geiger H (2002). QTL mapping of stay-green in two sorghum recombinant inbred populations. Theoretical and Applied Genetics 106(1):133-142. |
|
|
Henzell RG, Brengman R, Fletcher D, McCosker T (1992). Relationships between yield and non-senescence (stay-green) in some grain sorghum hybrids grown under terminal drought stress. In: Foale M A, Henzell R G, Vance P N. (eds). 2nd Australian Sorghum Conference. Gatton, Qld, Australia: Australian Institute of Agricultural Science, Melbourne pp. 355-359. |
|
|
Johnson HW, Robinson HF, Comstock RE (1955). Estimates of Genetic and Environmental Variability in Soya beans. Agronomy Journal 47(7):314-318. |
|
|
Jordan DR, Hunt CH, Cruickshank AW, Borrell AK, Henzell RG (2012). The relationship between the stay-green trait and grain yield in elite sorghum hybrids grown in a range of environments. Crop Science 52(3):1153-1161. |
|
|
Joshi AK, Kumari M, Singh VP, Reddy CM, Kumar S, Rane J, Chand R (2007). Stay green trait: variation, inheritance and its association with spot blotch resistance in spring wheat (Triticum aestivum L.). Euphytica 153(1):59-71. |
|
|
Kassahun B, Bidinger F, Hash C, Kuruvinashetti M (2010). Stay-green expression in early generation sorghum [Sorghum bicolor (L.) Moench] QTL introgression lines. Euphytica 172(3):351-362. |
|
|
Keneni G (2012). Genetic potential and limitations of Ethiopian chickpea (Cicer arietinum l.) germplasm for improving attributes of symbiotic nitrogen fixation, phosphorus upatke and use efficiency, and adzuki bean beetle (Callosobruchus chinensis l.) resistance. PhD thesis. |
|
|
Khera P, Pandey MK, Varshney RK (2013). Pest and diseases: Old and new threats - Modern breeding tools to tailor new crop cultivars. Secheresse 24(4):261-73. |
|
|
Luche HDS, Silva JAGD, Maia LCD, Oliveira ACD (2015). Stay-green: A potentiality in plant breeding. Ciência Rural 45(10):1755-1760. |
|
|
Mace E, Singh V, van Oosterom E, Hammer G, Hunt C, Jordan D (2012). QTL for nodal root angle in sorghum (Sorghum bicolor L. Moench) co-locate with QTL for traits associated with drought adaptation. Theoretical and Applied Genetics 124(1):97-109. |
|
|
Mahalakshmi V, Bidinger FR (2002). Evaluation of stay-green sorghum germplasm lines at ICRISAT. Crop Science 42(3):965-974. |
|
|
Mera GA (2018). Drought & its impacts in Ethiopia. Weather & climate extremes 22:24-35. |
|
|
Ministry of agriculture (MoA) (2018). Plant Variety release, protection and seed quality control directorate. Crop variety register, No. 21, Addis Ababa, Ethiopia, pp. 68-77. |
|
|
Mullan D, Mullan D (2012). Chlorophyll Content. In: Physiological breeding II. A field guide to wheat genotyping. Pask A, Pietragalla J, Mullan D, Reynolds M (eds). CIMMYT, pp. 41-43. |
|
|
Pask A, Pietragalla J (2012). Leaf area, green crop area and senescence. In: Physiological breeding II. A field guide to wheat genotyping. Pask A, Pietragalla J, Mullan D, Reynolds M. (eds). CIMMYT, pp. 58-62. |
|
|
Patterson HD, Thompson R (1971). Recovery of inter-block information when block sizes are unequal. Biometrika 58(3):545-554. |
|
|
Pelleschi S, Rocher JP, Prioul JL (1997). Effect of water restriction on carbohydrate metabolism and photosynthesis in mature maize leaves. Plant, Cell and Environment 20(4):493-503. |
|
|
R Core Team (2019). R: a Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/. |
|
|
Reddy NR, Madhusudhana R, Murali MS, Seetharama N, Jagannatha VP (2014). Detection and validation of stay-green QTL in post-rainy sorghum involving widely adapted cultivar, M35-1 and a popular stay-green genotype B35. BMC Genomics 15(909):1471-2164. |
|
|
Reguera M, Peleg Z, Abdel-Tawab YM, Tumimbang EB, Delatorre CA, Blumwald E (2013). Stress-induced cytokinin synthesis increases drought tolerance through the coordinated regulation of carbon and nitrogen assimilation in rice. Plant Physiology 163(4):1609-1622. |
|
|
