ABSTRACT
Correlation coefficients and stability of grain yield were determined using 6 extra-early quality protein maize (QPM) parental inbred lines and their F1 (15) single crosses evaluated in selected ecological zones of Ghana. The objectives were; to estimate the genetic correlation between grain yield and other agronomic traits and to determine the stability of the single cross hybrids across four locations. Randomized Complete Block Design (RCBD) with three replications was used for each location. Estimates of correlation coefficients and stability analysis of grain yield was done using Genstat 9.2 and additive main effects and multiplicative interaction (AMMI) statistical model (MATMODEL 2.0). Results from phenotypic correlation of grain yield showed highly positive correlation with thousand grain weight (TGW) and number of kernels per row (NKR) across all locations suggesting that selection efficiency could be improved through indirect selection. AMMI analysis revealed non-significant genotype by environment interaction (GEI) for grain yield whilst genotypic and environmental main effects were highly significant. However, the contribution of the environment was higher which suggests that anyone of the locations used in this study can be used for subsequent evaluations in order to manage the limited resources available for the testing program.
Key words: Correlation, stability, grain yield.
Maize (Zea mays L.) is one of the most important cereals cultivated in the world and a primary staple food in many developing countries. In most maize breeding programs, among all the agronomic traits, a particular attention is paid to grain yield. The knowledge of correlation between yield and its component characters and among the component characters is essential for yield improvement programmes (Vidya and Oommen, 2002). Correlation measures the degree of association between two or more characters and is measured by a correlation coefficient (Hallauer and Miranda, 1988). This could be influenced by genetic or environmental (non-genetic) effects. Genetic correlation is associated with the breeding values of two characters (Falconer, 1989) and their measurements can be identified directly in a number of individuals in a population (Hallauer and Miranda, 1988).
Yield is a complex trait determined by several component characters. Therefore, there is the need to consider other contributing traits when selecting for yield.
Grain yield in nature, routinely exhibits GXE Interaction (Khalil et al., 2011) which necessitates evaluation of cultivars in multiple environments (Kang, 2004; Fan et al., 2007). Crop cultivars are grown in diverse environments of different soil types, soil fertility levels, moisture levels, temperatures and cultural practices. During production, all these cumulated conditions constitute the growing environment for the crop varieties (Abdulai et al., 2007). This poses a serious challenge to plant breeders in the identification and selection of appropriate genotypes to perform consistently in multiple environments (Ngaboyisonga, 2008). Selection is therefore, usually ineffective since genotypes may fail to exhibit the same relative performance in varied environments (Knight, 1970). It has also been shown by Comstock and Moll (1963) that correlation between phenotypic and genotypic values was significantly reduced by GEI affecting progress of selection. This is because, relative rankings for major traits often varies across multiple environments hence possibility of identifying single superior genotype poses difficulties if not impossible (Khalil et al., 2011; Abdulai et al., 2007). Stability analysis defines the true performance of a cultivar when reproduced at distinct environments (Brown and Caligari, 2008). As indicated by Khalil et al. (2011), several stability statistic studies have been done to partition GEI which includes regression analysis (Gauch, 1988), multivariate analysis (Westcoff, 1987), cluster analysis (Crossa et al., 1991), additive main effect and multiplicative interaction (AMMI) model (Gauch, 1992) and GGE-biplot (Yan, 1999).
The information on genetic correlation and stability analysis could be beneficial for making breeding strategies as well as to manage available funds, resources and time to achieve the desired research goal. Therefore the objectives of this study were; to estimate the genetic correlation between grain yield and other agronomic traits and to determine the stability of the genotypes across various locations.