Ribaut JM, Bänziger M, Betran J, Jiang C, Edmeades GO (2002). Breeding: Drought tolerance improvement in tropical maize. Quantitative Genetics, Genomics, and Plant Breeding, pp. 85-99. |
|
|
Rosenow DT, Quisenberry JE, Wendt CE, Clark LE (1983). Drought tolerant sorghum and cotton germplasm. Agricultural Water Management 7(1-3):207-222. |
|
|
Sabadin PK, Malosetti M, Boer MP, Tardin FD, Santos FG, Guimara˜es CT, Gomide RL, Andrade CLT, Albuquerque PEP, Caniato FF, Mollinari M, Margarido GRA, Oliveira BF, Schaffert RF, Garcia AAF, van Eeuwijk SA, Magalhaes JV (2012). Studying the genetic basis of drought tolerance in sorghum by managed stress trials and adjustments for phenological and plant height differences. Theoretical and Applied Genetics 124:1389-1402. |
|
|
Sanchez AC, Subudhi PK, Rosenow DT, Nguyen HT (2002). Mapping QTLs associated with drought resistance in sorghum (Sorghum bicolor L. Moench). Plant Molecular Biology 48(5):713-726. |
|
|
Singh BD (2002). Plant Breeding: Principles and Methods. Kalyani Publishers, New Delhi-Ludhiana. |
|
|
Srinivas G, Satish K, Madhusudhana R, Reddy NR, Mohan M, Seetharama SN (2009). Identification of quantitative trait loci for agronomically important traits and their association with genic-microsatellite markers in sorghum. Theoretical and Applied Genetics 118(8):1439-1454. |
|
|
Subudhi PK, Rosenow DT, Nguyen HT (2000). Quantitative trait loci for the stay-green trait in sorghum (Sorghum bicolor L. Moench): consistency across genetic backgrounds and environments. Theoretical and Applied Genetics 101(5-6):733-741. |
|
|
Tao YZ, Henzell RG, Jordan DR, Butler DG, Kellu AM, McIntyre CL (2000). Identification of genomic regions associated with stay green in sorghum by testing RILs in multiple environments. Theoretical and Applied Genetics 100(8):1125-1232. |
|
|
Teshome A, Zhang J (2019). Increase of extreme drought over Ethiopia under climate warming. Advances in Meteorology 2019:1-18. |
|
|
Thomas H, Howarth CJ (2000). Five ways to stay-green. Journal of Experimental Botany 51(suppl_1):329-337. |
|
|
Thomas H, Ougham H (2014). The stay green trait. Journal of Experimental Botany 65(14):3889-3900. |
|
|
Thomas H, Smart CM (1993). Crops that stay-green. Annals of Applied Biology 123(1):193-233. |
|
|
USGS (2017). Indicating Food Security in Ethiopia. Master Thesis Information Management. Tilburg University Student name: Wesley van der Heijden. |
|
|
Vadez V, Deshpande SP, Kholova J, Hammer GL, Borrell AK, Talwar HS, Hash CT (2011). Stay green QTL effects on water extraction and transpiration efficiency in a lysimetric system: Influence of genetic background. Functional Plant Biology 38(7):553-566. |
|
|
van Oosterom EJ, Hammer GL (2008). Determination of grain number in sorghum. Field Crops Research 108(3):259-268. |
|
|
Vinodhana NK, Ganesamurthy K, Punitha D (2009). Genetic variability and drought tolerant studies in sorghum. International Journal of Plant Science 4(2):460-463. |
|
|
Wanous MK, Miller FR, Rosenow DT (1991). Evaluation of visual rating scales for green leaf retention in sorghum. Crop Science 31(6):1691-1694. |
|
|
Xu W, Rosenow DT, Nguyen HT (2000b). Stay-green trait in grain sorghum: relationship b/n visual rating and leaf chlorophyll concentration. Plant Breeding 119(4):365-367. |
|
|
Xu W, Subudhi PK, Crasta OR, Rosenow DT, Mullet JE, Nguyen NT (2000a). Molecular mapping of QTLs conferring stay-green in grain sorghum (Sorghum bicolor L. Moench). Genome 43(3):461-469. |
|
|
Younis ME, El?Shahaby OA, Abo?Hamed SA, Ibrahim AH (2000). Effects of water stress on growth, pigments and 14CO2 assimilation in three sorghum cultivars. Journal of agronomy and Crop Science 185(2):73-82. |
|
|
Zwack PJ, Rashotte AM (2013). Cytokinin inhibition of leaf senescence. Plant Signalling and Behaviour 8(7):247371-247377. |
|
Copyright © 2024 Author(s) retain the copyright of this article.
This article is published under the terms of the Creative Commons Attribution License 4.0