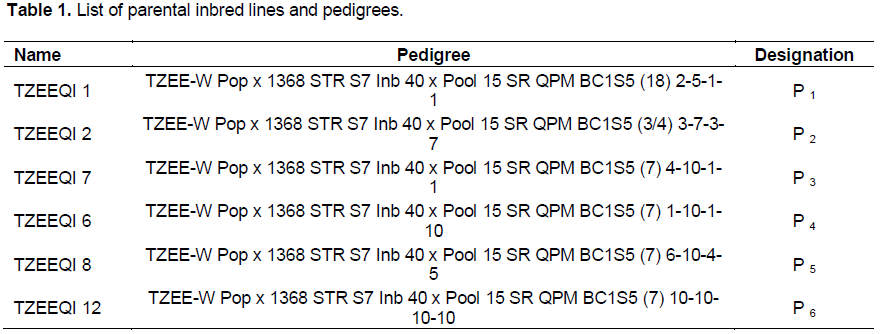
Six extra-early quality protein maize (QPM) F6 inbred lines were obtained from International Institute of Tropical Agriculture (IITA), Ibadan, Nigeria (Table 1). These were crossed in the major season of the year 2011 in partial diallel mating design to form 15 F1 hybrids using hand-pollination (Table 2). These single cross hybrids were produced and evaluated in the major season of 2012 at Crops Research Institute (CRI) – Fumesua in the forest ecological zone of Ghana with coarse sandy-loam soil. The field evaluation was replicated at Ejura in the forest transition zone with fine coarse sandy-loam soil, Pokuase and Akomadan in the coastal savannah and semi-deciduous forest ecological zones respectively with coarse sandy-loam soil for both locations (Sallah et al., 2004).
Randomized complete block design (RCBD) with three replications and two-row plot of 5 m long each with planting interval of 75 cm × 40 cm was used. Three seeds were sown per hill and later thinned to two plants per hill at three weeks after planting (WAP) to obtain a final planting density of approximately 66,000 plants ha-1 in each trial. Normal field management practices such as fertilization, weeding, pest and disease control were used for each location.
Data collection and analysis
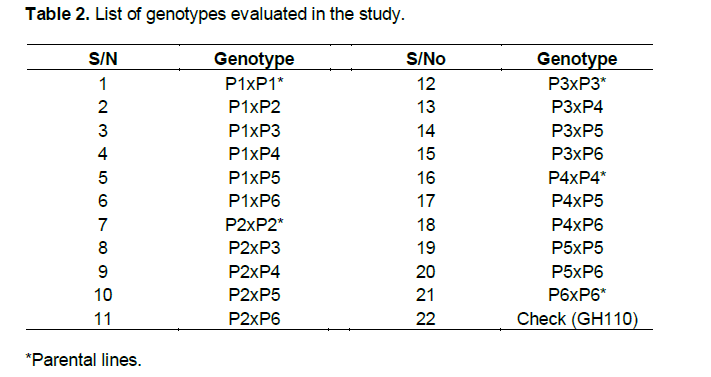
For each plot, five plants were randomly sampled for data on agronomic and morphological characters. Border plants on each row as well as non-competitive plants were excluded. Days to 50% anthesis and full emergence of silks were also recorded and designated as Anthesis (AD) and silking (SD), respectively. Anthesis–silking interval (ASI) was calculated as the difference between number of days to 50% silking and anthesis (SD–AD). A measuring pole was used to measure from the ground level to the node bearing the uppermost ear and flag leaf node and was considered as ear and plant heights respectively. At physiological maturity, root and stem lodging parameters were taken as the percentage of plants leaning at an angle greater than 45° from the vertical and percentage of plants with broken stalks at or below the main ear at maturity respectively. After harvesting, grain weight was obtained using weighing scale. Thousand (1000) kernel weights were determined for each plot. Cob diameter was measured at mid-portion along the cob (n=5) whilst cob length was measured as length of the cob (n=5) from the base to the tip using callipers. The kernel number per row and number of rows per cob were counted and averages recorded for each plot. Estimates of correlation coefficients were determined to show the degree of association between yield and its components, and among yield components using Genstat version 9.2. The genetic (rG) and phenotypic correlations (rP) between two characters, X and Y, were estimated according to Akhtar et al. (2011):
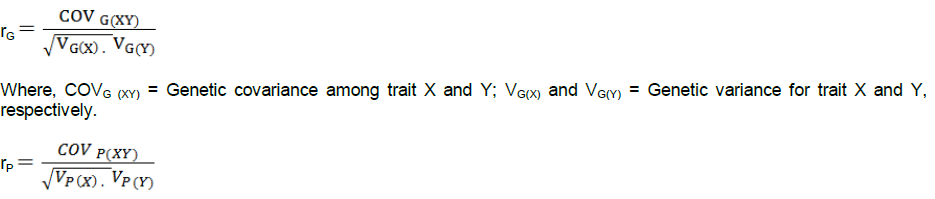
Where, COVP (XY) = Phenotypic covariance among traits X and Y; VP(X) and VP(Y) = Phenotypic variance for traits X and Y, respectively.
The grain yield of individual genotypes was analysed using additive main effects and multiplicative interactions (AMMI) statistical model (MATMODEL 2.0 (Gauch, 1993) to obtain analysis of variance and mean estimates of AMMI.
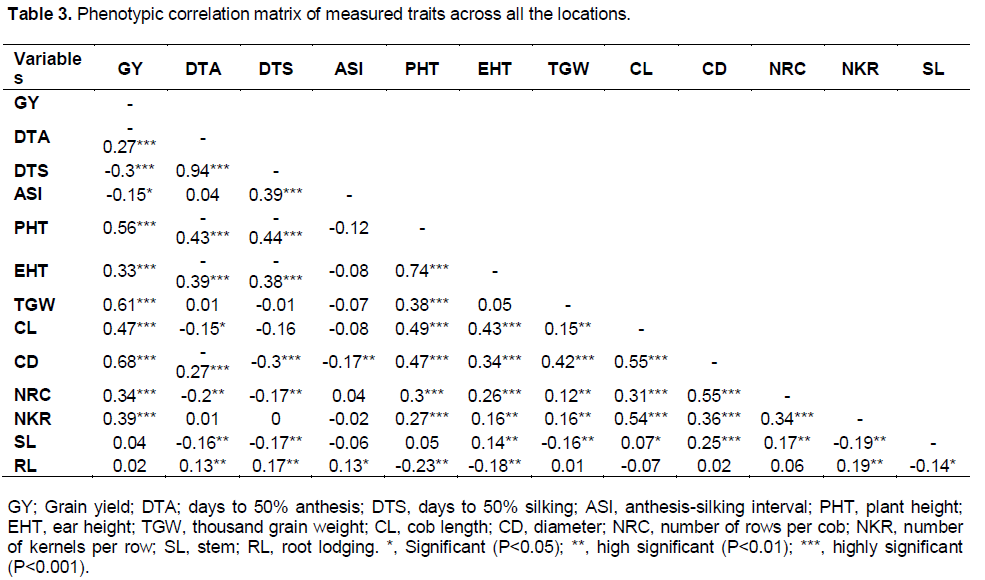
Results from phenotypic correlation showed that grain yield had a highly positive correlation (P<0.001) with plant height, ear height, cob length, cob diameter and number of rows per cob at Ejura and Akomadan (Data not shown). Grain yield showed highly positive correlation with thousand grain weight and number of kernels per row across all locations (Table 3). At flowering there was a highly significant (P<0.001) positive association between days to 50% anthesis and silking across locations. However, DTS and DTA correlated negatively (P<0.05) with plant height, ear height, cob length, cob diameter, and stem lodging. There was no association between days to silking and number of kernels per row. Plant height and ear height showed positive and highly significant (P<0.001) correlation, and both had similar association with cob length, cob diameter, number of rows per cob and number of kernels per row. On the other hand, plant height and ear height had negative and highly significant (P<0.01) correlation with root lodging. Thousand grain weight significantly (P<0.01) correlated positively with plant height, cob length, cob diameter, number of rows per cob and number of kernels per row across all the locations. It was however observed to have negative and highly significant (P<0.01) correlation with stem lodging. The association between cob length and cob diameter was significant (P<0.001) positive.
In the additive main effects and multiplicative interaction (AMMI) analysis of stability and adaptability for grain yield (Table 4), two principal component (PC) axes were generated to decompose genotype × environment interaction and both were not significant. By principle, AMMI analysis is used when genotype by environment interaction (GEI) is significant however this was done to identify the relative importance of these factors. The main effects of genotype and environment were significant at (P<0.01) and (P<0.001), respectively but the contribution of environment (35.8%) was higher than genotypes (10.4%).
Genetic correlation analysis is an important tool for estimating the value and association of various characters with grain yield (Edmeades et al., 1997). The genetic association among traits plays a vital role in improving selection efficiency in plant breeding programs. In selection programs, grain yield and some yield components (such as number of rows per cob, cob length and diameter) are among the most economic traits usually targeted by plant breeders. The studies on relationships among yield and related characters could be important strategy for crop improvement. Therefore, special preferences should be given to these parameters when formulating indirect selection indices for grain yield improvement in maize. The corroborative reports of significant positive correlation between grain yield and other yield components suggests that any one of the traits could be used to select indirectly for grain yield. For instance, Yousuf and Saleem (2001), affirmed the opportunity to select plant height, number of kernels per row cob length (Ali et al., 2010) thousand-grain weight, cob diameter, and number of rows per cob (Rafiq et al., 2010) indirectly for grain yield. On the other hand, the strong positive correlation between traits (days to anthesis and silking; plant height and ear height) suggests that each of two pairs of traits may either be controlled by the same or similar genes or by genes with pleiotropic effect on these traits or may be controlled by closely linked genes (Brown and Caligari, 2008). However, both days to anthesis and silking exhibited negative significant correlation with grain yield, plant height, ear height, cob diameter and stem lodging. Hence, flowering days seems undesirable for indirect selection for these traits. The negative significant association between days to flowering and grain yield agrees with Jayakumar et al. (2007). Depending on the breeder’s objectives, the strong positive association between plant height and ear height as well as their relationship with other traits (thousand-grain weight, cob length, cob diameter, number of rows per cob, number of kernels per row and root lodging) can play a major role in formulating selection indices. The positive correlation among some yield components (thousand-grain weight, cob length, cob diameter, number of rows per cob and number of kernels per row) suggests their usefulness for indirect selection.
The magnitude and nature of genotype by environment (GE) effect displayed by additive main effects and multiplicative interaction (AMMI) analysis of variance for grain yield in the main effects showed higher environmental effects than the genotypic effects. According to Easwari and Sheela (1998), and Cach et al. (2006) as cited by Ssemakula et al. (2007), the predominance of environmental effect over genotypic effect was because yield is a polygenic trait and, therefore, subject to much influence from the environment. As observed in the AMMI analysis, the non-significant GE interaction implies that the genotypes had similar responses across the environments in which they were evaluated and that all the genotypes can reliably be assessed under anyone of the locations used for this study in future or advance evaluation trials (Yan and Tinker, 2006). In other words, it is unnecessary to assess these genotypes simultaneously in the multi- environments used for the study in subsequent evaluations, thereby offering an opportunity to manage the limited resources available for the testing program (Tonk et al., 2011).
The correlation among studied traits especially the positive association between grain yield and other essential yield components (such as thousand-grain weight, cob length, cob diameter, number of rows per cob and number of kernels per row) gives a positive indication that these traits could be considered in developing selection indices in maize improvement programs. The non-significant genotype × environment interaction revealed by AMMI suggests that the relative performance of the genotypes in grain yield did not change across all environments hence anyone of the locations used in this study can be used for subsequent evaluations.
The authors have not declared any conflict of interest.
REFERENCES
|
Abdulai MS, Sallah PYK, Safo-Kantanka DO (2007). Maize grain yield stability analysis in full season lowland maize in Ghana. Int. J. Agric. Biol. 9(1):41-45. |
|
|
|
Akhtar N, Mehmood T, Ahsan M, Aziz A, Ashraf M, Ahmad S, Asif M, Safdar E (2011). Estimation of correlation coefficients among seed yield and some quantitative traits in wheat (Triticum aestivum L.). Afr. J. Agric. Res. 6(1):152-157. |
|
|
|
Ali AW, Azzam HK, AL-Ahmad SA, (2010). Genetic variances, heritability, correlation and path coefficient analysis in yellow maize crosses (Zea mays L.) Agric. Biol. J. N. Am. 1(4): 630-637. http://www.scihub.org/ABJNA (Accessed on 22-11-2011) |
|
|
Brown J, Caligari P (2008). An introduction to plant breeding. Blackwell Publishing Ltd, Oxford, UK.
Crossref |
|
|
Cach NT, Lenis JI, Perez JC, Morante N, Calle F, Ceballos H (2006). Inheritance of useful traits in cassava grown in sub-humid conditions. Plant Breed. J. 125:177-182.
Crossref |
|
|
|
Comstock RE, Moll RH (1963). Genotype-environment interactions. p. 164–196. In W.D. Hanson and H.F. Robinson (ed.) Statistical genetics and plant breeding. National Academy of Sciences– National Research Council Publication. 982. NAS-NRC, Washington, DC. |
|
|
Crossa JP, Fox N, Pfeiffer WH, Rajaram S, Gauch HG (1991). AMMI adjustment for statistical analysis of an international wheat yield trial. Theoret. Appl. Genet. J. 81: 27–37.
Crossref |
|
|
|
Easwari ACS, Sheela MM (1998). Genetic analysis in diallel cross of inbred lines of cassava. Madras Agric. J. 85:264-268. |
|
|
|
Edmeades GO, Bola˜nos J, Chapman SC (1997). Value of secondary traits in selecting for drought tolerance in tropical maize. CIMMYT, El Batán, Mexico, March 25–29. pp. 222–233. |
|
|
|
Falconer DS (1989). Introduction to quantitative genetics. 3rd edition. John Wiley and Sons Inc. New York. |
|
|
Fan X, Kang SM, Chen H, Zhang Y, Tan J, Xu C (2007). Yield stability of maize hybrids evaluated in multi-environment trials in Yunnan, China. Agron. J. 99:220-228.
Crossref |
|
|
|
Gauch HG (1993). Matmodel Version 2.0: AMMI and related analysis for two-way data matrices. Microcomputer power, Ithaca, New York, USA. |
|
|
Gauch HG Jr. (1988). Model selection and validation for yield trials with interaction. Biometrics 44:705-715.
Crossref |
|
|
|
Gauch HG Jr. (1992). AMMI and related models. In: Gauch, H.G. (ed.) Statistical analysis of regional trials. Elsevier Science Publishers, Netherlands. |
|
|
|
Hallauer AR, Miranda JB (1988). Quantitative genetics in Maize breeding. Iowa State Univ. Press, Ames. |
|
|
|
Jayakumar J, Sundaram T, Arun PD, Ragu RRA (2007). Correlation studies in maize (Zea mays L.) evaluated for grain yield and other yield attributes. Int. J. Agric. Sci.(2):57-60. |
|
|
|
Kang MS (2004). Breeding: Genotype-by-environment interaction. In R.M. Goodman (ed.) Encyclopedia of plant and crop science. Marcel-Dekker, New York pp. 218–221. |
|
|
|
Khalil IA, Rahman HU, Naveed-Ur-Rehman AM, Khalil IH, Iqbal M, Atullah H, Afridi K, Sajjad M, Ishaq M (2011). Evaluation of maize hybrids for grain yield stability in North-West Pakistan. Sarhad J. Agric. 27(2):213-218. |
|
|
Knight R (1970). The measurement and interpretation of genotype environment interactions. Euphytica 19:225–235.
Crossref |
|
|
|
Ngaboyisonga C (2008). Quality Protein Maize under stress environments: Gene action and Genotype × environment effects. Doctor of Philosophy Thesis, Department of Plant Science and Crop Protection, University of Nairobi, Kenya. |
|
|
|
Rafiq CM, Rafique M, Hussain A, Altaf M (2010). Studies on heritability, correlation and path analysis in maize (Zea mays L.) J. Agric. Res. 48(1):35-38. |
|
|
Sallah PYK, Abdulai MS, Obeng-Antwi K (2004). Genotypes and environment interactions in three maturity groups of maize cultivars. Afr. Crop Sci. J. 12(2):95 -104.
Crossref |
|
|
|
Ssemakula G, Dixon AGO, Maziya-Dixon B (2007). Stability of total carotenoid concentration and fresh yield of selected yellow-fleshed cassava (Manihot esculenta Crantz). J. Trop. Agric. 45(1-2):14-20. |
|
|
|
Tonk FA, Ilker E, Tosun M (2011) Evaluation of genotype x environment interactions in maize hybrids using GGE biplot analysis. Crop Breeding Appl. Biotechnol. 11:1-9. |
|
|
|
Vidya C, Oommen SK (2002). Correlation and path analysis in yard-long bean. J. Trop. Agric. 40:48-50. |
|
|
Westcoff B (1987). A method of analysis of the yield stability of crops. J. Agric. Sci. 108:267–274.
Crossref |
|
|
Yan W, Tinker NA (2006). Biplot analysis of multi-environment trial data: Principles and applications. J. Plant Sci. 86:623–645.
Crossref |
|
|
|
Yan W (1999). Methodology of cultivar evaluation based on yield trial data-with special reference to winter wheat in Ontario. Doctor Philosophy Thesis submitted to University of Guelph, Ontario, Canada. |
|
|
|
Yousuf M, Saleem M (2001). Correlation analysis of S1 families of maize for grain yield and its components International. J. Agric. Biol. 3(4):387-388. |